Abstract
The IL-17 cytokine family is composed of six members. IL-17F, discovered in 2001, recently has drawn increasing attention due to its greatest similarity to IL-17, a widely recognized inflammatory cytokine. The genes encoding IL-17 and IL-17F are localized in the same chromosomal region and are co-expressed by CD4+ and γδ T cells. IL-17F can be secreted as homodimers or heterodimers with IL-17. Similar to IL-17, IL-17F utilizes IL-17RA and IL-17RC as its receptor and employs Act1 and TRAF6 as its signal transducers to induce the expression of pro-inflammatory cytokines and chemokines in many different cell types. However, mice lacking either IL-17 or IL-17F exhibit distinct defects in experimental models of asthma and colitis. These results have laid the basis to understand the role of IL-17F in the pathogenesis of human diseases.
1. Introduction
The IL-17 family of cytokines contains six members, IL-17 (also called IL-17A), IL-17B, IL-17C, IL-17D, IL-17E (also known as IL-25) and IL-17F. These polypeptides consist of 163–202 amino acids with molecular masses of 20–30 kDa. They share four conserved cysteine residues at C-terminal region that may participate in the formation of intermolecular disulfide linkages [1]. While little is known about IL-17B, IL-17C and IL-17D, IL-25 with least sequence identity with IL-17 has been shown to regulate innate and adaptive allergic responses [2–4]. On the other hand, IL-17F, the most recently discovered cytokine in this family, shares strongest homology to IL-17 [5, 6].
IL-17 has been associated with the pathogenesis of multiple autoimmune diseases including rheumatoid arthritis, multiple sclerosis and inflammatory bowel diseases [7, 8]. IL-17 also plays a critical role in host defense upon bacterial and fungal infection by recruiting neutrophils and producing antimicrobial-peptide [7]. Recent progress in understanding IL-17 expression and regulation has led to the identification of a new subset of CD4+ helper T (TH) cells, TH17. IL-17F was also found to be co-expressed in TH17 cells [9]. Since many in vitro studies demonstrated that IL-17F has similar proinflammatory function as IL-17, IL-17F may contribute to the host defense and autoimmune function of TH17 cells. In this review, we will summarize the cellular sources, signaling pathways, in vivo and in vitro function of IL-17F based on recent publications.
2. IL-17F expression and regulation
IL-17F was originally identified in human genome sequence using IL-17 sequence [5, 6]. In addition to activated CD4 T cells, unlike IL-17, IL-17F mRNA was also reported to be associated with activated monocytes, basophils and mast cells [5, 10]. However, cellular sources of IL-17F were revealed by RT-PCR in these reports. Expression of IL-17F at protein levels was not assessed until recently when staining antibody or ELISA became available. When naïve CD4 T cells were activated under TH17 polarizing conditions (IL-6 or IL-21 plus TGFβ), they highly produced IL-17F and two-thirds of IL-17F-positive cells were co-stained with IL-17 [11]. Interestingly, T cells treated with only TGFβ to induce Foxp3 expression transiently upregulate the expression of IL-17F but not IL-17 at both mRNA and protein levels [12], the significance of which is not clear at this point. Intracellular cytokine staining using an antibody raised against IL-17F demonstrated that IL-17F is produced by TH17 cells and γδ T cells in vivo [11]. When lamina propria T cells and CD4+ T cells infiltrating into brain of mice subject to experimental autoimmune encephalitis (EAE) were isolated and stimulated with PMA and Ionomycin, these cells also secret IL-17F [11]. A recent report on an IL-17F-RFP reporter mouse demonstrated that differentiated TH17 cells, lamina propria T cells, memory CD4+ T cells, γδ T cells and NKT cells are cellular sources for IL-17F [12].
The gene encoding IL-17F is localized in adjacent to the IL-17 gene on chromosome. During TH17 differentiation, the IL-17F and IL-17 gene promoters associate with histone H3 that are hyperacetylated and tri-methylated at Lys-4 and several evolutionarily conserved non-coding regions in the IL-17-IL-17F gene locus also exhibit association with hyperacetylated H3 [13], indicating a coordinated regulation of the two cytokine genes at the epigenetic levels. Conservation of genomic organization and regulation of IL-17 and IL-17F undermines their restricted expression pattern, otherwise causing inappropriate host immune responses. Transcription factors RORγ, RORα and STAT3 are essential for TH17 development [14–17]. STAT3-deficient CD4+ cells are unable to produce IL-17 or IL-17F [15]. RORγ-deficient T cells exhibited greatly reduced expression of both IL-17 and IL-17F [14, 16]. Interestingly, while overexpression of RORα or RORγ activated IL-17 and IL-17F expression, RORα-deficient T cells did not have compromised IL-17F production while their IL-17 production was reduced [14]. These results suggest that IL-17 but not IL-17F expression may be more sensitive to the concentration of RORs.
In addition to CD4+ T cells and γδ T cells, CD8+ T cells in tumor-bearing mice or human tissues with tumors express equally high levels of IL-17 as CD4 T cells, and these CD8+ T cells are distinct from IFNγ-producing CD8 + T cells [18]. IL-17 was also found to be produced by dendritic cells (DCs), the major cell type in langerhans cell histiocytosis lesions [19]. It is not clear yet whether these CD8+ T cells and DCs also secret IL-17F. Further analysis is needed.
3. Biological function of IL-17F in vitro
IL-17F, very similar to IL-17, has been considered as an inflammatory cytokine since it induces many proinflammatory cytokines and chemokines. Induction of TGF-β and IL-2 was initially reported in vein endothelial cells, suggesting possible role of IL-17F on angiogenesis [10]. Moreover, IL-17F can also induce ICAM1 and GM-CSF expression in airway bronchial epithelial cells [5, 20]. IL-17F upregulates the expression of IL-6 and CXCL1 in fibroblasts and epithelial cells [11]. CCL2, CCL7, TSLP and MMP13 induction by IL-17F in lung fibroblast cells was reported to be less potent when compared to the same concentration of IL-17 [11]. These studies suggest potential overlapping functions for IL-17 and IL-17F that are normally co-expressed.
Synergistic effects of IL-17F with other cytokines have also been examined. IL-17F induces G-CSF expression in combination with TNFα [21]. Together with IL-22, IL-17F induces antimicrobial peptides, hBD-2, S100A7, S100A8 and S100A9 [22]. IL-17F can synergize with IL-23 in human eosinophils to promote the production of IL-1β and IL-6, different from IL-17 [23]. Molecular mechanisms that underlie the synergy of IL-17F with other cytokines are poorly understood. Nonetheless, some studies have pointed that the synergy of IL-17 with other cytokines is achieved by the stability of mRNA transcript of certain inflammatory genes. For example, IL-17 together with TNFα stabilizes IL-6 mRNA [24].
Crystallographic structure of IL-17F revealed that IL-17F adopts a cystine knot fold and dimerization mode is close to the neurotrophin family of cystine knot growth factors [6]. Since IL-17F shares high homology with IL-17, it becomes possible to reason that IL-17F forms heteromeric complexes with IL-17. The existence of human and mouse IL-17A/F heterodimer has recently been demonstrated using biochemical and physiochemical method [25, 26]. Mass spectrometry analysis of natural IL-17A/F heterodimer produced by primary human CD4+ T cells has shown the existence of interchain disulfide-linked peptides containing a peptide from IL-17F and another from IL-17 [25]. In the meantime, mouse IL-17A/F has been reported to be secreted from TH17 cells [26, 27]. While detected, the regulation of homo- and heterodimerization of IL-17 and IL-17F proteins have not been understood. Interestingly, while human CD4+ T cells expressed IL-17A/F heterodimer and IL-17F homodimer with almost no production of IL-17 homodimer [25], mouse TH17 cells secreted all three isoforms [26].
Relative potency of IL-17F is of interest since IL-17, IL-17A/F heterodimer and IL-17 are co-expressed in TH17 cells and may utilize the same receptors [26]. Earlier reports demonstrated weaker activity of IL-17F in inducing cytokines compared to that of IL-17 [21]. Although IL-17F can induce a diverse set of genes, its ability to upregulate transcription factors and subsequently proinflammatory molecules is lower than IL-17 when it is tested at the same concentration as IL-17 [11]. In addition, careful comparison of potency in vivo by instillation of recombinant proteins revealed that IL-17F exhibits the lowest biological activity among IL-17 and IL-17A/F heterodimer [27]. However, one should not overlook the roles of IL-17F since cytokines often exert its effect in concert with other molecules during diseases.
In vitro function of the IL-17A/F heterodimer is so far similar to homodimer IL-17 and IL-17F [27]. Since IL-17 exists as merely a heterodimer in human [25], it became important to explore further the role of heterodimer IL-17A/F. Also, considering that these cytokines may compete for using the same receptors, combined effects of these cytokines should be evaluated.
4. Receptors and signaling pathway of IL-17F
In vitro, IL-17F did not bind to purified IL-17RA protein or compete for IL-17 binding to IL-17RA [6]. Blocking IL-17RA using an antibody, however, is sufficient in removing IL-17 signaling but less efficiently for IL-17F [21]. Therefore, it has been speculated that IL-17F also utilizes IL-17RA but with much lower affinity compared to IL-17. Convincing observation was made when IL-17RA-deficient fibroblasts were reported to exhibit impaired responses to IL-17F in producing proinflammatory cytokines such as IL-6 and CXCL1 [11]. In vivo, when IL-17F was injected to peritoneum of IL-17RA-deficient mice, neutrophil recruitment to the peritoneum was defective in IL-17RA-deficient mice.
Despite that IL-17 elicits strong inflammatory response, the binding to IL-17RA was found to be of relatively low affinity with KD values in the range of 100–500nM while inhibition constant is about 5nM [6, 28]. Discrepancy between receptor affinity and potency of biological activity suggested other receptors may exist. Indeed, IL-17RC was recently reported to form a complex with IL-17RA and is essential to provide IL-17 signaling [29].
IL-17RC has also been shown as the receptor for IL-17F by using recombinant IL-17RC or overexpression of IL-17RC [30] and IL-17RC knockout mice [31]. Human IL-17 and IL-17F both binds to human IL-17RC with high affinity while mouse IL-17 did not bind to mouse IL-17RC [30]. However, since IL-17RA and IL-17RC form a heterodimer [29], lack of either molecules completely abrogate the inflammatory function of IL-17 and IL-17F.
Since IL-17RA and IL-17RC has different tissue expression, roles of IL-17 and IL-17F might be tissue-specific. IL-17RC has limited expression to non-hematopoietic cells while IL-17RA is expressed ubiquitously [30]. Also, IL-17RC exists as many isoforms including a soluble form, which may antagonize IL-17 and IL-17F signaling [32]. Careful comparison of IL-17RA- and IL-17RC-deficient mice will likely reveal additional complexity of IL-17 and IL-17F signaling in vivo.
How IL-17F mediates its signaling through the receptors has begun to be revealed based on the observation made with IL-17. TRAF6, an adaptor and E3 ubiquitin ligase, is essential in IL-17 signaling [33]. However, lack of TRAF6 binding residue in IL-17RA implied existence of other adaptors for IL-17RA. IL-17RA and IL-17R family belong to a new superfamily called STIR (SEFIR - similar expression to fibroblast growth factor (FGF) genes and IL-17Rs - and TIR), and IL-17R family member all contain a conserved sequence segment that shares similar residues with Toll-like receptor/IL-1R (TIR) domain [34]. Despite its similarity with TIR domain, IL-17 does not utilize MyD88 and IRAK4 for cytokine induction [11]. SEFIR domain is also observed in one cytoplasmic protein, Act1, also known as CIKS, a connection to IκB kinase and stress-activated kinase [35, 36]. Also, it contains a TRAF6 binding motif and exhibits TRAF6 association in vitro [37]. Act1 physically associates with IL-17RA through its SEFIR domain and is required for IL-17-induced gene expression [38]. Later, it was demonstrated that Act1 is essential in IL-17-dependent signaling in autoimmune and inflammatory disease [39].
IL-17F has been shown to also employ Act1 and TRAF6 as its adaptor [11]. It is not known whether these adaptor molecules are recruited through IL-17RA or IL-17RC, both of which contain the STIR domains. IL-17F was reported to utilize TRAF6 to ubiquitinate IL-17RA, whereas IL-17 does not require TRAF6 for at least ubiquitination [40]. IL-17F induces transcription factors such as C/EBPβ, C/EBPδ and NFκB in fibroblasts [11]. Downstream signaling pathways of IL-17F may vary depending on cell types. It activates Raf-MEK-extracellular signal-regulated kinase (ERK) 1/2, but not p38 and c-Jun N-terminal kinase in bronchial epithelial and HUVEC cells [41]. However, IL-17F did not activate any of MAPKs but p65 NFκB in gastric cancer cell line [42].
5. Function of IL-17F in human and animal disease models
a. Airway inflammation
IL-17F was originally found in bronchoalveolar lavage cells from allergic asthma patients upon ragweed allergen stimulation [5]. In addition, a coding region variant (His161Arg) of IL-17F gene, possibly encoding an antagonist for IL-17F, has been linked to asthma patients in Japanese populations [43]. Therefore, the role of IL-17F in human asthma and airway inflammation is of great interests.
To analyze the function of IL-17F in airway inflammation, adenoviral infection [44] or Lipofectamine-mediated gene transfer [45] were adopted to overexpress IL-17F in vivo, which resulted in tissue recruitment of neutrophils. However, inhalation of recombinant protein IL-17F alone was not able to trigger any neutrophils recruitment in a recent study [27]. Since IL-17F expression is associated with chronic lung inflammation, the effect of sustained IL-17F expression in vivo in its native form was addressed using lung-specific transgenic overexpression of IL-17F. Overexpression of IL-17F in lung epithelium did not lead to any neutrophil infiltration but resulted in infiltration of lymphocytes and macrophages and mucus hyperplasia [11]. The pathology was observed after several months of IL-17F expression in lung. The features of inflammatory pathology and mucus production in CC10-IL-17F mice are consistent with those observed in CC10-IL-17 mice [46], suggesting chronic expression of IL-17F or IL-17 can lead to similar pathology in lung.
An IL-17F-deficient mouse was generated and analyzed recently [11]. Protease extract from Aspergillus is one type of allergen and inhalation of this agent to mouse causes recruitment of neutrophils within 24 hrs [47]. When IL-17F-deficient mice were challenged with the Aspergillus allergen, neutrophil recruitment into lung tissues was significantly reduced just as IL-17RA-deficient mice, although IL-17-deficient mice did not have this defect [11]. These results indicate that IL-17F but not IL-17 plays an important role in mediating innate responses to allergens in an IL-17RA-dependent manner.
In OVA-alum induced asthma model, IL-17RA-deficient mice were first reported to exhibit reduced generation of TH2 cells [48]. IL-17-deficient mice phenocopied the receptor-deficient animals [11, 49]. However, cultured lung draining lymph nodes from OVA-challenged IL-17F KO mice produced significantly higher levels of IL-4, IL-5 and IL-13 than cells from WT or IL-17KO mice [11]. In spleen, IL-17KO mice showed greatly reduced production of IL-4, IL-5 and IL-13 whereas IL-17F KO mice exhibit enhanced IL-5 and IL-13 production. These analyses indicate that while IL-17 may promote TH2 response in lung through IL-17RA, IL-17F has a regulatory role in restricting allergic asthma development. Whether or not IL-17RC mediates IL-17F signaling in this case remains to be determined.
In addition to asthma, IL-17F has been linked to development of chronic obstructive pulmonary diseases [43] where the disease is manifested by emphysema and chronic bronchitis. However, direct evidence where IL-17F participates in disease initiation or progression does not exist. It will be interesting to see if IL-17F not only mediates inflammation but also actively involved in fibrosis or lung remodeling.
b. Intestinal inflammation
Recent studies have demonstrated that colonic IL-17F expression is associated with inflammatory bowel disease (IBD) and this inducible IL-17F expression is significantly higher in Crohn’s disease as compared with ulcerative colitis [50]. Similar observations were also made with the expression of IL-17 and IL-22 [51, 52]. However, the IL-17F His161Arg polymorphism was found in one report not associated with IBD susceptibility even though this mutation is strongly correlated with incidence of asthma [50]. Contradictory to this report, another study found this polymorphism correlated with the development of ulcerative colitis [53].
Role of IL-17 in the development of intestinal inflammation has been disputed since IL-17 did not contribute significantly to CD4+ T cell adoptive transfer colitis model [54, 55], while IL-17 plays a protective role in dextran sulfate sodium (DSS)-induced colitis [11, 56]. IL-17F, at least in DSS induced colitis model, appears to play a pathogenic role. In IL-17F-deficient mice, CCL2, CCL5, and CCL7 expression were drastically reduced upon DSS treatment, associated with greatly improved pathology [11]. These results indicate IL-17 plays a protective role, whereas IL-17F may exacerbate the intestinal inflammation. The signaling mechanisms of this differential function are unclear at this moment.
c. Neuronal inflammation
While IL-17 was detected in human patients suffering from multiple sclerosis [57], detection of IL-17F in disease lesions has not been reported. However, IL-17F expression was increased in active lesion sites in experimental autoimmune encephalitis (EAE), an animal model of multiple sclerosis (our unpublished observation). IL-17F-deficient mice displayed similar onset of EAE while IL-17–deficient mice had significantly delayed disease [11]. In late stage of EAE, IL-17F-deficient mice exhibited attenuated diseases that were associated with reduced recruitments of CD4+ T cells in the brain and spinal cords in EAE model [11]. However, IFNγ- or IL-17-producing cells in spleen or lymph nodes remained similar or showed little differences between WT and IL-17F-deficient mice [11]. These results indicate that IL-17 may be a more important initiating factor in EAE than IL-17F while both contribute to the chronic inflammation.
d. Other autoimmune diseases: arthritis and psoriasis
Despite its close tie with IL-17, contribution of IL-17F to development of arthritis has not been assessed. However, IL-17F was capable of inducing significant cartilage matrix release and inhibited new cartilage matrix synthesis as efficiently as IL-17 in vitro [6].
IL-17F was elevated in mouse model of psoriasis-like skin inflammation [59] and human psoriasis samples were associated with elevated levels of IL-17F [60]. However, the role of IL-17F in the development psoriasis has not been reported yet.
e. Infections
Upon Klebsiella pneumonia infection, lung tissues of infected mice readily expressed IL-17F within 12 hrs [61]. Also, IL-17F was significantly elevated in sputum of cystic fibrosis patients who were colonized with Pseudomonas aeruginosa undergoing a pulmonary exacerbation [21]. In addition, IL-17F polymorphism is significantly associated with the development of inflammatory changes in the gastric mucosa in H. pylori-infected Japanese subjects [62]. Despite the presence of IL-17F in various inflamed tissues upon infections, little information is known regarding its biological function in host defense.
6. Conclusions
Characterization of IL-17 cytokine family members was started with the cytokine IL-17 and now it is extended to IL-17F and IL-25 (IL-17E). IL-17F could be merely deceived as another inflammatory cytokine, whose expression and function overlap with those of IL-17, sharing similar regulation, signaling and functions. However, detailed experimental studies with appropriate tools such as staining antibody and knockout mice have been instrumental in revealing unexpected role of IL-17F in pathogenesis of asthma and intestinal inflammation. Although in some cases, such studies have identified considerable redundancy of IL-17 and IL-17F, in others, they have also indicated unique functions for IL-17F in the immune system. In addition, the presence of IL-17A/F heterodimers substantially influences the potency, specificity and the spectrum of the activity of these cytokines. Therefore, future studies should be directed to understand the mechanism and role of IL-17F and its related cytokines and provide a platform for development of therapeutic intervention for inflammatory diseases.
Figure 1.
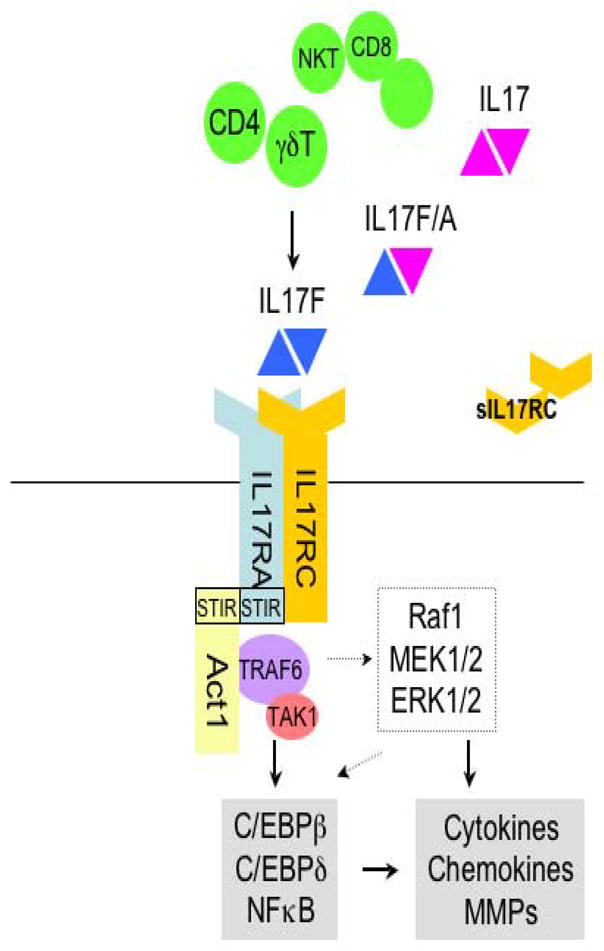
Overview of IL-17F signaling. Once IL-17F is secreted from CD4 and γδT cells, it is recognized by IL-17RA/RC heteromeric complex. IL-17RA, then, recruits the adaptor molecule, Act1. Act1 activates TRAF6 as well as other transcription factors, leading to induction of cytokines, chemokines and MMPs (matrix metalloproteinase) genes (solid arrow indicates direct influence and dashed arrow indicates possible pathways).
Table 1.
In vivo function of IL-17F in comparison to IL-17
| Diseases | IL-17 | IL-17F | References | |
|---|---|---|---|---|
| Arthritis | pathogenic | ND | [58] | |
| EAE | pathogenic | pathogenic (lesser extent) | [11,46] | |
| Chronic lung inflammation | overexpression | pathogenic | pathogenic | [11,46] |
| Asthma | pathogenic or protective | protective | [11,48,49] | |
| COPD | ND | ND | ||
| IBD | CD4- transfer colitis | not required or pathogenic (in combination with IL-6) | ND | [54,55] |
| DSS-induced colitis | protective | pathogenic | [11,56] | |
| Innate allergen challenge | not required | neutrophil recruitment | [11] | |
| Bacterial and fungal infection | protective and pathogenic | ND | [7] | |
The above is a list of various models of disease involving inflammation and/or autoimmunity where IL-17 and IL-17F play pathogenic or protective roles. EAE, experimental autoimmune encephalomyelitis; COPD, chronic obstructive pulmonary disease; IBD, inflammatory bowel disease; DSS, dextran sulfate sodium; ND, not determined.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–74. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 2.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–16. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–9. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchi M, Onuchic LF, Li XD, Essayan DM, Schroeder J, Xiao HQ, Liu MC, Krishnaswamy G, Germino G, Huang SK. Identification of a novel cytokine, ML-1, and its expression in subjects with asthma. J Immunol. 2001;167:4430–5. doi: 10.4049/jimmunol.167.8.4430. [DOI] [PubMed] [Google Scholar]
- 6.Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, Maruoka M, Mao W, Foster J, Kelley RF, Pan G, Gurney AL, de Vos AM, Starovasnik MA. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. Embo J. 2001;20:5332–41. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–34. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 9.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 10.Starnes T, Robertson MJ, Sledge G, Kelich S, Nakshatri H, Broxmeyer HE, Hromas R. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol. 2001;167:4137–40. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- 11.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–75. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J Biol Chem. 2007;282:5969–72. doi: 10.1074/jbc.C600322200. [DOI] [PubMed] [Google Scholar]
- 14.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–63. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–3. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 19.Coury F, Annels N, Rivollier A, Olsson S, Santoro A, Speziani C, Azocar O, Flacher M, Djebali S, Tebib J, Brytting M, Egeler RM, Rabourdin-Combe C, Henter JI, Arico M, Delprat C. Langerhans cell histiocytosis reveals a new IL-17A-dependent pathway of dendritic cell fusion. Nat Med. 2008;14:81–7. doi: 10.1038/nm1694. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi M, Kokubu F, Odaka M, Watanabe S, Suzuki S, Ieki K, Matsukura S, Kurokawa M, Adachi M, Huang SK. Induction of granulocyte-macrophage colony-stimulating factor by a new cytokine, ML-1 (IL-17F), via Raf I-MEK-ERK pathway. J Allergy Clin Immunol. 2004;114:444–50. doi: 10.1016/j.jaci.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 21.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ, Pirhonen J, Kolls JK. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175:404–12. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung PF, Wong CK, Lam CW. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation. J Immunol. 2008;180:5625–35. doi: 10.4049/jimmunol.180.8.5625. [DOI] [PubMed] [Google Scholar]
- 24.Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, Qiu Y, Whitters MJ, Tomkinson KN, Dunussi-Joannopoulos K, Carreno BM, Collins M, Wolfman NM. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem. 2007;282:13447–55. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- 26.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–40. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 27.Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams CM, Wright JF, Fouser LA. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179:7791–9. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 28.Yao Z, Spriggs MK, Derry JM, Strockbine L, Park LS, VandenBos T, Zappone JD, Painter SL, Armitage RJ. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine. 1997;9:794–800. doi: 10.1006/cyto.1997.0240. [DOI] [PubMed] [Google Scholar]
- 29.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–9. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 30.Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, Aujla SJ, Reardon B, Moore M, Shea P, Schreckhise R, Bukowski TR, Presnell S, Guerra-Lewis P, Parrish-Novak J, Ellsworth JL, Jaspers S, Lewis KE, Appleby M, Kolls JK, Rixon M, West JW, Gao Z, Levin SD. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179:5462–73. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–9. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 32.You Z, Shi XB, DuRaine G, Haudenschild D, Tepper CG, Lo SH, Gandour-Edwards R, de Vere White RW, Reddi AH. Interleukin-17 receptor-like gene is a novel antiapoptotic gene highly expressed in androgen-independent prostate cancer. Cancer Res. 2006;66:175–83. doi: 10.1158/0008-5472.CAN-05-1130. [DOI] [PubMed] [Google Scholar]
- 33.Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med. 2000;191:1233–40. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci. 2003;28:226–9. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 35.Leonardi A, Chariot A, Claudio E, Cunningham K, Siebenlist U. CIKS, a connection to Ikappa B kinase and stress-activated protein kinase. Proc Natl Acad Sci U S A. 2000;97:10494–9. doi: 10.1073/pnas.190245697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Commane M, Nie H, Hua X, Chatterjee-Kishore M, Wald D, Haag M, Stark GR. Act1, an NF-kappa B-activating protein. Proc Natl Acad Sci U S A. 2000;97:10489–93. doi: 10.1073/pnas.160265197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanamori M, Kai C, Hayashizaki Y, Suzuki H. NF-kappaB activator Act1 associates with IL-1/Toll pathway adaptor molecule TRAF6. FEBS Lett. 2002;532:241–6. doi: 10.1016/s0014-5793(02)03688-8. [DOI] [PubMed] [Google Scholar]
- 38.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of IL-17 receptor. J Biol Chem. 2006;281:35603–7. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 39.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–56. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 40.Rong Z, Cheng L, Ren Y, Li Z, Li Y, Li X, Li H, Fu XY, Chang Z. Interleukin-17F signaling requires ubiquitination of interleukin-17 receptor via TRAF6. Cell Signal. 2007;19:1514–20. doi: 10.1016/j.cellsig.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 41.Kawaguchi M, Kokubu F, Matsukura S, Ieki K, Odaka M, Watanabe S, Suzuki S, Adachi M, Huang SK. Induction of C-X-C chemokines, growth-related oncogene alpha expression, and epithelial cell-derived neutrophil-activating protein-78 by ML-1 (interleukin-17F) involves activation of Raf1-mitogen-activated protein kinase kinase-extracellular signal-regulated kinase 1/2 pathway. J Pharmacol Exp Ther. 2003;307:1213–20. doi: 10.1124/jpet.103.056341. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y, Toh ML, Zrioual S, Miossec P. IL-17A versus IL-17F induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in AGS gastric adenocarcinoma cells. Cytokine. 2007;38:157–64. doi: 10.1016/j.cyto.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Hizawa N, Kawaguchi M, Huang SK, Nishimura M. Role of interleukin-17F in chronic inflammatory and allergic lung disease. Clin Exp Allergy. 2006;36:1109–14. doi: 10.1111/j.1365-2222.2006.02550.x. [DOI] [PubMed] [Google Scholar]
- 44.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–53. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 45.Oda N, Canelos PB, Essayan DM, Plunkett BA, Myers AC, Huang SK. Interleukin-17F induces pulmonary neutrophilia and amplifies antigen-induced allergic response. Am J Respir Crit Care Med. 2005;171:12–8. doi: 10.1164/rccm.200406-778OC. [DOI] [PubMed] [Google Scholar]
- 46.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiss A, Montes M, Susarla S, Jaensson EA, Drouin SM, Wetsel RA, Yao Z, Martin R, Hamzeh N, Adelagun R, Amar S, Kheradmand F, Corry DB. A new mechanism regulating the initiation of allergic airway inflammation. J Allergy Clin Immunol. 2007;120:334–42. doi: 10.1016/j.jaci.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 48.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–25. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–87. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 50.Seiderer J, Elben I, Diegelmann J, Glas J, Stallhofer J, Tillack C, Pfennig S, Jurgens M, Schmechel S, Konrad A, Goke B, Ochsenkuhn T, Muller-Myhsok B, Lohse P, Brand S. Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): upregulated colonic IL-17F expression in active Crohn’s disease and analysis of the IL17F p. His161Arg polymorphism in IBD Inflamm Bowel Dis. 2008;14:437–45. doi: 10.1002/ibd.20339. [DOI] [PubMed] [Google Scholar]
- 51.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, Leclair S, Herrmann K, Seiderer J, Ochsenkuhn T, Goke B, Auernhammer CJ, Dambacher J. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–38. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 52.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arisawa T, Tahara T, Shibata T, Nagasaka M, Nakamura M, Kamiya Y, Fujita H, Nakamura M, Yoshioka D, Arima Y, Okubo M, Hirata I, Nakano H. The influence of polymorphisms of interleukin-17A and interleukin-17F genes on the susceptibility to ulcerative colitis. J Clin Immunol. 2008;28:44–9. doi: 10.1007/s10875-007-9125-8. [DOI] [PubMed] [Google Scholar]
- 54.Noguchi D, Wakita D, Tajima M, Ashino S, Iwakura Y, Zhang Y, Chamoto K, Kitamura H, Nishimura T. Blocking of IL-6 signaling pathway prevents CD4+ T cell-mediated colitis in a T(h)17-independent manner. Int Immunol. 2007;19:1431–40. doi: 10.1093/intimm/dxm114. [DOI] [PubMed] [Google Scholar]
- 55.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 57.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–8. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 58.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 59.Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, Nickerson-Nutter C, Fouser LA, Young DA. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 61.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–9. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arisawa T, Tahara T, Shibata T, Nagasaka M, Nakamura M, Kamiya Y, Fujita H, Yoshioka D, Arima Y, Okubo M, Hirata I, Nakano H. Genetic polymorphisms of molecules associated with inflammation and immune response in Japanese subjects with functional dyspepsia. Int J Mol Med. 2007;20:717–23. [PubMed] [Google Scholar]


