Abstract
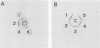
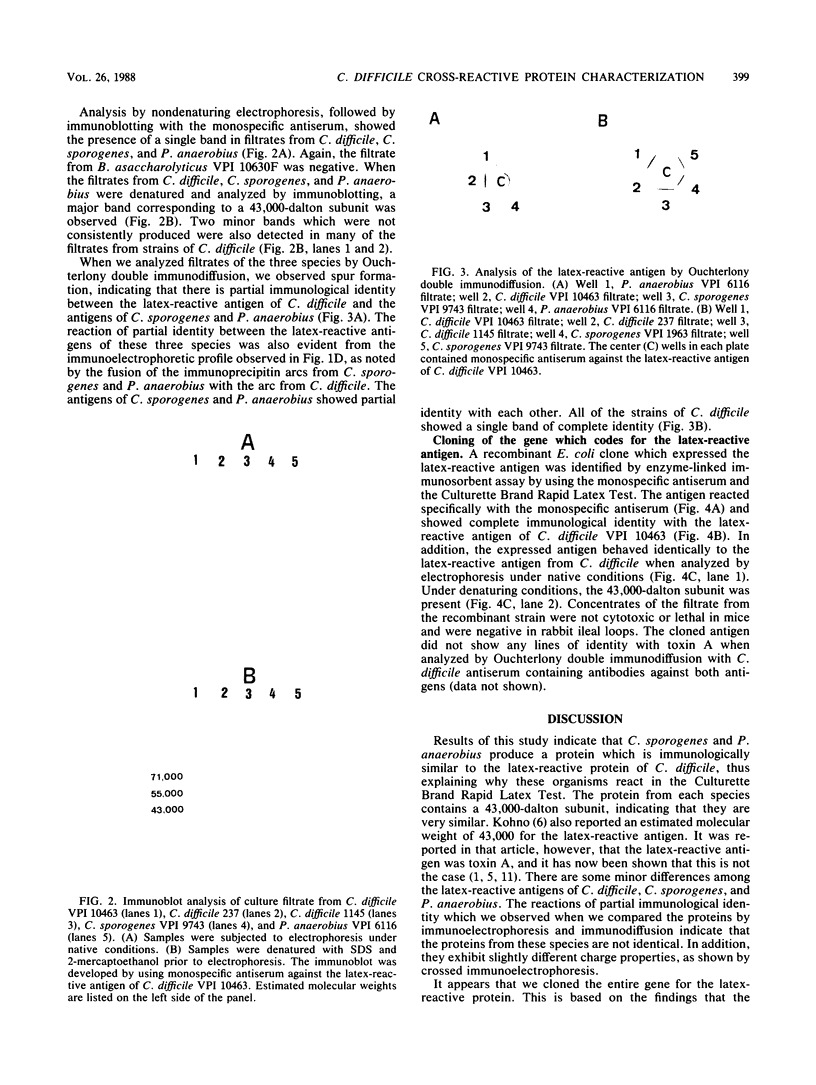
Clostridium sporogenes, Peptostreptococcus anaerobius, and Bacteroides asaccharolyticus have been reported to react in the Culturette Brand Rapid Latex Test (Marion Scientific, Div. Marion Laboratories, Inc., Kansas City, Mo.) for Clostridium difficile. From the results of this study we showed that C. sporogenes and P. anaerobius produce a protein which is very similar biochemically and immunologically to the protein of C. difficile that is detected by the test. Thus, the positive latex reactions observed with C. sporogenes and P. anaerobius are due to a cross-reactive protein. We did not detect this cross-reactive protein in filtrates from B. asaccharolyticus, indicating that this bacterium reacts with the latex reagent by some other mechanism. We cloned the C. difficile gene that codes for the cross-reactive protein and showed that the protein produced by the recombinant organism is nontoxic and distinct from toxin A, thus confirming our earlier findings.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borriello S. P., Barclay F. E., Reed P. J., Welch A. R., Brown J. D., Burdon D. W. Analysis of latex agglutination test for Clostridium difficile toxin A (D-1) and differentiation between C difficile toxins A and B and latex reactive protein. J Clin Pathol. 1987 May;40(5):573–580. doi: 10.1136/jcp.40.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich M., Van Tassell R. L., Libby J. M., Wilkins T. D. Production of Clostridium difficile antitoxin. Infect Immun. 1980 Jun;28(3):1041–1043. doi: 10.1128/iai.28.3.1041-1043.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya S., Nakamura S., Yamakawa K., Nishida S. Evaluation of a commercially available latex immunoagglutination test kit for detection of Clostridium difficile D-1 toxin. Microbiol Immunol. 1986;30(2):177–181. doi: 10.1111/j.1348-0421.1986.tb00932.x. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Wilkins T. D. Purification of Clostridium difficile toxin A by affinity chromatography on immobilized thyroglobulin. Infect Immun. 1987 Aug;55(8):1873–1877. doi: 10.1128/iai.55.8.1873-1877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lyerly D. M., Lockwood D. E., Richardson S. H., Wilkins T. D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Sullivan N. M., Wilkins T. D. Enzyme-linked immunosorbent assay for Clostridium difficile toxin A. J Clin Microbiol. 1983 Jan;17(1):72–78. doi: 10.1128/jcm.17.1.72-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Wilkins T. D. Commercial latex test for Clostridium difficile toxin A does not detect toxin A. J Clin Microbiol. 1986 Mar;23(3):622–623. doi: 10.1128/jcm.23.3.622-623.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. R., Holter J. J., Shanholtzer C. J., Garrett C. R., Gerding D. N. Detection of Clostridium difficile toxins A (enterotoxin) and B (cytotoxin) in clinical specimens. Evaluation of a latex agglutination test. Am J Clin Pathol. 1986 Aug;86(2):208–211. doi: 10.1093/ajcp/86.2.208. [DOI] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]