Abstract
During locomotion, the human body exhibits inherent dynamic properties such as mass (M), stiffness (K) and damping (B). During the gait cycle, foot contact with the ground progresses from the heel to the toe. Contact forces between the foot and ground are defined as ground reaction forces (GRF). It is unclear how body dynamics are affected by foot landing position. If the shape of GRF is indicative of body dynamics, our understanding of gait patterns in normal and pathologic conditions may improve. The aims of this study were to determine: (1) whether foot landing position affects the inherent dynamics of the human body and (2) the extent to which the GRF curve reflects the response of inherent body dynamics to sudden loading.
Eight non-disabled control volunteers performed a series of small jumps and landed on one leg with a fully-extended knee in three foot landing positions: heel, mid-foot, and toe. They then walked at self-paced velocity over force plates. For each foot landing position, values of K, B and the dimensionless damping coefficient, ξ, were calculated from the period of vertical body oscillations, T, and compared with an ANOVA test. In addition, the time between the two peaks of the vertical GRF, TGRF, was compared with T. We found that that K and B decreased and ξ did not change (p < 0.01) between heel to toe landing positions. TGRF was not different than T for the toe-landing position, which suggests that the dynamic body response has major impact on the shape of GRF.
Keywords: body dynamics, stiffness, damping, foot position, toe landing
1. Introduction
Human body dynamics along the vertical longitudinal axis (Z) can be modeled as a second order system when reduced to the properties of body mass (M), stiffness (K) and damping (B). This second order system is represented by:
| (1) |
where g represents gravitational acceleration.
During locomotion, human body dynamics is a function of joint dynamics, activation of involved muscles, joint position, and the properties of the foot interface with the ground. Values of human body stiffness and damping have been reported for single-leg landing in a toe contact position (Cavagna 1970, Ferris et al. 1997, Bach et al. 1983); however, during walking, foot position with respect to the ground shifts from dorsiflexion (heel contact, at initial stance) to plantarflexion (toe off, at terminal stance). The impact of foot position on the vertical body dynamics is unknown. Throughout the paper, we will use the term “body dynamics” to refer to vertical body dynamics during single leg loading, unless otherwise specified.
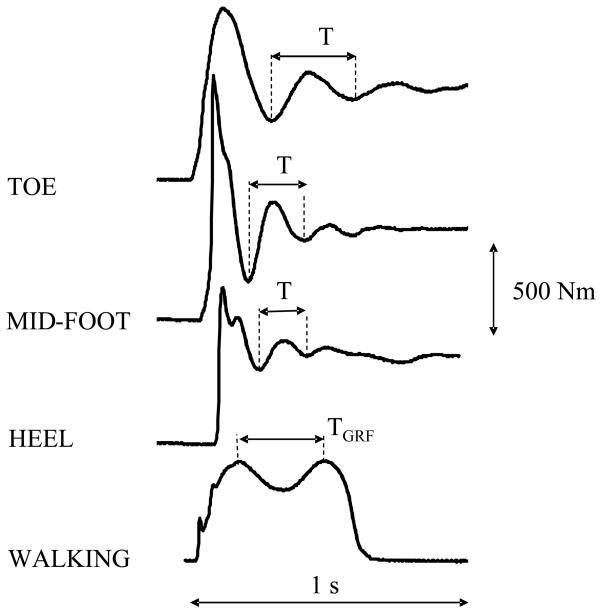
Ground reaction forces (GRF) reflect the force history of the human body’s contact with its environment; specifically, the foot’s contact with the ground. A typical plot of the vertical component of GRF reveals two peaks separated by a single trough (Figure 1, solid line). It is commonly assumed that the GRF plot shape is a direct reflection of net moments of forces generated by the muscles around the ankle, knee and hip joints. It is recognized that ankle plantarflexors play a role on the second peak of GRF knee flexion plays a role on the trough, and body mass and acceleration play a role on the first peak (Winter, 1990; Alexander, 1979).
Fig. 1.
The determination of the period of oscillation (T) for different foot landing positions, (TOE, MID-FOOT, HEEL) and the time between peaks of vertical GRF during self-paced walking (TGRF).
Considering that the response of this second order system (Eq. 1) to sudden loading (e.g., stance phase of walking) should result in damped oscillation, one would expect the shape of the GRF during walking to reflect inherent body dynamics, as has been observed during running (Farley et al., 1996).
Both the position of the foot at initial contact and the shape of the GRF curve are altered in individuals with musculoskeletal abnormalities and/or prosthetic limbs. Determining the impact of foot position on body dynamics as well as the extent to which body dynamics influence the shape of the GRF curve could enhance the clinical interpretation of gait and potentially lead to more effective treatment of musculoskeletal diseases and gait disturbances.
The aims of this study were to determine: (1) whether foot landing position affects the inherent dynamics of the human body and (2) the extent to which the GRF curve reflects the response of inherent body dynamics to sudden loading.
2. Materials and Method
Eight non-disabled subjects (4 men and 4 women, ages 23–54 years) volunteered to take part in this study. A “non-disabled” subject was defined as an individual having no known musculoskeletal disease or abnormality and having not had any prior musculoskeletal manipulation, such as a surgical procedure. Subjects with any current or chronic musculoskeletal complaint, such as stiffness, joint pain or instability, were excluded from the study. The body height of subjects ranged from 1.60 to 2.02 m and their body mass ranged from 48.5 to 96.8 kg. All subjects were enrolled or employed with the Southern Illinois University School of Medicine in Springfield, IL and gave written consent to participate in this study. The protocol was approved by the Springfield Committee for Research Involving Human Subjects (SCRIHS) at Southern Illinois University School of Medicine. The subjects performed three foot loading landing tasks (heel, toe, and mid-foot landing positions) and one self-paced walking task, barefoot and wearing loose, comfortable shorts. They were asked to perform each task 20–50 times. Each subject was instructed to remain balanced in the landing position for approximately 1–2 seconds after ground contact, allowing the body to display an oscillatory motion following the sudden deceleration.
We were studying in the dynamic response of the body after loading the foot with the entire body weight in three different positions. For the first task, each subject stood with all of their body weight on the left foot which was atop a 14-cm wooden block and with their right leg hanging freely. Subjects gently hopped off the block and landed on the ball of the right foot (“toe landing position”, i.e. in full plantarflexion) on the force plate. For the second task, the subject resumed the starting position on top of wooden block with all body weight on the left foot, next the subjects gently hopped off the block and landed on the right heel (“heel landing position”, i.e. in full dorsiflexion) on the force plate. For the third task, the wooden block was removed; the subject began by standing on both feet, 25–35 cm apart. The right foot was initially placed on the force place, and the subject was instructed to stand on the left foot while shifting the body mass from right to left foot, and next shift the body weight to the right leg, landing with right leg on the force place, when the data was collected (“mid-foot landing” position). The subjects were given sufficient time to practice each task in order to learn to perform it correctly.
For all landing tasks, the right foot landed on a force measuring platform (Advanced Medical Technology, Inc.) fixed within the floor. Force measurements recorded by the AMTI plate were processed and plotted on a PC computer using the Vicon Workstation and Polygon software (Oxford Metrics Ltd.) as a function of time, at a sampling frequency of 100Hz. These forces, representing the vertical GRF, generated a graph similar to that of damped harmonic motion (see Fig. 1). Any trial that generated indiscernible oscillations was excluded from our analysis. In addition, any trial in which the subject was unable to hold the landing position for 1–2 seconds or flexed the right knee was excluded. Since no structure was provided to aid or support the subjects, maintaining of balance following impact was challenging. Further, it was vital that the right knee remained fully extended at all times, permitting only minimal movement about the knee joint and thus minimal influence of the knee on the resulting GRF. Consequently, after visual inspection, approximately 10–20 trials for each landing position were accepted for further analysis.
For the final task, the subjects were asked to walk a distance of 20 meters at a self-paced velocity across AMTI force plates for 5–6 trials. GRF data was collected in order to determine the extent to which the GRF curve reflected the response of inherent vertical leg dynamics to sudden loading.
3. Analysis
For each trial analyzed, the peaks of two consecutive oscillations (without the first peak) (Figure 1) were used to determine the period of oscillation (T). Using this measured period and the subject’s mass (M), total body stiffness (K) was calculated as:
| (2) |
The damping (B) of the body in the vertical direction was calculated as:
| (3) |
where A1 and A2 are the amplitudes of consecutive oscillations.
The dimensionless damping factor was calculated as:
| (4) |
In order to compare our result with the published data, we also calculated the frequency of oscillation as f = T−1.
Mean values of variables were calculated for all trials analyzed. A three factor ANOVA test and a post-hoc Tukey HSD test for multiple comparisons were used to study the effect of foot position on body dynamics. A t-test with Bonferroni correction was used to compare the TGRF and the period of body oscillation (T) for each landing position. Statistical analysis was performed using Statistica software (StatSoft Ltd.) with the level of significance p < 0.017.
3. Results
Foot position affected the period of oscillation, T (F(2,23)=18.9, 0<0.001), stiffness, K (F(2,,23)=19.3, p=0.001), and damping, B (F(2,23)=4.7, p<0.018)), but not the dimensionless damping ξ (F(2,23)=0.39, p=0.68).
The lowest periods of oscillation and the highest values of body stiffness and body damping were observed in the heel landing position (Table 1). Stiffness and damping were higher in the heel landing position than in the toe, and they were higher in the mid-foot position than in the toe position. The time between two peaks of GRF TGRF (0.34 ± 0.04 s) was larger (p<0.01) than T for the heel landing and mid-foot landing positions, but was not statistically significantly different from the toe landing position.
Table 1.
The effect of foot landing position on body dynamics in non-disabled controls.
| HEEL (mean ± SD) |
MID-FOOT (mean ± SD) |
TOE (mean ± SD) |
|
|---|---|---|---|
| Period of Vertical Body oscillations T(s) | 0.23 ± 0.041 | 0.24 ± 0.032 | 0.32 ± 0.041,2 |
| Frequency f=T −1 of Vertical Body oscillations (s−1) | 4.35 ±0.751 | 4.17 ± 0.522 | 3.13 ± 0.391,2 |
| Vertical Body Stiffness (kN m−1) | 61.63 ± 21.521 | 56.25 ± 15.282 | 29.77 ± 12.051,2 |
| Vertical Body Damping (kN s m−1) | 1.11 ± 0.331 | 1.06 ± 0.24 | 0.73 ± 0.271 |
| Dimensionless Vertical Body damping ξ | 0.26 ± 0.08 | 0.24 ± 0.05 | 0.26 ± 0.03 |
- statistically significant difference between toe and heel landing positions, p<0.01
- statistically significant difference between toe and midfoot landing positions, p<0.01
SD=standard deviation
4. Discussion
The major findings of this study were that: (1) foot landing position affects inherent body dynamics (e.g. stiffness, damping); and (2) inherent body dynamics may be the important factor determining the shape of the GRF vertical component.
In our study, the body stiffness and damping were highest for the heel landing position; it decreased slightly for the mid-foot landing position, and decreased almost two fold for the toe landing position. The changes of body dynamics as a function of foot landing position have not been previously systematically reported, although Farley et al. (1999) reported that ankle joint is the major determinant of leg stiffness and that leg stiffness changes with the foot landing position. The decrease in stiffness for the toe landing position could be related to ankle dorsiflexion after foot contact with the ground. Our results for body stiffness and damping in the toe landing position are similar to data published by Cavagna, 1970 (K = 24.80 ± 1.70 kNm−1; B = 0.48 ± 0.07 kNsm−1), Bach et al. 1983 (K = 31 ± 8.7 kNm−1) and Farley et al. 1999 (K = 23 kNsm−1). The frequency found for the toe landing position in our study (f = 3.13 ± 0.39 Hz) was close to that reported by Cavagna, 1970 (f = 2.86 ±0.17 Hz) and Bach et al., 1983 (f = 2.99 ± 0.32 Hz). The higher frequency of the leg during heel contact found in our study (f = 4.35 ± 0.75 Hz) has not been previously reported. The higher frequency observed during heel contact implies while the leg may be inherently less sensitive to small disturbances during heel contact than during toe contact (due to a higher stiffness), it is comparatively more responsive to disturbances (due to a faster response). These properties help the leg to automatically adjust to changes in the ground surface during walking.
Despite the differences in body dynamics observed with changing foot landing position, the dimensionless damping coefficient, ξ, remained essentially unchanged (0.24 to 0.26). This suggests that stability of the leg was not affected by the foot landing position in our experiment. The term “stability” used here refers to the ability of the system to reach a different state (or limited cycle of oscillation). The fact that we did not control the height from which the subjects hopped on the force plate could confound the results of our study, especially considering that Farley and Morgenroth (1999) reported that for almost a threefold increase in hop height a twofold increase in ankle stiffness. However it is unclear if stiffness is affected the same way in cyclic (as in Farley and Morgenroth, 1999 study) as it is in single hops (our study) and this should be addressed in future studies.
These changes in body dynamics should be taken into consideration during the design of prosthetics and orthotics that attempt to reproduce “normal” ankle and leg motion Miller et al. (1997) and Bartonek et al (2007).
The foot contact with a ground that takes place at the beginning of the gait cycle is an example of rapid (step-type) loading of the rigid ground with the body’s dynamic system, described by Eq. 1 as a second order system. The typical response of the system consists of an initial transition in loading from 0 to body weight that subsequently either exhibits oscillations or is critically damped. The number of oscillations, or lack thereof, is characterized by the dimensionless damping coefficient, ξ, and the period of the oscillations, T, (or frequency f= T−1), namely by the stiffness and mass (Eq. 2).
In our study, the time between the two peaks of GRF (TGRF) during self-paced walking (TGRF = 0.34 ± 0.04 s) was not statistically significantly different from the period of oscillations (T) for the toe landing position (T = 0.32 ± 0.04 s) (Fig. 1); this suggests that the vertical GRF may be considered as resulting from the response of the global body dynamics to sudden loading. We use the term “global body dynamics” (and stiffness in particular) to refer to the body property that accounts for evident oscillatory patterns of the body’s center of mass. Farley et al. (1999), suggested that angle joint stiffness is the major determinant of leg stiffness, but added that its exact contribution to the global body stiffness is not clear. The finding that the global body stiffness (characterized by TGRF) corresponds to the body dynamics in the toe landing position may be due to the differences between our experimental conditions and walking. During walking, the leg stiffness should be lower than during our experimental conditions because knee flexion is commonly present throughout the gait cycle, (McMahon et al., 1987), whereas, in our tasks the knee was locked. The flexed knee during walking decreases total leg stiffness because the total leg stiffness is equal to the summation of the in-series stiffnesses of all joint elements, the heel pad and foot arch; therefore, total leg stiffness is smaller than the smallest stiffness of the elements. Other possible reasons for decreased stiffness during walking are that the single leg angle varies with respect to the vertical during walking, the single leg interactions are only present during the single support phase of walking, and the dynamics is changed by muscle activation. Further studies should clarify the role of each of these components in body dynamics.
Our data not only reports the foot position-related changes in body dynamics, but also provides another argument to emphasize the role of body dynamics in the execution of the movement task. The concept that a body’s dynamics limits its performance in the time domain was introduced to human ambulation by Cavagna et al. (1966) and Alexander (1979, 2005) and to cyclic movements by Kelso et al. (1987). Human walking may be considered an example of the simplified control of a complex, nonlinear system that terminates in the cyclic, smooth motion of the body’s center of mass (“limited cycle of oscillation” in Poincare’s sense, Gibson, 1963; Clark and Phillips, 1993).
The efficiency and dynamic stability of a cyclic system is maximized when it is driven at its resonant frequency (Rosenblum et al., 1988). The self-tuning of frequency f (f = T−1) of voluntary repetitive movements to the resonant frequency has been shown to occur in the ankle joint (Jones et al., 1971; Bach et al., 1983); in the forearm (Neilson, 1972); in the knee (Lebiedowska, 2008) and during running (Farley et al., 1996, 1999; Ahlborn et al., 2002). Farley et al. (1999) reported that the preferred frequency of one leg hopping was 2.17 ± 0.07 rad s−1 (SE), which is very close to the natural frequency of the leg in toe landing position found in our study (ω = 2.01 ± 0.26 rad s−1, Table 1).
Walking at a self-paced velocity might reflect the tuning of walking velocity (through cadence and/or stride length) to the global body dynamics in order to minimize energy consumption by utilizing the energy stored in the body (Holt et al., 1991). The elastic rebound of the body that occurs with each step may play a role in the efficient exchange of kinetic and potential energy by the center of mass, as has been reported in running (Schepens et al., 1998) and walking (Biewener, 2006). Interestingly, symmetry of the GRF peaks, minimum energy consumption and minimum walking variability all occur at the self-paced velocity of 1.3 m s−1 (Burdett et al., 1983; Zarrugh et al., 1974, Breit et al., 1997; Jordan et al., 2007).
During walking, the characteristics of body structure (i.e. the spatial relationships between body segments) provides sufficient stiffness to prevent structural collapse and sufficient damping for both planned (voluntary) and unplanned (reflex) interactions with the structure’s environment. Thus, muscle activation patterns may emerge through the training of neural and biomechanical network specific for a movement tasks that utilizes the body dynamics most optimal for the given task; rather than result from a series of net torque generations at the joints created to counteract external forces acting on a body.
Alexander (2005) estimated that a family of robots modeling the utilization of body dynamics (so-called “passive walkers”) exhibited a more energetically-similar cost of walking to human beings to than robots propelled by generating torque at the level of the each joint. This concept is compelling, particularly when interpretation using inverse dynamics fails; for example, when co-activation of antagonistic muscles is present, which would lead to zero net torques at a joint. This concept might also lend explanation to the commonly-reported activation of plantarflexors muscles during the terminal stance phase of walking (Winter, 1990), which may be a way to decrease the ξ at terminal stance and equalize the two peaks of GRF - in sum, to ensure a constant energy flow. A neural block of the gastrocnemius muscle (major ankle plantarflexor) tends to decrease, but not eliminate the second peak of GRF (Sutherland, 1980), and plantarflexors’ role in propulsion has been reported to be minor, (Michel and Do, 2002) even in gait initiation.
Some clinical observations support the view that the central gait generator may be driven by body dynamics through peripheral inputs from proprioceptors rather than by a robust central drive (Grillner, 2006). For example, various clinical conditions that affect body dynamics at the ankle joint (e.g., talipes equinus seen in cerebral palsy, foot drop secondary to tibial nerve or other nervous system damage, ankle fusion surgery, artificial limbs) result in abnormal gait patterns and the shape of GRF. Cook et al. (1997) showed that knee restriction using a stiff brace resulted in decreased time T between the peaks of the vertical GRF, and that foot equinus in patients with cerebral palsy often results in a third oscillation. The decrease in cadence observed when an artificial foot is loaded with additional mass may be a reflection of optimization in response to changing leg dynamics (Donn et al., 1989).
Changes in gait parameters secondary to the particular disease may thus be considered as an optimal tuning (adaptation) to abnormal body dynamics. Wagenaar and Van Emmerik (1994) suggested that identification of body dynamics and the ability of a patient to facilitate transition into new movement patterns are crucial for effective therapy. Perhaps one way to improve the abnormal kinematics patterns observed during pathological gait would be to ensure that the comprehensive treatment include steps to compensate for abnormal body dynamics. Any attempt to train a repetitive movement pattern without addressing the abnormal body dynamics may not be effective long term. Such optimization of gait patterns to body dynamics has been suggested for children with cerebral palsy by Jeng et al. (1996) and for patients with other movement disorders by Wagenaar and Van Emmerik (1994). Future studies should clarify if the long term effect of training that incorporates dynamic (mechanical network) changes is indeed advantageous over training that is based solely on the emergence of neural networks.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlborn BK, Blake RW. Walking and running at resonance. Zoology (Jena) 2002;105(2):165–174. doi: 10.1078/0944-2006-00057. [DOI] [PubMed] [Google Scholar]
- Alexander M. Walking Made Simple. Science. 2005;308(5718):58–59. doi: 10.1126/science.1111110. [DOI] [PubMed] [Google Scholar]
- Alexander RM, Maloiy GMO, Hunter B, Jayes AS, Nturibi J. Mechanical stresses in fast locomotion of buffalo (Syncerus-Caffer) and elephant (Loxononta-Africana) Journal of Zoology. 1979;189:135–144. [Google Scholar]
- Bach TM, Chapman AE, Calvert TW. Mechanical resonance of the human body during voluntary oscillations about the ankle joint. Journal of Biomechanics. 1983;16:85–90. doi: 10.1016/0021-9290(83)90049-0. [DOI] [PubMed] [Google Scholar]
- Bartonek A, Eriksson M, Gutierrez-Farewik EM. A new carbon fibre spring orthosis for children with plantarflexor weakness. Gait & Posture. 2007;25(4):652–656. doi: 10.1016/j.gaitpost.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Patterns of mechanical energy change in tetrapod gait: pendula, springs and work. Journal of Experimental Zoology. 2006;305A:899–911. doi: 10.1002/jez.a.334. [DOI] [PubMed] [Google Scholar]
- Breit GA, Whalen RT. Prediction of human gait parameters from temporal measures of ground contact. Medicine and Science in Sports and Medicine. 1997;29(4):540–547. doi: 10.1097/00005768-199704000-00017. [DOI] [PubMed] [Google Scholar]
- Burdett RG, Skrinar GS, Simon SR. Comparison of mechanical energy work and metabolic energy consumption during normal gait. Journal of Orthopaedic Research. 1983;1(1):63–72. doi: 10.1002/jor.1100010109. [DOI] [PubMed] [Google Scholar]
- Cavagna GA. Elastic bounce of the body. Journal of Applied Physiology. 1970;29(3):279–282. doi: 10.1152/jappl.1970.29.3.279. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Margaria R. Mechanics of walking. Journal of Applied Physiology. 1966;21(1):271–278. doi: 10.1152/jappl.1966.21.1.271. [DOI] [PubMed] [Google Scholar]
- Clark JE, Phillips SJ. A longitudinal study of intralimb coordination in the first year of independent walking: a dynamical systems analysis. Child Development. 1993;64:1143–1157. [PubMed] [Google Scholar]
- Donn JM, Porter D, Roberts VC. The effect of footwear mass on the gait patterns of unilateral below-knee amputees. Prosthetics and Orthotics International. 1989;13:140–144. doi: 10.3109/03093648909079422. [DOI] [PubMed] [Google Scholar]
- Farley CT, Gonzalez O. Leg stiffness and stride frequency in human running. Journal of Biomechanics. 1996;29(2):181–186. doi: 10.1016/0021-9290(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Farley CT, Morgenroth DC. Leg stiffness primarily depends on ankle stiffness during human hopping. Journal of Biomechanics. 1999;32(3):267–273. doi: 10.1016/s0021-9290(98)00170-5. [DOI] [PubMed] [Google Scholar]
- Farley CT, Blickhan R, Saito J, Taylor CR. Hopping frequency in humans: a test of how springs set stride frequency in bouncing gaits. Journal of Applied Physiology. 1991;71(6):2127–2132. doi: 10.1152/jappl.1991.71.6.2127. [DOI] [PubMed] [Google Scholar]
- Ferris DP, Farley CT. Interaction of leg stiffness and surface stiffness during human hopping. Journal of Applied Physiology. 1997;82(1):15–22. doi: 10.1152/jappl.1997.82.1.15. discussion 13–4. [DOI] [PubMed] [Google Scholar]
- Gibson JE. Nonlinear Automatic Control. New York: McGraw-Hill Book Company; 1963. [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52(5):751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Holt KG, Hamill J, Andres RO. Predicting the minimal energy costs of human walking. Medicine and Science in Sports and Exercise. 1991;23(4):491–498. [PubMed] [Google Scholar]
- Holt KG, Wagenaar RC, LaFiandra ME, Kubo M, Obusek JP. Increased musculoskeletal stiffness during load carriage at increasing walking speeds maintains constant vertical excursion of the body center of mass. Journal of Biomechanics, Apr. 2003;36(4):465–471. doi: 10.1016/s0021-9290(02)00457-8. [DOI] [PubMed] [Google Scholar]
- Jeng S, Holt K. Self-Optymazation of Walking in Nondisabled Childern and Children with Spastic Hemiplegia Cerebral Palsy. Journal of Motor Behavior. 1996;28:15–27. doi: 10.1080/00222895.1996.9941729. [DOI] [PubMed] [Google Scholar]
- Jones GM, Watt DG. Observations on the control of stepping and hopping movements in man. Journal of Physiology. 1971;219(3):709–727. doi: 10.1113/jphysiol.1971.sp009684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan K, Challis JH, Newell KM. Walking speed influences on gait cycle variability. Gait & Posture. 2007;26(1):128–34. doi: 10.1016/j.gaitpost.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Kelso JAS, Schöner G. In: Toward a physical (synergetic) theory of biological coordination. Graham R, Wunderlin A, editors. Vol. 9. New York: Lasers and synergetics, Springer Proceedings in Physics; 1987. pp. 224–237. [Google Scholar]
- Lebiedowska MK. The kinematic consequences of invariant dynamics in children 6 to 18 years of age. Journal of Biomechanics. 2008;41(11):2458–64. doi: 10.1016/j.jbiomech.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon TA, Valiant G, Frederick EC. Groucho running. Journal of Applied Physiology. 1987;62:2326–2337. doi: 10.1152/jappl.1987.62.6.2326. [DOI] [PubMed] [Google Scholar]
- Michel V, Do MC. Are stance ankle plantar flexor muscles necessary to generate propulsive force during human gait initiation? Neuroscience Letters. 2002 Jun 7;325(2):139–43. doi: 10.1016/s0304-3940(02)00255-0. [DOI] [PubMed] [Google Scholar]
- Miller LA, Childress DS. Analysis of a vertical compliance prosthetic foot. Journal of Rehabilitation Research & Development. 1997;34:52–58. [PubMed] [Google Scholar]
- Neilson PD. Frequency-response characteristics of the tonic stretch reflexes of biceps brachii muscle in intact man. Medical & Biological Engineering. 1972;10(4):460–472. doi: 10.1007/BF02474194. [DOI] [PubMed] [Google Scholar]
- Rosennblum LD, Turvey MT. Maintance tendency in cooordinated rhythmic movements: relative fluctuation and phase. Neuroscience. 1988;27:289–300. doi: 10.1016/0306-4522(88)90238-2. [DOI] [PubMed] [Google Scholar]
- Schepens B, Willems PA, Cavagna GA. The mechanisms of running in children. Journal of Physiology. 1998;509(3):927–940. doi: 10.1111/j.1469-7793.1998.927bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland DH. The role of plantar flexors in normal walking. Journal of Bone and Joint Surgery (American) 1980;62:354–363. [PubMed] [Google Scholar]
- Wagenaar RC, van Emmerik REA. Dynamics of pathological gait. Human Movement Sciences. 1994;13:441–471. [Google Scholar]
- Winter DA. Biomechanics and Motor Control of Human Movement. 2. Wiley Interscience; New York: 1990. 1990. [Google Scholar]
- Zarrugh MY, Todd FN, Ralston HJ. Optimization of Energy Expenditure during level walking. European Journal of Applied Physiology. 1974;33:293–306. doi: 10.1007/BF00430237. [DOI] [PubMed] [Google Scholar]