Abstract
The Tg2576 transgenic mouse is an extensively characterized animal model for Alzheimer’s disease (AD). Similar to AD, these mice suffer from progressive decline in several forms of declarative memory including contextual fear conditioning and novel object recognition (NOR). Recent work on this and other AD animal models suggests that initial cognitive deficits are due to synaptic dysfunction that, with the correct intervention, are fully treatable. We recently reported that acute calcineurin (CaN) inhibition with FK506 ameliorates one form of declarative memory (contextual fear conditioning) impairment in 5 months old Tg2576.
This study tested whether acute CaN inhibition rescues deficits in an additional form of declarative memory, spontaneous object recognition, by employing the NOR paradigm. Furthermore, we determined whether FK506 rescue of NOR deficits depends on the retention interval employed and therefore is restricted to short-term, intermediate-term, or long-term memory (STM, ITM or LTM, respectively). In object recognition, Tg2576 are unimpaired when NOR is tested as a STM task and CaN inhibition with FK506 does not influence NOR STM performance in Tg2576 or WT mice. Tg2576 were impaired in NOR compared to WT mice when a 4 or 24 hour retention interval was employed to model ITM and LTM, respectively. Acute CaN inhibition prior to and during the training session reversed these deficits in Tg2576 mice with no effect on WT performance. Our findings demonstrate that aberrant CaN activity mediates object recognition deficits in 5 months old Tg2576 when NOR is employed as a test for ITM and LTM. In human AD, CaN inhibition may lead the way for therapeutics to improve declarative memory performance as demonstrated in a mouse model for AD.
INTRODUCTION
The transgenic animal model Tg2576 is an extensively characterized mouse model for Alzheimer’s disease (AD; (Hsiao 1998; Kobayashi et al. 2005). The transgene expressed by these mice is a human 695 amino acid splice-variant of APP that contains the double mutation K670N, M671L that leads to elevated Aβ production and accumulation (Hsiao et al., 1996). By all measures thus far, Tg2576 mice are cognitively normal at 3 months of age and cognitive performance declines from the age of 5–6 months onward (Dineley et al. 2002; Westerman et al. 2002). Recent work on this animal model and wildtype (WT) rodents suggests that Aβ-induced cognitive deficits can be reversed in Tg2576 and are transient in WT rodents; suggesting that initial cognitive deficits induced by excess Aβ are due to synaptic dysfunction that, with the correct intervention, are fully treatable (Arendash et al. 2001; Cleary et al. 2005; Dineley et al. 2007; Kotilinek et al. 2002; Lesne et al. 2006).
In support of this notion, we previously reported that the contextual fear conditioning deficit in 5 months old Tg2576 is reversed following a single treatment with the Ca2+/calmodulin-dependent protein phosphatase calcineurin (CaN) inhibitor FK506 (Dineley et al., 2007). This occurs concomitantly with normalization of CaN enzymatic activity yet no change in Aβ levels in the CNS (Dineley et al. 2007). CaN is a key protein phosphatase involved in phosphorylation-dependent kinase activity that is crucial for synaptic plasticity and proper memory function. CaN is up-regulated in the CNS of 5 months old Tg2576; up-regulation of CaN enzymatic activity occurs in several brain regions with the common feature that each of those brain regions also exhibit increased Aβ(Dineley et al. 2007). In vitro, treatment of neuroblastoma cells with oligomeric, but not fibrillar, Aβ1–42 leads to CaN activation that is blocked by antibodies selective for the oligomeric conformation of the peptide (Dineley et al., 2007; Reese et al., 2008). Based on these findings, our current model posits that high levels of oloigomeric Aβ in the CNS of adult Tg2576 mice lead to increased CaN activity and that such excess activity is directly involved in the cognitive deficits observed in these mice.
Cognitive tasks, by virtue of the retention interval employed, test short-, intermediate-, and long-term memory (STM, ITM, and LTM, respectively). STM, ITM, and LTM have temporal constraints that are characterized by unique molecular profiles (Backman et al. 2005; Hawkins et al. 2006; Kandel 2001; Stough et al. 2006). STM typically transpires on a time-scale of minutes and is independent of protein synthesis and gene transcription; rather, STM depends upon modification of pre-existing substrates (e.g. kinases and phosphatases). ITM transpires over the course of several hours, is protein synthesis-dependent, and is gene transcription-independent. LTM is gene transcription-dependent and typically exhibits retention intervals of ≥24 hours. Thus, the training-testing interval is extremely informative regarding the mechanism of action underlying the type of memory impairment.
The novel object recognition (NOR) paradigm is especially well-suited to study STM, ITM, and LTM because first, training and testing occur as single sessions each 10 minutes in duration and, second, simply by manipulating the retention interval one can test STM (2 minute retention interval), ITM (4 hour retention interval), and LTM (24 hour retention interval; (Stough et al. 2006). The retention interval is the amount of time the animals must retain the memory of the identical 3D objects presented during the training session prior to the testing session when one of the familiar objects is replaced with a novel one. We chose to adapt the NOR paradigm to test whether Tg2576 exhibit deficits in an additional form of declarative memory, whether these deficits are restricted to one or more temporal forms of memory, and if CaN inhibition with FK506 ameliorates these deficits in a retention time-dependent manner. This work furthers our knowledge on the specificity and selectivity of rescue of cognitive deficits with CaN inhibition in a mouse model of AD.
MATERIAL AND METHODS
Materials
Novel object recognition was performed in a 40 cm × 40 cm open field chamber with opaque walls (Fig. 1). Floor inserts were constructed upon which 2 cm2 Lego platforms were glued down so that objects with a Lego base could be attached. Object Lego platforms were placed 16 cm apart and 12 cm from the back and side walls of the open field box. Objects ranged from 1.6 × 1.6 × 3.7 to 4.6 × 4.6 × 4.7 cm width × depth ×, height.
Figure 1.
Animal placement for object recognition paradigm. Sample object exposure phase (a) and novel object test (b). (a) Following habituation to the open field box, animals are initially placed into the open field box facing the opposite direction to object placement. A 10 minute training session follows during which time the mice are exposed to sample objects that are identical. (b) After a designated retention period (2 minutes, 4 hours, 24 hours) the sample objects are replaced with one identical to the training object, the other a novel object. Novel object placement is alternated between animals. During both the training and testing phases, the amount of time exploring each object was scored. Top-down view of object recognition box with object perimeters designated (c), and Clever Sys ObjectScan arena set-up (d). Entry of the mouse nose into the object arena is scored as object exploration; the duration of time that the animal‘s nose remains within the arena is measured.
FK506 (Prograf tacrolimus, Fujisawa Healthcare Inc.) was purchased from the UTMB hospital pharmacy. The stock solution (5 mg/mL in 80% ethanol with 200 mg polyoxyl 60 hydrogenated castor oil) was diluted to 2 mg/ml with sterile 0.9% saline and administered at 10 mg/kg i.p. Vehicle injections consisted of 0.9% sterile saline after confirming that the FK506 diluent had no effect on locomotor activity or performance in NOR. All other reagents were molecular biology purity grade and were purchased from Sigma Chemicals.
Animals
Tg2576 and wildtype littermates were bred in the UTMB animal care facility and maintained under USDA standards and IACUC-approved protocols. Dams were C6/SJL F1 females purchased from Jackson Laboratories and sires were Tg2576 (B6SJLF1/J) males from our colony (Hsiao et al. 1996). Males and female mice were used for experimentation. No sex differences were detected; therefore results are reported for male and female mice combined. Mice were tested at 5 months of age; fifteen to thirty animals per experimental group were used. Naïve mice were tested in each experimental paradigm such that each group of mice was tested only once.
Novel Object Recognition
Each mouse was habituated to an empty novel object recognition open field box. Habituation also served to test each animal for normal locomotion. Each mouse was placed in the open field box for two 10 min test sessions 24 hr apart during which the TopScan (Clever Sys. Inc.) video tracking software quantifies various locomotor parameters: total distance traveled, time spent moving ≥50mm/sec, number of rears, number of entries into and time spent in the center 1/9th of the locomotor arena. Prior to performing the experiments reported herein, we ran wildtype animals in which we simultaneously scored object exploration manually and using the ObjectScan (ClverSys) automated software. The parameters used to score object exploration with the automated software tracked very well with the values obtained with manual scoring (i.e., statistically equivalent; two-tailed Student’s t-test p>0.05, t(24)= 1.67.
The object recognition procedure was based on that published by (Bevins et al. 2006). Twenty-four hours after the last habituation session, mice were subjected to training in a 10 minute session of exposure to two identical, non-toxic objects (metal or hard plastic items) in the open field box (Fig. 2). The time spent exploring each object was recorded using ObjectScan (Clever Sys. Inc.); an area 2 cm2 surrounding the object is defined such that nose entries within 2 cm of the object was recorded as time exploring the object (Fig. 1). After the training session, the animal was returned to its home cage. After a variable retention interval of 2 min to 24 hrs, the animal was returned to the arena in which two objects, one identical to the familiar object but previously unused (to prevent olfactory cues and prevent the necessity to wash objects during experimentation) and one novel object. The animal was allowed to explore for 10 minutes, during which the amount of time exploring each object was recorded. Objects were randomized and counterbalanced across animals. Animals that spent< 7 sec exploring the objects during the 10 min test session were omitted from analysis (de Bruin et al. 2006). Objects and arenas were thoroughly cleaned with 70% isopropanol between trials. Mice were tested in NOR only once; we observed reduced exploratory activity if mice were tested repeatedly (data not shown).
Figure 2.
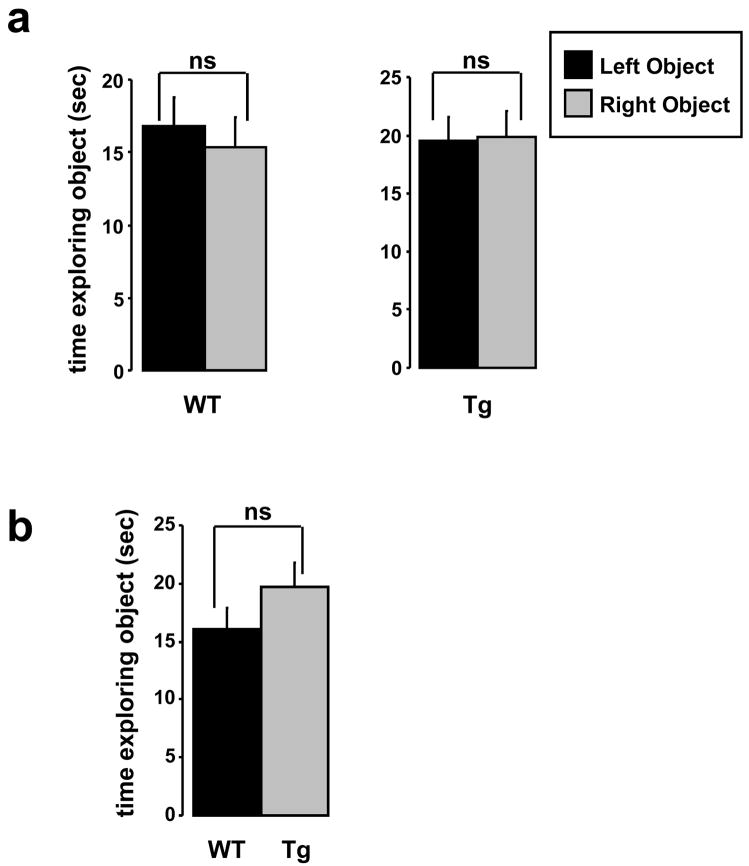
During the training phase of object recognition, genotype influences neither right versus left nor total object exploration time. (a) There was no significant (ns) difference between the amount of time WT or Tg2576 mice explored identical objects placed on the left versus right side of the open field box during exposure to the identical sample objects. p=0.28 and 0.76 Student’s paired two-tail t-test, WT and Tg2576, respectively. (b) The total amount of time WT and Tg2576 explored sample objects during the training phase did not differ. p=0.09 Student’s unpaired two-tailed t-test.
Drug Injections
FK506 was injected at a dose of 10 mg/kg, i.p. approximately 12 hours prior to the NOR training session. This dose and injection regimen of FK506 effectively inhibits CNS CaN activity for at least 36 hours following injection, as described in previous studies (Dineley et al. 2007). Age and sex matched animals were injected with a comparable volume of 0.9% saline.
Data Analysis
For novel object recognition tests, the percent time exploring each object (familiar versus novel) is reported as an object discrimination ratio (ODR) and is calculated using the following formula:
Data is reported as the mean +/− SEM.
Statistics
NOR data was analyzed using GraphPad Prism software. One-sample t-tests were used to determine whether the object discrimination ratio was different than chance (0.50). Two-way ANOVA was used to determine if treatment, genotype or both parameters had significant effect on NOR performance. One-way ANOVA with post hoc Newman-Keuls Multiple Comparison Test was used to determine which treatment groups differed.
As a control, the amount of time each genotypic group explored the left or right (identical) object during the training session was analyzed with paired Student’s t-test in order to determine if genotype influenced spatial preference for object exploration. As well, the mice were analyzed as one group in order to test whether the training set-up differentially influenced left versus right object exploration preference.
RESULTS & DISCUSSION
Validation of the NOR training paradigm
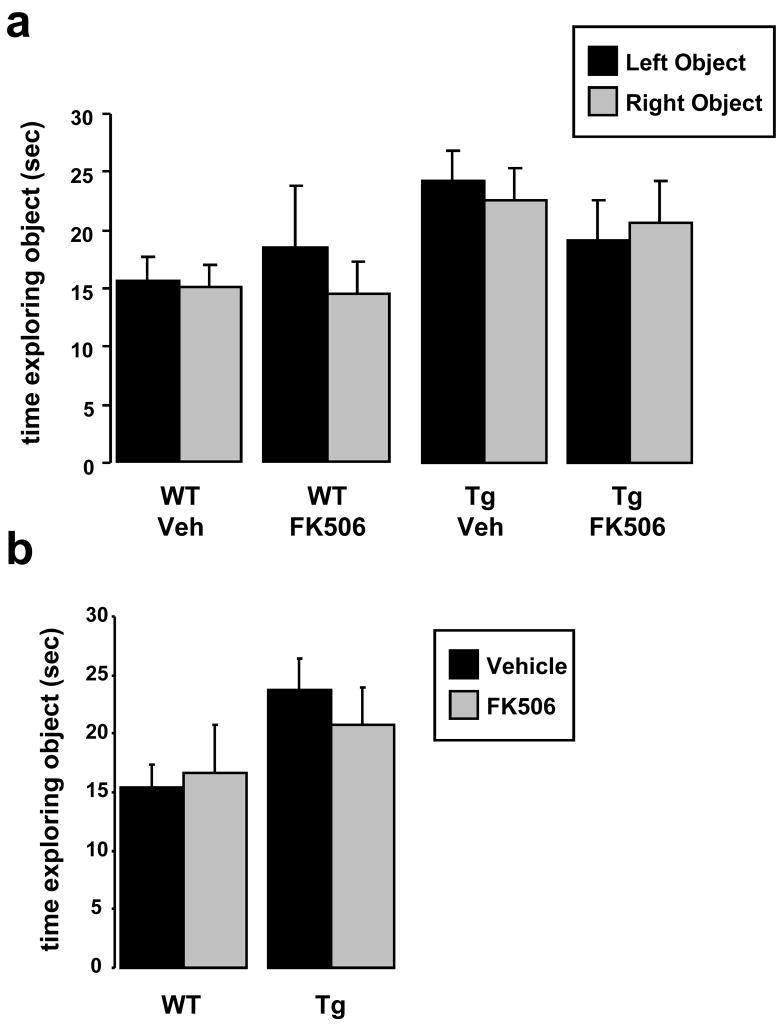
During set-up of the NOR apparatus and training paradigm we wished to validate that we had not introduced any artifactual preferences for right versus left object exploration. We ran 5 months old WT and Tg2576 through training for NOR and analyzed the amount of time each group of animals explored objects placed on the right versus the left of the open field chamber (Figs. 1 & 2). We found no significant difference in exploration time of objects situated on the right versus left of the chamber (Fig 3a); furthermore, we found that genotype had no influence on the total amount of time mice explored identical objects under NOR training conditions (Fig. 3b). We also tested whether FK506 affected right versus left object exploration in WT or Tg2576 and again found no significant difference either in right versus left object exploration in the vehicle-treated groups and no effect of FK506 (Fig. 4a). In addition, FK506 had no effect in the total amount of time WT and Tg2576 explored identical objects under NOR training conditions (Fig. 4b).
Figure 3.
During the training phase of the object recognition paradigm, FK506 treatment did not affect sample object exploration. (a) WT or Tg2576 treated with FK506 (or vehicle) showed no exploratory preference for identical objects placed on the left versus right side of the open field box during sample exposure. Two-tailed t-test found no significant difference between the time WT or Tg2576 mice treated with vehicle or FK506 explored the left versus right sample object during the training phase. p=0.67, 0.29, 0.33, 0.53 for WT-vehicle, WT-FK506, Tg2576-vehicle, and Tg2576-FK506 respectively. One-way ANOVA also did not detect any effect of treatment on object exploration: p=0.10, F(3,101)=2.17 for objects in the left position; p=0.13, F(3,101)=1.95 for objects in the right position. (b) The amount of time WT and Tg2576 explored identical sample objects during the training phase did not differ under conditions of vehicle versus FK506 treatment. Two-tailed t-test found no significant difference between the time WT or Tg2576 mice treated with vehicle or FK506 explored the left versus right sample object during the training phase. p=0.70, 0.41, WT and Tg2576 respectively. Two-way ANOVA found no significant effect of treatment or genotype on object exploration during the exposure phase of object recognition training p=0.58 (F=0.30), 0.23 (F=1.4), and 0.43 (F=0.63) for treatment, genotype and interaction respectively.
Figure 4.
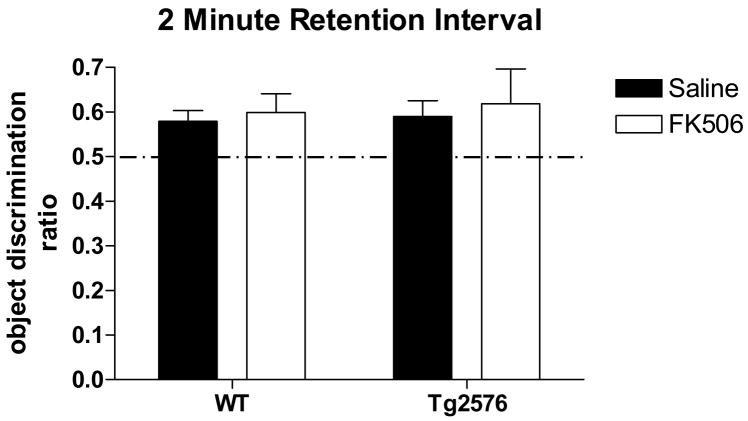
Following a two minute retention interval, Tg2576 exhibit no deficit in novel object recognition; FK506 treatment does not enhance performance in either genotype. WT and Tg2576 mice preferentially explored the novel object after a two minute delay between sample object exploration and the introduction of a novel object for the test phase. Two-way ANOVA found neither a treatment nor genotype effect. Treatment: p=0.56; F(4,87)=0.34. Genotype: p=0.72; F=0.13. Interaction: p=0.92; F=0.01. One-way ANOVA also found no significant difference between the performance of WT or Tg2576 given saline or FK506 (p>0.05).
Based on these careful analyses, we were confident that our NOR apparatus and training paradigm did not introduce any artifactual bias for object exploration; there also was no exploratory bias based on genotype or FK506. We were then ready to use NOR to test for differences in recognition memory between WT and Tg2576 mice.
Tg2576 exhibit NOR deficit depending on the retention interval
NOR training was performed on 5 months old WT and Tg2576 mice injected either with saline or FK506 four hours previously. NOR testing was performed either 2 min, 4 hours, or 24 hours later to test whether Tg2576 exhibit deficits in NOR and whether FK506 treatment reversed any observed deficits.
Utilizing a 2 min retention interval to test for NOR STM, WT and Tg2576 exhibited object recognition by preferentially exploring the novel object during the testing phase as indicated by a statistically significant ODR > 0.50 (Table I; Fig. 5). Two-way ANOVA found neither a treatment nor a genotype effect. One-way ANOVA and post hoc Newman-Keuls analysis found no significant difference between the performance of WT or Tg2576 given saline or FK506. Therefore, when a short (minutes long) retention interval is utilized, Tg2576 perform as well as WT mice; CaN inhibition with FK506 has no effect on either WT or Tg2576 performance. Tg2576 are apparently normal in NOR when it is set up as a STM task and performance of this task is CaN independent.
Table I.
Mean object discrimination ratio and SEM for each of the experiments reported. WT-S = wildtype animals given saline, WT-F= wildtype animals given FK506; Tg-S=Tg2576 animals given saline; Tg-F=Tg2576 animals given FK506. p value reported for one-sided Student’s t-test for whether each group explored the novel object more than chance (0.50).
| WT-S | WT-F | Tg-S | Tg-F | ||
|---|---|---|---|---|---|
| 2 min | Mean | 0.57 | 0.59 | 0.62 | 0.69 |
| SEM | 0.025 | 0.041 | 0.031 | 0.081 | |
| p | 0.035 | 0.036 | 0.004 | 0.003 | |
| 4 hr | Mean | 0.54 | 0.55 | 0.45 | 0.63 |
| SEM | 0.018 | 0.021 | 0.029 | 0.057 | |
| p | 0.042 | 0.001 | 0.319 | 0.013 | |
| 24 hr | Mean | 0.55 | 0.60 | 0.47 | 0.59 |
| SEM | 0.016 | 0.0166 | 0.030 | 0.032 | |
| p | 0.009 | 0.008 | 0.312 | 0.00003 |
Figure 5.
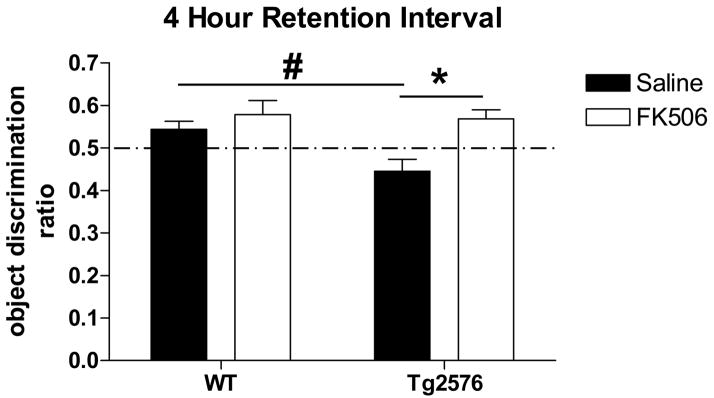
Using a four hour retention interval, Tg2576 exhibit a novel object recognition deficit; FK506 treatment reverses this deficit. Two-way ANOVA detected a treatment, genotype and an interaction. Treatment: p=0.003; F(1,121)=8.97. Genotype: p=0.039; F=4.32. Interaction: p=0.05; F=4.88. One-way ANOVA found no significant difference between the performance of WT given saline or FK506 (p>0.05; F(3,106)=5.51) whereas Tg2576 treated with saline performed significantly worse than WT (#p<0.05); Tg2576 treated with FK506 performed significantly better than Tg2576 given saline (*p<0.01).
WT and Tg2576 mice were then tested for object recognition memory 4 hours after training. In contrast to WT animals, 5 months old Tg2576 exhibit a NOR deficit with a 4 hour retention interval as indicated by an ODR that is significantly less than 0.5 (Table I; Fig. 6). CaN inhibition with FK506 reversed this deficit. Two-way ANOVA detected a treatment, genotype and an interaction effect. Interaction can be interpreted as the treatment not having the same effect on both genotypes, supporting the notion that Tg2576, but not WT, improve in NOR performance when CaN activity is inhibited with FK506. As such, post-hoc Newman-Keuls analysis found no significant difference between the performance of WT given saline or FK506 whereas Tg2576 treated with saline performed significantly worse than WT; Tg2576 treated with FK506 performed significantly better than Tg2576 given saline. Therefore, Tg2576 mice are impaired in NOR when it employs ITM; NOR ITM is modulated by CaN specifically in Tg2576 mice since CaN inhibition improves Tg2576 performance but not that of WT mice.
Figure 6.
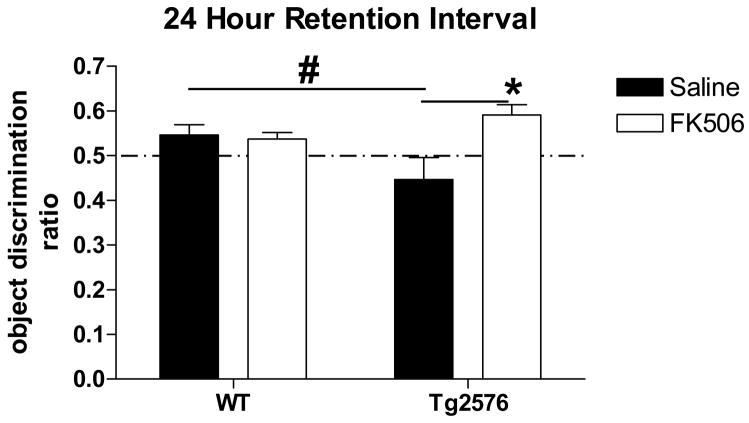
Utilizing a 24 hour retention interval, Tg2576 exhibit a novel object recognition deficit; FK506 treatment reverses this deficit. Two-way ANOVA detected an interaction but no indication of a genotype or a treatment effect. Treatment: p=0.068; F(1,38)=3.54. Genotype: p=0.524; F=0.41. Interaction: p=0.038; F=4.64. One-way ANOVA found no significant difference between the performance of WT given saline or FK506 (p>0.05; F(3,78)=6.23); WT performed significantly better than Tg2576 given saline (#p<0.05) and Tg2576 treated with FK506 performed significantly better than Tg2576 given saline (*p<0.001).
Finally, WT and Tg2576 mice were tested for object recognition memory 24 hours after training in order to test for NOR LTM. As indicated by an ODR significantly less than 0.50, Tg2576 mice at 5 months of age were unable to perform object recognition when the retention interval between training and testing was 24 hours (Table I; Fig. 7). However, as was the case for NOR ITM, CaN inhibition with FK506 improved Tg2576 performance. Two-way ANOVA detected a significant interaction effect suggesting that FK506 treatment differentially affected WT versus Tg2576. In fact, post hoc Newman-Keuls analysis concluded that there was no significant difference between NOR in WT mice given saline or FK506. In addition, statistical analyses found that WT mice performed significantly better than Tg2576 given saline and Tg2576 treated with FK506 performed significantly better than Tg2576 given saline. Therefore, CaN negatively modulates NOR LTM selectively in Tg2576 mice. The ability of FK506 to selectively improve NOR performance in Tg2576 mice supports the idea that the observed increase in CaN activity in Tg2576 brain interferes with NOR as both an ITM and a LTM task.
An earlier study of Tg2576 utilizing a paradigm similar to ours have analyzed NOR and found no differences between Tg2576 and WT littermates when retention intervals of 2 or 30 min retention interval was utilized (Hale et al. 2005). However, in contrast to our findings, Hale and Good (2005) found that both WT and Tg2576 mice preferentially explored the novel object with a 24 hour retention interval. While there were no procedural differences between the present study and that of Hale and Good (2005), this study utilized 14 month old animals in contrast to the 5 month old subjects in this study. In addition, there are noteworthy differences between the background strain upon which their Tg2576 line was maintained. Based on the breeding strategy described, their Tg2576 and WT littermates contain a greater proportion of C57Bl/6 than ours; this difference may underlie the contrasting results obtained in the two studies.
The strength of synaptic transmission and the induction of memory is thought to depend on the balance between opposing molecular signaling factors, such as protein kinases and phosphatases (Wang et al. 1996). As such, CaN is considered an inhibitory constraint on the formation of long-lasting phases of memory. Tg2576 exhibit elevated CaN activity and deficits in NOR ITM and LTM. These cognitive deficits are reversed following acute treatment with FK506; acute FK506 also normalizes CNS CaN activity (Dineley et al., 2007). These observations suggest that the CaN cascade plays an important role in the transition of object recognition from STM to ITM and ITM to LTM. Previous work utilizing genetically altered animals and pharmacological manipulation of CaN provides strong support that this is the case for other cognitive tasks as well (Dineley et al., 2007; Ikegami et al. 2000; Mansuy et al., 1998; Malleret et al, 2001; Sharma et al., 2003).
It must be noted, however, that the CaN inhibitor FK506 generates various downstream biological activities by disruption of the natural FKBP-containing complexes or by formation of novel complexes, such as FKBP12–FK506–calcineurin (Kissinger et al. 1995; Lam et al. 1995; Liu et al. 1991). The FKBP-FK506 complexes result in immunosuppressive activity that may be undesirable in the context of a therapy in which chronic exposure to FK506 would be necessary. In addition, FK506 binds multiple immunophilin isoforms, increasing the risk that off-target activities will lead to unanticipated effects (Cameron et al. 1995; Lam et al. 1995). The fact that a single injection of FK506 was effective in reversing cognitive deficits in Tg2576 and FK506 had no effect on WT NOR (and contextual fear conditioning, Dineley et al., 2007) performance argues against these as potential mechanisms.
In summary, WT and Tg2576 mice perform equivalently well in NOR when short retention intervals were utilized; FK506 had no effect on WT or Tg2576 performance under these conditions. The absence of CaN modulation of a task that typically depends upon the modification (e.g., phosphorylation and dephosphorylation) of existing substrates suggests that CaN-dependent dephosphorylation events are not critical to NOR STM. Tg2576 were impaired in NOR compared to WT mice when a 4 hour retention interval was employed to model ITM. FK506 reversed this deficit signifying that Tg2576 suffer from impaired NOR ITM that is CaN-dependent. Tg2576 mice were also unable to establish NOR when a 24 hour retention interval was used unless CaN inhibition with FK506 was applied. Therefore, CaN appears to be a negative regulator of recognition memory under conditions that require protein synthesis or gene transcription, or both. However, since FK506 treatment had no effect on WT performance regardless of the retention interval employed, CaN’s contribution to NOR is revealed only under conditions of elevated CaN activity resulting from aberrant Aβ accumulation, such as that found in Tg2576 brain. These studies provide additional evidence that synaptic dysfunction underlies early cognitive impairment in AD and may be fully reversible if the appropriate intervention occurs.
Acknowledgments
This work was funded by R21NS053986 from the NIH/National Institute of Neurological Diseases and Stroke to GT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arendash GW, Gordon MN, Diamond DM, et al. Behavioral assessment of Alzheimer’s transgenic mice following long-term Abeta vaccination: task specificity and correlations between Abeta deposition and spatial memory. DNA Cell Biol. 2001;20(11):737–44. doi: 10.1089/10445490152717604. [DOI] [PubMed] [Google Scholar]
- Backman L, Jones S, Berger AK, et al. Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology. 2005;19(4):520–31. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1(3):1306–11. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Cameron AM, Steiner JP, Roskams AJ, et al. Calcineurin associated with the inositol 1,4,5-trisphosphate receptor-FKBP12 complex modulates Ca2+ flux. Cell. 1995;83(3):463–72. doi: 10.1016/0092-8674(95)90124-8. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8(1):79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- de Bruin N, Pouzet B. Beneficial effects of galantamine on performance in the object recognition task in Swiss mice: deficits induced by scopolamine and by prolonging the retention interval. Pharmacol Biochem Behav. 2006;85(1):253–60. doi: 10.1016/j.pbb.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Hogan D, Zhang WR, et al. Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol Learn Mem. 2007;88(2):217–24. doi: 10.1016/j.nlm.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KT, Xia X, Bui D, et al. Accelerated plaque accumulation, associative learning deficits, and up-regulation of alpha 7 nicotinic receptor protein in transgenic mice co-expressing mutant human presenilin 1 and amyloid precursor proteins. Journal of Biological Chemistry. 2002;277(25):22768–80. doi: 10.1074/jbc.M200164200. [DOI] [PubMed] [Google Scholar]
- Hale G, Good M. Impaired visuospatial recognition memory but normal object novelty detection and relative familiarity judgments in adult mice expressing the APPswe Alzheimer’s disease mutation. Behav Neurosci. 2005;119(4):884–91. doi: 10.1037/0735-7044.119.4.884. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Kandel ER, Bailey CH. Molecular mechanisms of memory storage in Aplysia. Biol Bull. 2006;210(3):174–91. doi: 10.2307/4134556. [DOI] [PubMed] [Google Scholar]
- Hsiao K. Transgenic mice expressing Alzheimer amyloid precursor proteins. Exp Gerontol. 1998;33(7–8):883–9. doi: 10.1016/s0531-5565(98)00045-x. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294(5544):1030–8. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kissinger CR, Parge HE, Knighton DR, et al. Crystal structures of human calcineurin and the human FKBP12-FK506-calcineurin complex. Nature. 1995;378(6557):641–4. doi: 10.1038/378641a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi DT, Chen KS. Behavioral phenotypes of amyloid-based genetically modified mouse models of Alzheimer’s disease. Genes Brain Behav. 2005;4(3):173–96. doi: 10.1111/j.1601-183X.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, et al. Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J Neurosci. 2002;22(15):6331–5. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E, Martin MM, Timerman AP, et al. A novel FK506 binding protein can mediate the immunosuppressive effects of FK506 and is associated with the cardiac ryanodine receptor. J Biol Chem. 1995;270(44):26511–22. doi: 10.1074/jbc.270.44.26511. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–7. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Liu J, Farmer JD, Jr, Lane WS, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66(4):807–15. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Reese LC, Zhang W, Dineley KT, Kayed R, Taglialatela G. Selective induction of calcineurin activity and signaling by oligomeric amyloid beta. Aging Cell. 2008;7(6):824–35. doi: 10.1111/j.1474-9726.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stough S, Shobe JL, Carew TJ. Intermediate-term processes in memory formation. Curr Opin Neurobiol. 2006;16(6):672–8. doi: 10.1016/j.conb.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Wang JH, Kelly PT. The balance between postsynaptic Ca(2+)-dependent protein kinase and phosphatase activities controlling synaptic strength. Learn Mem. 1996;3(2–3):170–81. doi: 10.1101/lm.3.2-3.170. [DOI] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, et al. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2002;22(5):1858–67. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]