Abstract
A rapid method using capillary electrophoresis with laser-induced fluorescence detection (CE-LIF) was developed to determine free and protein-bound glutathione (GSH) in human HepG2 hepatocarcinoma cells. The samples were derivatized with 5-iodoacetamidofluorescein (5-IAF), and analyzed at 22 kV using sodium phosphate buffer (10 mM, pH 11.4) and an uncoated 58 cm × 75 µm I.D. fused silica capillary. The analysis time was less than 10 min and N-acetylcysteine was used as internal standard. The derivatization conditions, such as reaction time, 5-IAF concentration, running buffer and cartridge temperature were optimized. Argon gas was used in the study to prevent the oxidization of GSH during sample preparation. The optimized method required only 30–40 nl sample per analysis and was fast and sensitive. The method was applied to the analyses of HepG2 cells treated with the small metal chelating agent, pyrrolidine dithiocarbamate (PDTC). The results demonstrate that the amount of protein-bound GSH, which reflects the amount of protein S-glutathionylation, increased in a time-dependent manner upon cell treatment with PDTC, reaching a maximum of over 50% increase 2 h post-PDTC.
Keywords: Glutathione, Pyrrolidine dithiocarbamate, 5-Iodoacetamidofluorescein, HepG2 cell, Capillary electrophoresis, Laser-induced fluorescence
1. Introduction
The small metal chelator compound, pyrrolidine dithiocarbamate (PDTC), has been used as a cytoprotective agent for reducing organ injury induced by ischemia-reperfusion in rats [1–3], alleviating apoptosis and cell cycle arrest under oxidative stress [4] and improving myocardial contractility in rats with cirrhotic cardiomyopathy [5]. The antitumorigenic and antiinflammatory properties of PDTC have been associated with its free radical scavenging activities and ability to inhibit activation of nuclear factor-kappa B (NF-κB) [6]. PDTC also inhibits activation of STAT3 signal transduction pathways in response to interleukin-6 in human HepG2 hepatoma cells and rat hepatocytes, and restores their insulin responsiveness [7].
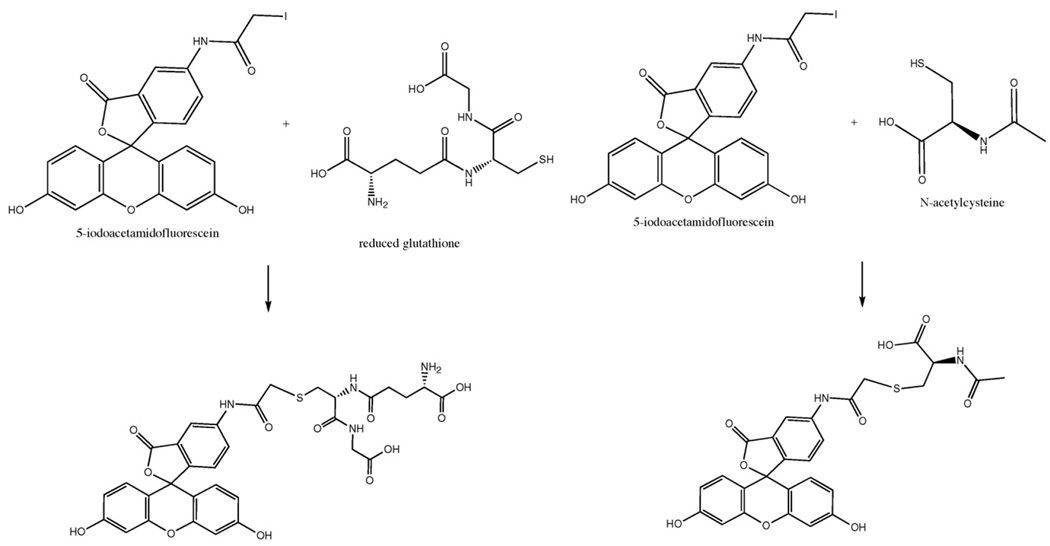
Both NF-κB and STAT3 are redox-sensitive transcription factors whose activities depend on the intracellular redox state. This state is reflected by the cellular levels of the tripeptide glutathione (GSH), Fig. 1, which acts as a powerful antioxidant and a major redox regulator. GSH is found almost exclusively in its reduced form; however, it can undergo oxidation by forming glutathione disulfide (GSSG) in response to oxidative stress. In addition, GSH is also capable of forming mixed disulfide bonds with proteins, a reversible reaction known as S-glutathionylation [8,9]. Protein S-glutathionylation is implicated in cell signaling and metabolic pathways by modulating protein function in response to mild oxidative stress.
Fig. 1.
Derivatization reactions using 5-IAF to label GSH and NAC.
The objective of this study was the development of an analytical method for the determination of intracellular concentrations of protein-bound GSH. Previous studies employed capillary electrophoresis with laser-induced fluorescence detection (CE-LIF) to measure GSH in red blood cells [10], plasma [11], serum [12], bacteria [13] and various cell lines [14]. However, these methods were designed to determine only the concentrations of GSH and (or) other thiols. In this study, a CE-LIF method was developed and optimized for the analysis of protein-bound GSH concentration in intact cells. In addition, the sample preparation procedure was investigated in this study, and argon was found to be an effective way to prevent GSH from oxidation.
After the optimization, the CE-LIF method was applied to the analysis of free and protein-bound GSH levels in crude extracts of control and PDTC-treated HepG2 cells. It was found that PDTC elicited a time-dependent increase in S-glutathionylation of proteins. To the best of our knowledge, the reciprocal regulation of free GSH and protein S-glutathionylation levels by PDTC have not been reported. Therefore, this method provides more information to help understanding of the full extent of the role of PDTC in signal transduction. By determining the change of free and protein-bound GSH levels in cells, this method provides useful information to the biochemists to investigate the effect of oxidative events on the detoxification capacities of the cells.
2. Experimental
2.1. Chemicals
l-Glutathione (GSH), N-acetylcysteine (NAC), tri-n-butylphosphine (TBP), sodium phosphate, hydrochloric acid, dimethyl sulfoxide (DMSO), dimethylformamide (DMF) and acetonitrile were purchased from Sigma–Aldrich (St. Louis, MO, USA). 5-Iodoacetamidofluorescein (5-IAF) was purchased from Invitrogen (Carlsbad, CA, USA). The deionized water was obtained from a Milli-Q system (Millipore, Milford, MA, USA).
2.2. Solutions and derivatization procedure
GSH and NAC aqueous stock solutions were prepared at concentrations of 10 and 3.2 mg/ml, respectively. The stock solutions were stored at −20 °C and used within one month. A 5-IAF stock solution (12.5 mg/ml) was prepared in DMSO, and diluted with sodium phosphate buffer (10 mM, pH 11.4) to desired concentration prior to the derivatization. The phosphate buffer was prepared by dissolving 1.4 g Na2HPO4 in 1000 ml water, and the pH was adjusted to 11.4 using 0.1M NaOH.
Standard solutions with GSH concentrations ranging from 4 to 160 µM and NAC concentration of 10 µM were prepared in water with GSH and NAC stock solution. For the measurement of free GSH, 100 µl of each standard solution was transferred to a 0.5 ml centrifuge tube and mixed with 100 µl 400 µM 5-IAF solution, then the mixture was kept in dark at room temperature for 1 h for the derivatization to complete.
2.3. HepG2 cell culture and treatment procedure
Human HepG2 hepatoma cells were purchased from the American Type Cell Collection (ATCC, Manassas, VA, USA). Cells were cultured in minimal essential medium (MEM): F12 (1:1) supplemented with 10% fetal bovine serum, 1 mM pyruvate, 50 units/ml penicillin/50 µg/ml streptomycin, and 2 mM l-glutamine. Cells were expanded and maintained in a humidified atmosphere of 5% CO2 at 37 °C. The cells were grown on 60 mm tissue culture dishes. At confluency, the number of cells in each dish reaches ~3 million. Confluent cells were treated in serum-free medium in the presence or absence of 50 µM PDTC for 30 and 120 min, after which they were washed twice with ice-cold phosphate-buffered saline and snap frozen in liquid nitrogen. Cells were stored at −80 °C until analysis.
2.4. Sample preparation
HepG2 cells were lysed in 1 ml 2% acetonitrile in water, and transferred to a 1.5 ml centrifuge tube. An additional 200 µl 2% acetonitrile was used to rinse the dish and was combined with the cell extract. To measure the free GSH concentration, three aliquots of 80 µl cell extract were immediately transferred to 0.5 ml centrifuge tubes after a quick mix. Then 20 µl 50 µM NAC was added, followed by the addition of 100 µl 400 µM 5-IAF to the mixture. The derivatization was conducted at room temperature in dark for 1 h, after which the mixtures were centrifuged at 9000 × g for 5 min to sediment the insoluble proteins. 10 µl supernatant was diluted 100× with water and analyzed immediately by CE-LIF.
2.5. Protein-bound GSH analysis
The rest of the cell extract (~700 µl) was vortex mixed and incubated on ice for 10 min, followed by centrifugation at 9000 × g for 5 min at 4 °C.
The protein pellet was rinsed with 1 ml 10% acetonitrile in water. The supernatant was discarded and the protein pellet was dried in a SPD1010 Speedvac system (Thermo Fisher Scientific, Waltham, MA, USA) at 60 °C under reduced pressure for 1 h. The dried protein pellet was dissolved in 400 µl 50 mM NaOH at 60 °C for 30 min, and the protein solution was transferred to two 0.5 ml centrifuge tubes with 200 µl solution in each tube. One tube was spiked with 1 µl 3.2 mM GSH while the second tube received 1 µl water. Then 1 µl 1 mM NAC and 20 µl 10% TBP were added to each tube and incubated for 10 min at room temperature, and followed by the addition of 900 µl acetonitrile to each tube. After centrifugation (9000 × g for 5 min), a 1 ml aliquot of the supernatant was collected, dried in Speedvac system at 60 °C for 4h, 100 µl 400 µM 5-IAF was added and the derivatization reaction was allowed to proceed for 90 min in the dark. The mixture was diluted 100× with water prior to CE analysis.
2.6. CE instrumentation and experimental parameters
All the analyses were conducted with a Beckman P/ACE capillary electrophoresis system(Beckman, Palo Alto, CA, USA) equipped with laser-induced fluorescence detector. The excitation and emission wavelength were 488 and 520 nm, respectively. A 58 cm uncoated fused silica capillary with the I.D. 75 µm from Polymicro (Polymicro Technologies, Phoenix, AZ, USA) was employed. Unless indicated, the separation buffer was sodium phosphate (10 mM, pH 11.4). The sample was injected by pressure (0.5 psi for 5 s) at the anodic end of the capillary. The separation voltage was 22 kV at normal polarity, resulting in an electrophoretic current of 35 µA at 25 °C.
2.7. Protein quantification
The protein content in cell lysates was determined using bicinchoninic acid (BCA) protein assay reagent (Pierce Biotechnology, Rockford, IL, USA) with bovine serum albumin as standard.
3. Results
3.1. Optimization of the derivatization procedure
3.1.1. Reaction time
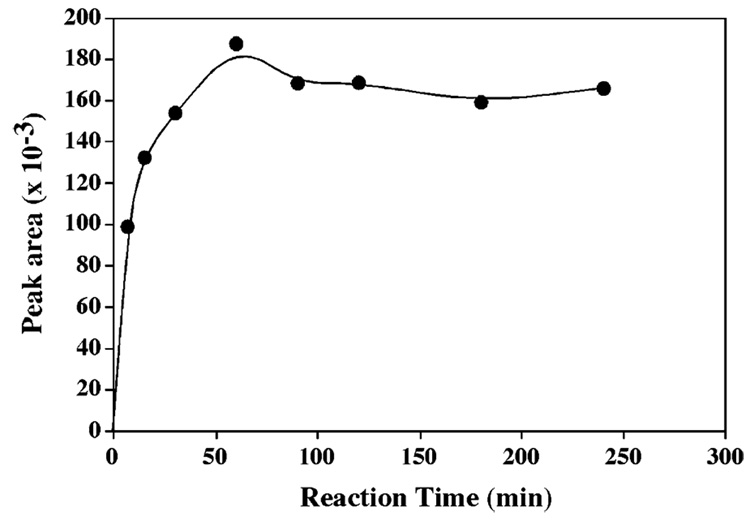
The optimum derivatization time was determined by examining the reaction time profile (Fig. 2). 32 µM GSH was reacted with 400 µM 5-IAF at room temperature, and the GSH peak area was plotted vs reaction time as showed in Fig. 2. When reaction time increased from 5 to 60 min, the peak area increased. After 1 h, the peak area maintained at a similar level. Increasing the reaction time up to 4 h did not improve the result. The initial reactions were carried at room temperature. Increasing the reaction temperature to 40 °C shortened the time required to reach the optimum signal intensity. However, the increasing on the background peaks could be observed. Therefore, the derivatization conditions used in this study were 1 h and room temperature.
Fig. 2.
Reaction time profile. GSH 32 µM, 5-IAF 400 µM.
3.1.2. Optimization of 5-IAF concentration
5-IAF concentration was also optimized. An 8 µM GSH sample solution was derivatized with 8, 80, 200, 400, 800, 1600, 2000, 2500 µM 5-IAF, respectively and analyzed with CE-LIF. The highest signal intensity was obtained with 400 and 800 µM 5-IAF. Considering the increased background peaks when higher IAF concentration was used, 400 µM was chosen for this study.
3.2. CE experimental conditions
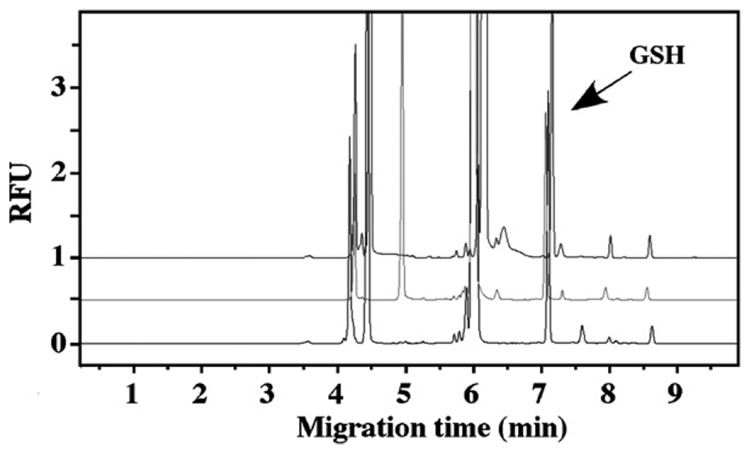
Three different separation buffers were compared (Fig. 3) under the same separation voltage (22 kV, normal polarity) and cartridge temperature (25 °C). It was observed that both sodium phosphate buffers, (10 mM, pH 11.4) and (10 mM, pH 12.5), showed similar separation results. When borate buffer (10 mM, pH 9.0) was used, a small background peak appeared very close to the GSH peak. Based on this observation, sodium phosphate buffer (10 mM, pH 11.4) was chosen. Higher cartridge temperature (40 °C) was also tested. It was observed that when the cartridge temperature was set at 25 °C, the reproducibilities between runs were better than those using 40 °C (results not shown). Therefore, the cartridge temperature was set at 25 °C for all the experiments.
Fig. 3.
Comparison of separation buffers. Upper panel: sodium tetraborate buffer (10 mM, pH 9.0); middle panel: sodium phosphate buffer (10 mM, pH 12.5); bottom panel: sodium phosphate buffer (10 mM, pH 11.4). GSH 32 µM. CE-LIF parameters are indicated in the text.
3.3. Optimization of sample preparation
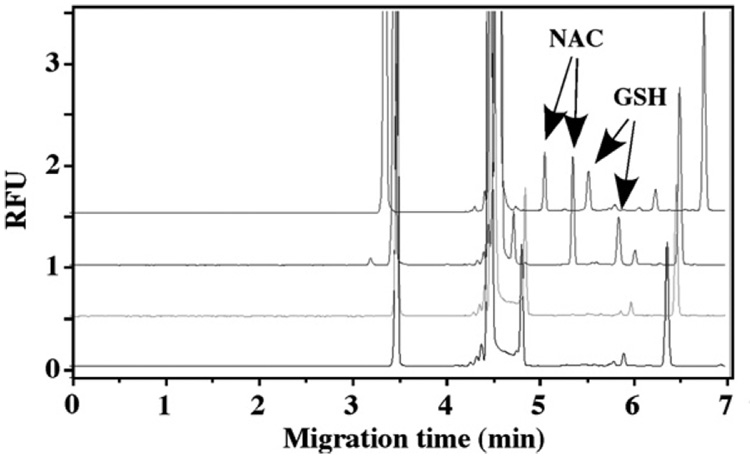
Since GSH can be easily oxidized when pH value is greater than 7, it is very important to address this issue to obtain the accurate GSH concentration [15]. To investigate the effective method to protect the sample from oxidation, 8 µM GSH solutions supplemented with 10 µM NAC as internal standard were prepared in four different ways, derivatized and analyzed (Fig. 4). The results demonstrate that both GSH and NAC are rapidly oxidized when exposed to the air. Continuous nitrogen flow could not prevent GSH and NAC oxidation. TBP was reported as an effective antioxidant and disulfide-cleaving agent [16]. When TBP was added to the reaction mixture, both GSH and NAC could be detected. When argon was used to prevent the oxidation, the centrifuge tubes were filled with argon before the addition of all the solutions. Since argon is heavier than air, it stays at the bottom of the tubes, and insulate the sample solution from the air. The results from argon protection were similar to those obtained with TBP protection. Since this assay was not designed to measure the total GSH in cell extract, argon protection was chosen as the way to prevent the auto-oxidation of the samples. In addition, deionized water and other solutions were degassed for 20 min then filled with argon prior to sample analysis.
Fig. 4.
Optimization of sample preparation procedure. From top to bottom: argon protection; TBP protection; continue nitrogen flow protection; and expose to air for 10 min without protection. GSH 8 µM. CE-LIF parameters are indicated in the text.
3.4. Standard curve
GSH standard solutions with the concentrations ranging between 4 and 160 µM were analyzed in triplicate. An eight-point calibration curve was obtained by plotting the GSH/NAC peak area ratio against the corresponding GSH concentrations. The regression equation obtained was y = 0.0396x – 0.1476, and the r2 value was 0.9976 (n = 8). The regression equation was used for the determination of the free and protein-bound GSH in the cell samples.
3.5. Reciprocal effects of PDTC on the cellular levels of free and protein-bound GSH
Control, 30 min and 2 h PDTC-treated HepG2 cells in quadruplicate were lysed and processed for the analysis of free and protein-bound GSH levels in the presence of argon gas. The procedure was described as above. In short, to measure the free GSH, three aliquots of each cell lysates were incubated with 5-IAF and NAC, and the derivatization was carried out in the dark for 90 min to ensure it was complete. The samples were analyzed by CE-LIF immediately after the derivatization. The RSDs for most of the samples were between 2.3% and 15.5%, except one 30 min PDTC-treated sample (30%). The recovery values for each sample were 115%, 115% and 119% for control, PDTC 30 min and 2 h, respectively. Free GSH concentration of each sample was obtained from the average of three replicas. And the free GSH levels in control, 30 min and 2 h PDTC-treated cells were the average of four samples respectively (Fig. 5). Although not significant, there was a trend toward a reduction in cellular free GSH levels with increasing incubation time with PDTC.
Fig. 5.
Free (left) and protein-bound (right) GSH in PDTC-treated HepG2 cells (n = 4).
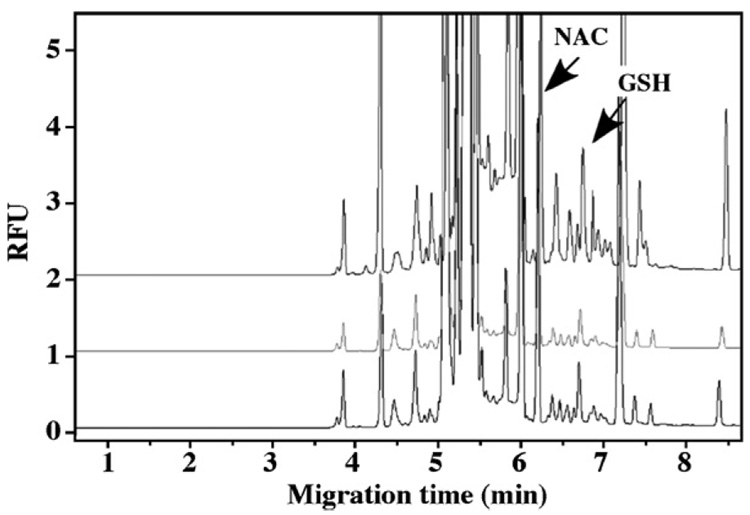
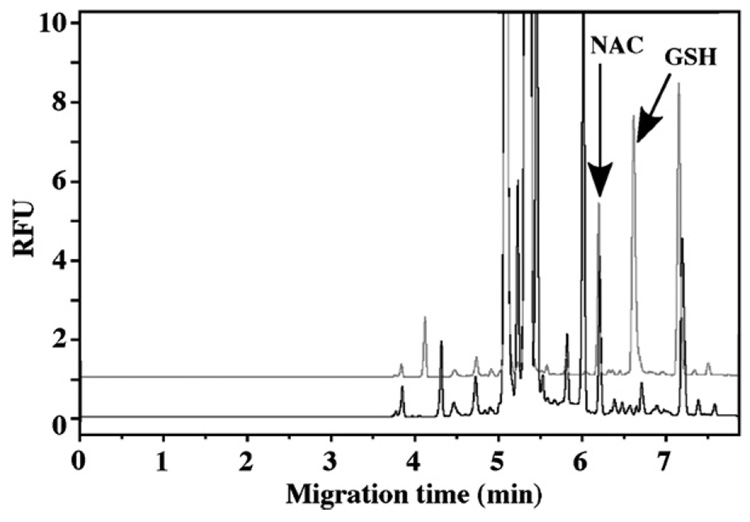
The protein content of the pellets obtained during the measurement of the free GSH was assayed using the BCA protein assay. The results indicated that these pellets contained over 95% of the cellular proteins and therefore, the pellets were used to determine the protein-bound GSH. In these studies, TBP was employed to cleave the disulfide bond to release protein-bound GSH, then derivatized as described in Section 2. The protein-bound GSH in control, 30 min and 2 h PDTC-treated HepG2 cells were analyzed and shown in Fig. 6. GSH peak was confirmed by spiking the sample with 1 µl 64 µM GSH standard solution (Fig. 7). The results (Fig. 5) show a significant increase (>50%) in the amount of protein-bound GSH after a 2-h incubation with PDTC. This suggests that PDTC elicited a time-dependent increase in protein S-glutathionylation. This observation is consistent with PDTC’s ability to protect reactive protein thiols through this reversible posttranslational modification.
Fig. 6.
Electropherograms of protein-bound GSH released from HepG2 cells. Upper panel: PDTC treated 2 h; middle panel: PDTC treated 30 min; lower panel: control.
Fig. 7.
Electropherograms of protein-bound GSH released from HepG2 cells. Upper panel: control spiked with 64 µM GSH; lower panel: control.
4. Conclusions
A simple and sensitive CE-LIF method for the determination of free and protein-bound GSH in intact cells was developed in this study. The experimental parameters, such as the reaction time, the GSH/5-IAF ratio, and separation buffer were optimized. The use of argon was demonstrated to be an efficient way to protect GSH from oxidation during the sample preparation procedure. Unlike previous published papers in which only GSH concentrations were determined, this study was focused on the change of both free GSH and protein-bound GSH concentrations in HepG2 cells. With increase in the PDTC treatment time, a decrease in free GSH concentration, together with a dramatic increase in protein-bound GSH concentration could be observed. This suggests that PDTC might have the ability to promote protein S-glutathionylation. And this observation helps explain PDTC’s ability to attenuate proapoptotic pathways leading to cell death. To the best of our knowledge, the reciprocal regulation of free GSH and protein S-glutathionylation levels by PDTC has not been reported. This information helps to fully understand the role of PDTC in signal transduction.
This method could also be used to evaluate the impact of other chemicals on protein S-glutathionylation. And this information could help interpret the effect of oxidative events on the detoxification capacities of the cells.
Acknowledgements
This work was supported by funds from the Intramural Research Program of the National Institute on Aging of the National Institutes of Health. The authors would like to thank Dr. Heeseung Kim for his kind help with the CE instrument.
References
- 1.El Eter E, Hagar HH, Al-Tuwaijiri A, Arafa M. Can. J. Physiol. Pharmacol. 2005;83:483. doi: 10.1139/y05-034. [DOI] [PubMed] [Google Scholar]
- 2.Tian XF, Yao JH, Li YH, Gao HF, Wang ZZ, Yang CM, Zheng SS. Hepatobiliary Pancreat. Dis. Int. 2006;5:90. [PubMed] [Google Scholar]
- 3.Kabay B, Teke Z, Aytekin FO, Yenisey C, Bir F, Sacar M, Erdem E, Ozden A. World J. Surg. 2007;31:1707. doi: 10.1007/s00268-007-9112-5. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Wu LJ, Tashiro S, Onodera S, Ikejima T. Free Radic. Res. 2008;42:1. doi: 10.1080/10715760701762407. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Lee SS. Liver Int. 2008 doi: 10.1111/j.1478-3231.2008.01692.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Senftleben U. Intensive Care Med. 2003;29:1873. doi: 10.1007/s00134-003-1932-7. [DOI] [PubMed] [Google Scholar]
- 7.He HJ, Zhu TN, Xie Y, Fan J, Kole S, Saxena S, Bernier M. J. Biol. Chem. 2006;281:31369. doi: 10.1074/jbc.M603762200. [DOI] [PubMed] [Google Scholar]
- 8.Dalle-Donnea I, Rossi R, Giustarinib D, Colombo R, Milzania A. Free Radic. Biol. Med. 2007;43:883. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Niwa T. J. Chromatogr.B. 2007;855:59. [Google Scholar]
- 10.Zinellu A, Sotgia S, Usai MF, Chessa R, Deiana L, Carru C. Electrophoresis. 2005;26:1963. doi: 10.1002/elps.200400042. [DOI] [PubMed] [Google Scholar]
- 11.Carru C, Deiana L, Sotgia S, Pes GM, Zinellu A. Electrophoresis. 2004;25:882. doi: 10.1002/elps.200305768. [DOI] [PubMed] [Google Scholar]
- 12.Causse E, Issac C, Malatray P, Bayle C, Valdiguie P, Salvayre R, Couderc F. J. Chromatogr. A. 2000;895:173. doi: 10.1016/s0021-9673(00)00672-5. [DOI] [PubMed] [Google Scholar]
- 13.Musenga A, Mandrioli R, Bonifazi P, Kenndler E, Pompei A, Raggi MA. Anal. Bioanal. Chem. 2007;387:917. doi: 10.1007/s00216-006-0980-6. [DOI] [PubMed] [Google Scholar]
- 14.Zinellu A, Sotgia S, Posadino AM, Pasciu V, Perino MG, Tadolini B, Deiana L, Carru C. Electrophoresis. 2005;26:1063. doi: 10.1002/elps.200406191. [DOI] [PubMed] [Google Scholar]
- 15.Anderson ME. Methods Enzymol. 1985;113:548. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 16.Tang D, Wen L-S, Santschi PH. Anal. Chim. Acta. 2000;408:299. [Google Scholar]