Abstract
Partially or completely unfolded polypeptides are highly prone to aggregation due to nonspecific interactions between their exposed hydrophobic surfaces. Extracellular proteins are continuously subjected to stresses conditions, but the existence of extracellular chaperones remains largely unexplored. The results presented here demonstrate that one of the most abundant extracellular proteins, fibrinogen has chaperone-like activity. Fibrinogen can specifically bind to nonnative form of citrate synthase and inhibit its thermal aggregation and inactivation in an ATP-independent manner. Interestingly, fibrinogen maintains thermal-denatured luciferase in a refolding competent state allowing luciferase to be refolded in cooperation with rabbit reticulocyte lysate. Fibrinogen also inhibits fibril formation of yeast prion protein Sup35 (NM). Furthermore, fibrinogen rescues thermal-induced protein aggregation in the plasma of fibrinogen-deficient mice. Our studies demonstrate the chaperone-like activity of fibrinogen, which not only provides new insights into the extracellular chaperone protein system, but also suggests potential diagnostic and therapeutic approaches to fibrinogen-related pathological conditions.
Keywords: Fibrinogen, Chaperone, Extracellular, Aggregation, Misfolding, Fibril formation
Partially or completely unfolded polypeptides are highly prone to aggregation due to nonspecific interactions between their exposed hydrophobic surfaces [1,2]. Living systems have evolved elaborate mechanisms to prevent such interactions and help proteins fold correctly. Of particular significance in this regard are various chaperones present in abundance intracellularly [3,4]. Extracellular proteins are continuously subjected to stresses, such as free radicals, shear stress in blood, and elevated body temperature. However, the existence of extracellular chaperones that modulate the folding and stabilization of extracellular proteins remains largely unexplored [5]. A few extracellular proteins, such as clusterin, haptoglobin, and serum amyloid P component (SAP), have been reported to have certain chaperone-like activity in humans [6–8]. More extracellular chaperones still remain unknown.
Human fibrinogen (FG) is a circulating 340 kDa glycoprotein, with a concentration of 2–4.5 mg/ml in the plasma [9]. FG is not only a vital part of the ‘‘common pathway” of the coagulation process [10], but also an acute-phase protein, the level of which increases under stress conditions [11]. FG binds to other extracellular matrix molecules and can act as a reservoir for growth factors, proteases and protease inhibitors. Elevated plasma FG is associated with age, atherosclerotic disease, acute myocardial infarction, and stroke [12,13]. However, the roles of FG in many of these physiological and pathological conditions are still not clear.
Here we show that FG has a chaperone-like activity. The chaperone-like property of FG was tested using model proteins for chaperone studies, such as citrate synthase (CS) [14] and luciferase [15,16]. Interestingly, FG can interact with partially denatured CS and protect it from thermal-induced aggregation and inactivation. Furthermore, FG can maintain thermal-denatured luciferase in a refolding competent state. FG also inhibits fibril formation of Sup35 (NM), the prion-determining domain of yeast prion protein Sup35 [17]. Moreover, FG rescues thermal-induced protein aggregation in the plasma of FG-deficient (FG−/−) mice. Taken together, these studies indicate that FG has chaperone-like activity, which provides new insights into the extracellular chaperone protein system.
Materials and methods
Materials
Human plasma FG, fibronectin (FN), IgG, transferrin, pig heart CS, firefly luciferase, heat shock protein 90 (HSP90), and GroEL were purchased from Sigma (USA). Bovine serum albumin was obtained from Roche (CH). Purified rabbit polyclonal antibodies against CS were purchased from Nordic Immunology (NL). The generation of FG−/− mice has been described previously [18]. Polyclonal goat anti-rabbit immunoglobulin/HP was from DakoCytomation (DK). All other antibodies were from Protgen (CN).
Chaperone activity assays with CS
Light scattering and activity assays of CS were carried out as described [14]. To determine the aggregation kinetics, light scattering was measured in an FL-4500 fluorescence spectrophotometer (Hitachi, JP).
Co-immunoprecipitation (IP) was carried out as follows: CS with or without FG was incubated at 25 °C or 43 °C. The reactions were stopped after different time courses of incubation, and the samples were centrifuged. The complex of FG and CS in the supernatant was pulled down with polyclonal antibody against FG, which was subjected to Western blotting with polyclonal antibody against CS.
Luciferase reactivation experiments
Luciferase reactivation experiments were carried out as described [15]. Luciferase (1 µM) was incubated with 10 µM FG, BSA, human IgG, transferrin, or lysozyme, respectively, in the presence of 50 mM sodium phosphate, pH 7.5 (100 µl total) at 43 °C for 20 min, then cooled down to room temperature. The heated mixture was diluted by 40-folds into solutions containing 30 µl of rabbit reticulocyte lysate (RRL), 25 mM Hepes (pH 7.5), 5 mM MgCl2, 10 mM KCl, 2 mM dithiothreitol (DTT), 2 mM ATP (50 µl total volume). In the parallel control studies, the heated mixture of luciferase and FG was diluted by 40-folds into Hepes buffer. Reactions were carried out at 30 °C. Aliquots were withdrawn at various time points, and luciferase activity was then measured using Centro LB 960 Luminometer from the Berthold Technologies GmbH & Co. KG. The activity of luciferase before incubation at 43 °C was assumed as 100% activity.
Thermal-induced protein aggregation in plasma of FG−/− mice was performed at 43 °C. After incubation for 48 h, the plasma from FG−/− mice and wild type mice was centrifuged at 20,000g for 15 min, and the precipitations were detected by SDS–PAGE. Bands in the gel were identified with liquid chromatography–tandem mass chromatography (LC–MS/MS).
Amyloid formation assays
The assay for amyloid formation was carried out as described [19]. Conversion to fibril was monitored by a change of thioflavin T based fluorescence intensity. Basically, concentrated stocks of Sup35 (NM), stored in 8 M urea, were diluted at least 50-fold into 5 mM KH2PO4, 150 mM NaCl, and pH 7.4. At indicated time, aliquots were measured in a FL-4500 fluorescence spectrophotometer (Hitachi), excited at 442 nm. Emission fluorescence at 485 nm was recorded. FG was added at distinct time points. Buffer only containing FG served as a control.
Results
Fibrinogen inhibits the thermal-induced aggregation and inactivation of citrate synthase
The ability to prevent aggregation of nonnative proteins is one of the fundamental biochemical properties for molecular chaperones [20]. To test whether FG has a chaperone-like activity, we employed citrate synthase (CS). CS is a model protein for chaperone studies, and the influence of the major classes of molecular chaperones on CS folding has been characterized [14].
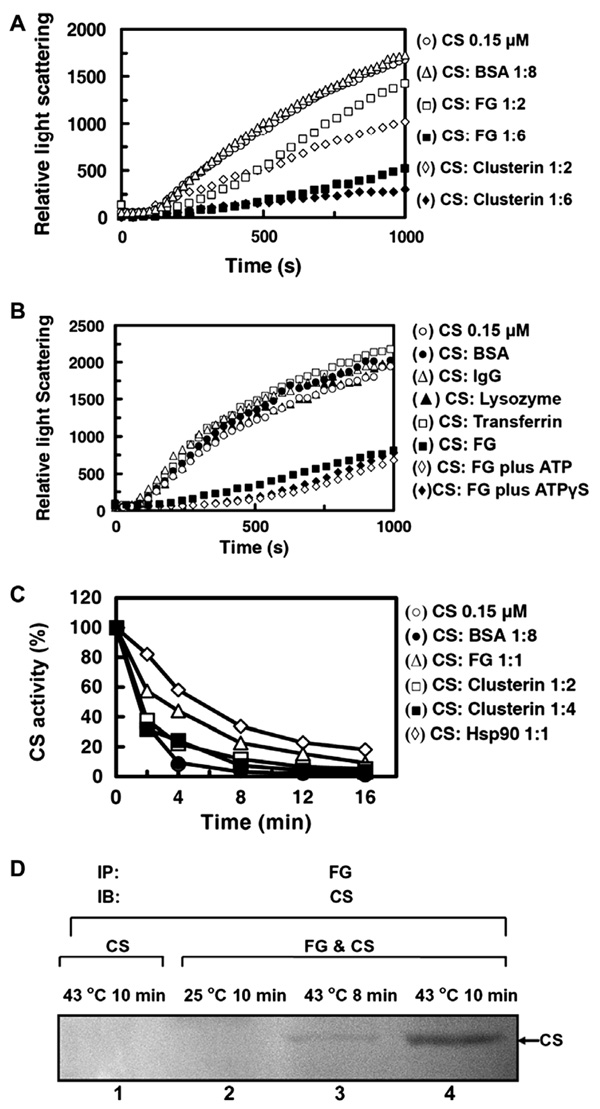
The thermal-induced aggregation of 0.15 µM CS at 43°C was suppressed by FG in a dose-dependent manner, to an extent comparable to that found when clusterin, a previously reported extra-cellular chaperone protein [6], was present. On the other hand, high level (1.2 µM) of BSA showed no inhibition of CS aggregation (Fig. 1A). Other plasma proteins such as IgG, transferrin, and lysozyme were also tested, but no inhibitory effect was found (Fig. 1B). The suppression of CS aggregation by FG was not affected by ATP or its analog, ATPγS (Fig. 1B), and ATPase activity was not detected in FG (data not shown).
Fig. 1.
Chaperone activity of FG tested with citrate synthase (CS). (A) Thermal-induced CS aggregation. CS alone (○) or with 1.2 µM BSA (Δ), 0.3 µM FG (□), 0.3 µM clusterin (◊), 0.9 µM FG (■), 0.9 µM clusterin (♦). (B) The influence of ATP on thermal-induced CS aggregation. CS alone (○) or with 1.2 µM BSA (●), IgG (Δ), lysozyme (▲), transferrin (□), 0.9 µM FG (■), 0.9 µM FG plus 2 mM Mg ATP (◊), 0.9 µM FG plus 2 mM ATPγS (♦) at 43 °C. (C) Thermal-induced inactivation of CS at 43 °C. CS alone (○) or with 0.15 µM FG (Δ), 0.3 µM clusterin (□), 0.6 µM clusterin (■), 0.15 µM HSP90 (◊), 1.2 µM BSA (●). (D) Co-immunoprecipitation (IP) of FG and thermal-denatured CS. IP with antibody against FG; IB with antibody against CS.
We also compared the ability of FG, clusterin, and HSP90 to protect the enzymatic activity of CS at 43 °C. Both 0.15 µM FG and 0.15 µM HSP90 dramatically attenuated the inactivation of CS, while 0.6 µM clusterin had significantly less protecting effect (Fig. 1C). To further confirm the specificity of the interaction between FG and partially denatured proteins, we tested whether FG selectively binds to the denatured form of proteins. Co-immuno-precipitation (IP) experiment was performed between FG and thermal-denatured CS. FG specifically binds to thermal-denatured CS at 43 °C but not native CS at 25 °C after co-incubation of these two proteins (Fig. 1D). These results indicate that FG can specifically interact with partially denatured CS and protect it from thermal-induced aggregation and inactivation.
Heat-denatured firefly luciferase or citrate synthase bound to fibrinogen are competent for refolding
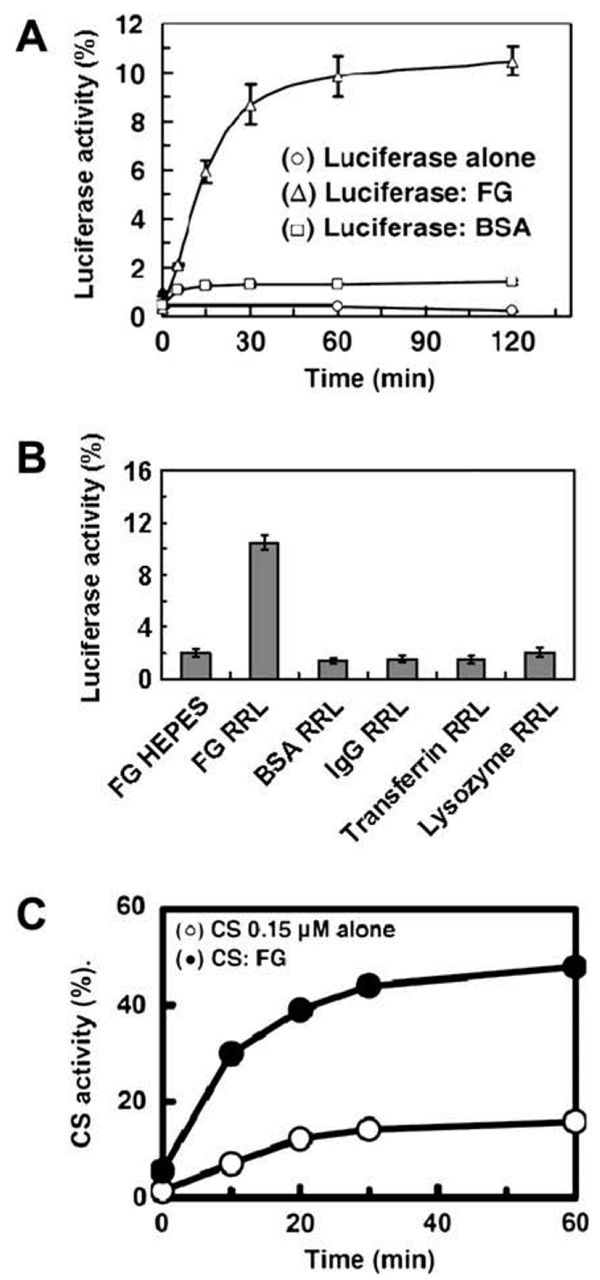
We used firefly luciferase to further test whether a heat denatured protein bound to FG could still be competent for refolding. Luciferase was well studied as a model substrate for chaperone studies [15,16]. We found that FG inhibited thermal-induced aggregation of luciferase and formed complex with thermal-denatured luciferase (data not shown). After co-incubated with FG at 43 °C, the heated mixture of luciferase and FG was then added into rabbit reticulocyte lysate (RRL) to monitor the refolding process. RRL is rich in components needed for protein synthesis and folding, including molecular chaperones such as hsp70, hsp90, and TRiC [21]. Reactivation from the heated mixture of luciferase and FG reached nearly 12% of the original activity of luciferase before incubation (Fig. 2A). If luciferase was incubated alone without FG, it irreversibly aggregated and no activity could be recovered by RRL (Fig. 2A). When luciferase was co-incubated with BSA, IgG, transferrin, or lysozyme, little reactivation of the heated mixture was obtained (Fig. 2B), thus the reactivation of luciferase was FG-dependent. However, FG itself alone was not enough to refold heat-denatured luciferase. When the heated mixture of luciferase and FG was added into Hepes buffer instead of RRL, little reactivation was obtained (Fig. 2B). In another thermal-induced inactivation test, CS was incubated without or with 0.6 µM FG, at 43 °C, until less than 5% of the original activity remained, and then temperature was reduced from 43°C to 25°C. Approximately 50% of the original activity of CS was recovered after 60 min, if initially incubated with FG. In contrast, less than 15% of the original activity of CS was regained, when this protein was initially incubated at 43 °C in the absence of FG (Fig. 2C). These results indicate that FG may serve as a reservoir, which maintains denatured proteins in a refolding competent form, so that the proteins can later be refolded either spontaneously or in conjunction with other chaperones. This property of FG is similar to some small heat shock proteins [15].
Fig. 2.
Heat-denatured firefly luciferase and CS bound to FG is competent for refolding. (A) Heated mixture of luciferase and FG added into rabbit reticulocyte lysate (RRL) (Δ), heated mixture of luciferase and BSA added into RRL (□), heated luciferase alone added into RRL (○). Error bars represent the standard error from duplicated measurements. (B) Heated mixture of luciferase and FG added into FG solutions, heated mixture of luciferase and FG added into RRL, heated mixture of luciferase and BSA, IgG, transferrin, lysozyme added into RRL. The height of the column represents the highest activity recovered. (C) Reactivation of CS. CS alone (○) or with 0.9 µM FG (●).
Fibrinogen inhibits fibril formation of Sup35 (NM)
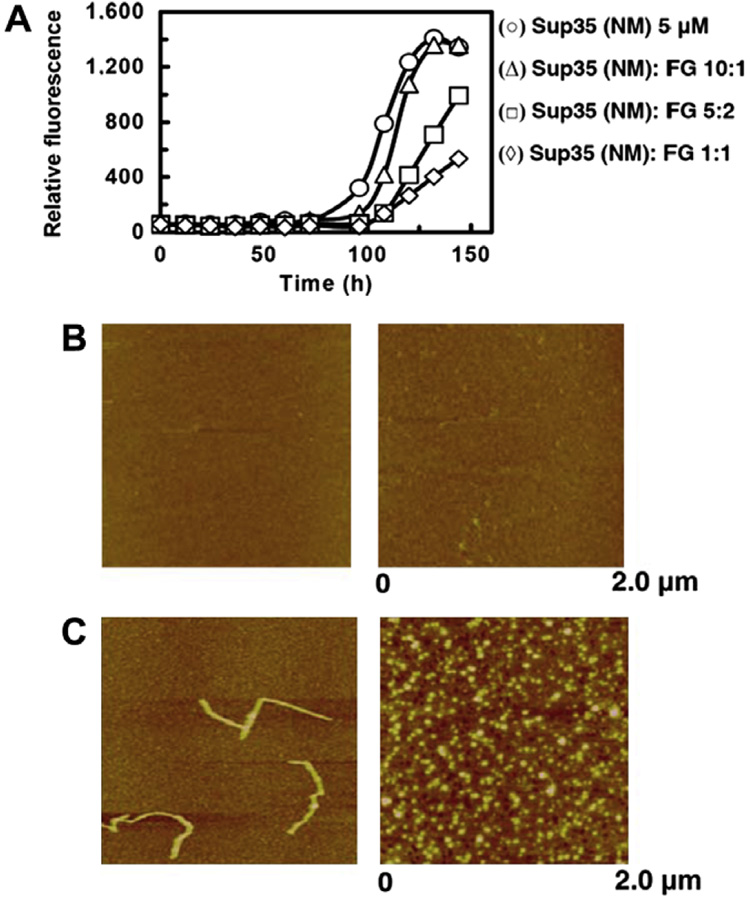
Besides the protection to stressed proteins, FG was also found to have an inhibitory effect on the spontaneously protein misfolding and fibril formation. Yeast prion protein, Sup35 (NM), was employed for the study, because it can quickly form fibrils under physiological conditions and shares key features of amyloid fibrils of mammalian prions or amyloid proteins [17,22]. The fibril formation process of Sup35 (NM) was monitored by thioflavin T fluorescence, which was inhibited by FG in a dose-dependent manner (Fig. 3A). The inhibitory effect of FG on the fibril formation process was further observed by AFM (Fig. 3B and C). There were no differences between Sup35 (NM) alone and Sup35 (NM) co-incubated with FG at the beginning of incubation (Fig. 3B). Sup35 (NM) formed fibrils in the absence of FG (Fig. 3C, left panel) after 96 h, but mainly amorphous aggregates in the presence of FG (Fig. 3C, right panel). The result indicates that FG can modulate the aggregation process of fibril formation.
Fig. 3.
Inhibitory effect of FG on the process of fibril formation of yeast prion protein Sup35 (NM). (A) Fibril formation of Sup35 (NM) without FG (○) and with 0.5 µM (Δ), 2 µM (□), and 5 µM (◊) FG. (B) AFM profile of Sup35 (NM) without or with 5 µM FG after 24 h incubation. (C) AFM profile of Sup35 (NM) without or with 5 µM FG after 96 h incubation. In (B) and (C), left, without FG; right, with FG.
The inhibitory effect of FG on the fibril formation of yeast prion Sup35 (NM) once again demonstrates the chaperone-like activity of FG and is similar to the previous reported results of molecular chaperones [23,24].
FG prevents thermal-induced protein aggregation of plasma proteins
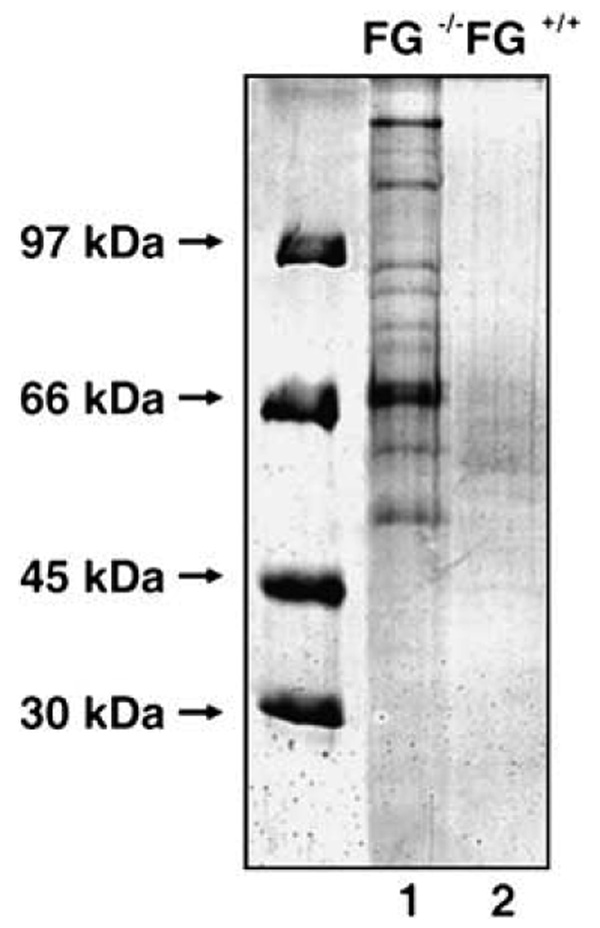
The general inhibition by FG of thermal-induced protein aggregation was further confirmed using plasma of FG−/− and wild type mice. After incubation at 43 °C for 48 h, a substantially larger amount of protein precipitate was found in the plasma of FG−/− mice, when compared to plasma of wild type mice (Fig. 4). This precipitation could also be rescued by adding exogenous FG into the plasma of FG−/− mice before incubation (data not shown). More than 12 proteins were identified in the precipitate by liquid chromatography–tandem mass chromatography (LC–MS/MS), which can be classified into four categories: coagulation proteins, extra-cellular matrix proteins, enzymes and other abundant plasma proteins (Table S1). Among these proteins, the binding of human plasminogen to fibrin and FG has already been studied [25]. Misfolding and amyloid fibrillogenesis of lysozyme variants associated with human amyloid disease has been characterized [26]. The protective effect of FG on the thermal-stressed precipitation of plasma proteins strongly suggests the biological relevance of FG.
Fig. 4.
SDS–PAGE of precipitated proteins from plasma of FG−/− mice and wild type mice. Lane 1, precipitation in plasma from FG−/− mice; lane 2, precipitation in plasma from wild type mice.
Additional studies showed that purified FG significantly reduced the aggregation of chemical-denatured Escherichia coli-expressed nerve growth factor (NGF), PEX, and oxidatively damaged serum proteins (data not shown). Interactions of FG with such a wide spectrum of nonnative proteins portray its general chaperone-like feature.
Discussion
We show here that FG specifically interacts with and suppresses the aggregation of a wide spectrum of stressed proteins. To our knowledge, this is so far the first study to demonstrate the chaperone-like activity of FG. As a vital part of the coagulation system, human FG constitutively presents with a high concentration of 2–4.5 mg/ml in the plasma [9], and its level could increase by about 200% during the acute phase, such as inflammation and oxidative stress conditions [11]. Elevated expression of FG may be considered not only as a strategy for survival during unfavorable times but also as a potential mechanism of defense against misfolding events. Consistent with this point, misfolded proteins have been implicated for inflammatory events in atherosclerosis [27], and Alzheimer’s disease [28]. Given the high concentration and important physical and pathological processes that FG gets involved, the chaperone-like activity of FG is of special significance to the large amount of extracellular proteins.
It is striking that FG can inhibit the formation of Sup35 (NM) fibril, which shares key features of amyloid fibrils of mammalian prions or amyloid proteins [22], indicating a potential role of FG on prion or other misfolding diseases. AFM study suggests that FG could modulate the aggregation process of Sup35 (NM) fibril to form amorphous aggregates (Fig. 3). Our result also indicates that FG more effectively inhibits Sup35 (NM) fibril formation at the early stage of fibril formation (data not shown). The property that FG can interact with the prefibrillar species during the fibril formation process not only supports the previous conception that chaperones can redirect the aggregation process toward the formation of amorphous deposits, thereby sequestering potentially toxic species from the bulk solution [23,24], but also provide strong therapeutic implication to the proposition that early prefibrillar soluble oligomers from proteins are inherently cytotoxic [29].
Recent observations revealed connections between the amyloid and hemostasis systems in humans [30–32]. It is particularly interesting that fibrin itself has high β-sheet content and fibrin-derived peptides can form amyloid fibers [32]. Several genetic variants in the region of the α-chain residues 522–554 in human FG were also reported to result in the formation of amyloid deposits [33–35]. FG was found to interact with non-digested PrPSc [36]. In FG-deficient mice, more advanced plaque formation in the artery was detected [37], supporting the contention that atherosclerosis may be a protein misfolding disease [27]. Interestingly, FG was also discovered to be associated with type II and cystic fibrosis-related diabetes [38,39], whereas type II diabetes is well known as being related to islet amyloid [40]. High levels of FG are also related to both Alzheimer’s disease and vascular dementia [41]. It is plausible that the increased high level of FG may help to compensate the additional request of extracellular chaperone functions under such pathological conditions. Other possibilities cannot be excluded, such as the possibilities that FG molecules in Alzheimer’s disease and vascular dementia may be mutated or contain abnormal post-translational modifications. Besides, human FG contains three potential integrin binding sites, two RGD sequences within the Aα chain and a non-RGD sequence in the γ chain. FG can interact not only with endothelia cells but also with immune cells such as monocytes, neutrophils, and B-lymphocytes, through both integrin and nonintegrin receptors [42]. Therefore, FG may also facilitate the cellular uptake and degradation of misfolded proteins to which it binds to.
Taken together, the novel chaperone-like activity of FG may not only provide new insights into the extracellular chaperone system but also have indications on FG-related physiological and pathological conditions.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbrc.2008.11.112.
Acknowledgments
This study was supported by grants from the National Science Fund for Distinguished Young Scholars in China (No. 30225014), the Major Program of National Natural Science Foundation of China (No. 30490171), the State Key Development Program for Basic Research of China (No. 2006CB910305), and Grant HL073750 from the National Institutes of Health (to F.J.C.).
References
- 1.Horwich A. Protein aggregation in disease: a role for folding intermediates forming specific multimeric interactions. J. Clin. Invest. 2002;110:1221–1232. doi: 10.1172/JCI16781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobson CM. Principles of protein folding, misfolding and aggregation. Semin. Cell Dev. Biol. 2004;15:3–16. doi: 10.1016/j.semcdb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Frydman J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu. Rev. Biochem. 2001;70:603–647. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- 5.Yerbury JJ, Stewart E, Wyatt A, Wilson MR. Quality control of protein folding in extracellular space. EMBO Rep. 2005;6:1131–1136. doi: 10.1038/sj.embor.7400586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphreys D, Carver J, Easterbrook-Smith SB, Wilson MR. Clusterin has chaperone-like activity similar to that of small heat-shock proteins. J. Biol. Chem. 1999;274:6875–6881. doi: 10.1074/jbc.274.11.6875. [DOI] [PubMed] [Google Scholar]
- 7.Kanekiyo T, et al. Lipocalin-type prostaglandin D synthase/β-trace is a major amyloid β-chaperone in human cerebrospinal fluid. Proc. Natl. Acad. Sci. USA. 2007;104:6412–6417. doi: 10.1073/pnas.0701585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coke AR, Purvis A, Baker D, Pepys MB, Wood SP. Molecular chaperone properties of serum amyloid P component. FEBS Lett. 2000;473:199–202. doi: 10.1016/s0014-5793(00)01530-1. [DOI] [PubMed] [Google Scholar]
- 9.Putnam FW. The Plasma Proteins. second ed. New York: Academic Press; 1975. [Google Scholar]
- 10.Doolittle RF. Fibrinogen and fibrin. Annu. Rev. Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- 11.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 12.Wilhelmsen L, et al. Fibrinogen as risk factor for stroke and myocardial infarction. N. Engl. J. Med. 1984;311:501–505. doi: 10.1056/NEJM198408233110804. [DOI] [PubMed] [Google Scholar]
- 13.Fowkes FG, Lee AJ, Lowe GD, Riemersma RA, Housley E. Inter-relationships of plasma fibrinogen, low density lipoprotein cholesterol, cigarette smoking and the prevalence of cardiovascular disease. J. Cardiovasc. Risk. 1996;3:307–311. [PubMed] [Google Scholar]
- 14.Buchner J, Grallert H, Jakob U. Analysis of chaperone function using citrate synthase as nonnative substrate protein. Methods Enzymol. 1998;290:323–338. doi: 10.1016/s0076-6879(98)90029-5. [DOI] [PubMed] [Google Scholar]
- 15.Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- 17.Glover JR, Kowal AS, Schirmer EC, Patino MM, Liu JJ, Lindquist S. Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell. 1997;89:811–819. doi: 10.1016/s0092-8674(00)80264-0. [DOI] [PubMed] [Google Scholar]
- 18.Ploplis VA, et al. A total fibrinogen deficiency is compatible with the development of pulmonary fibrosis in mice. Am. J. Pathol. 2000;157:703–708. doi: 10.1016/S0002-9440(10)64582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chien P, DePace AH, Collins SR, Weissman JS. Generation of prion transmission barriers by mutational control of amyloid conformations. Nature. 2003;424:948–951. doi: 10.1038/nature01894. [DOI] [PubMed] [Google Scholar]
- 20.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 21.Frydman J, Nimmesgern E, Ohtsuka K, Hartl FU. Folding of nascent polypeptide in a high molecular weight complex with molecular chaperones. Nature. 1994;370:111–117. doi: 10.1038/370111a0. [DOI] [PubMed] [Google Scholar]
- 22.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem. Sci. 1999;24:329–332. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 23.Barral JM, Broadley SA, Schaffar G, Hartl FU. Roles of molecular chaperones in protein misfolding diseases. Semin. Cell Dev. Biol. 2004;15:17–29. doi: 10.1016/j.semcdb.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Kumita JR, Poon S, Caddy GL, Hagan CL, Dumoulin M, Yerbury JJ, Stewart EM, Robinson CV, Wilson MR, Dobson CM. The extracellular chaperone clusterin potently inhibits human lysozyme amyloid formation by interacting with prefibrillar species. J. Mol. Biol. 2007;369:157–167. doi: 10.1016/j.jmb.2007.02.095. [DOI] [PubMed] [Google Scholar]
- 25.Lucas MA, Fretto LJ, McKee PA. The binding of human plasminogen to fibrin and fibrinogen. J. Biol. Chem. 1983;258:4249–4256. [PubMed] [Google Scholar]
- 26.Booth DR, Sunde M, Bellotti V, Robinson CV, Hutchinson WL, Fraser PE, et al. Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature. 1997;385:787–793. doi: 10.1038/385787a0. [DOI] [PubMed] [Google Scholar]
- 27.Ursini F, Davies KJA, Maiorino M, Parasassi T, Sevanian A. Atherosclerosis: another protein misfolding disease? Trends Mol Med. 2002;8:370–374. doi: 10.1016/s1471-4914(02)02382-1. [DOI] [PubMed] [Google Scholar]
- 28.Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol and misfolded proteins. Lancet. 2004;363:1139–1146. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- 29.Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 30.Fowler DM, Koulov AV, Balch WE, Kelly JW. Functional amyloid-from bacteria to humans. Trends Biochem. Sci. 2007;32:217–224. doi: 10.1016/j.tibs.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Shibayama Y, et al. Zinc-dependent activation of the plasma kinin-forming cascade by aggregated beta amyloid protein. Clin. Immunol. 1999;90:89–99. doi: 10.1006/clim.1998.4621. [DOI] [PubMed] [Google Scholar]
- 32.Kranenburg O, et al. Tissue-type plasminogen activator is a multiligand cross-beta structure receptor. Curr. Biol. 2002;12:1833–1839. doi: 10.1016/s0960-9822(02)01224-1. [DOI] [PubMed] [Google Scholar]
- 33.Benson MD, Liepnieks J, Uemichi T, Wheeler G, nea R. Hereditary renal amyloidosis associated with a mutant fibrinogen alpha-chain. Nat. Genet. 1993;3:252–255. doi: 10.1038/ng0393-252. [DOI] [PubMed] [Google Scholar]
- 34.Uemichi T, Liepnieks JJ, Benson MD. Hereditary renal amyloidosis with a novel variant fibrinogen. J. Clin. Invest. 1994;93:731–736. doi: 10.1172/JCI117027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uemichi T, et al. A frame shift mutation in the fibrinogen Aα chain gene in a kindred with renal amyloidosis. Blood. 1996;87:4197–4203. [PubMed] [Google Scholar]
- 36.Fischer MB, Roeckl C, Parizek P, Schwarz HP. Binding of disease-associated prion protein to plasminogen. Nature. 2000;408:479–483. doi: 10.1038/35044100. [DOI] [PubMed] [Google Scholar]
- 37.Iwaki T, Sandoval-Cooper MJ, Brechmann M, Ploplis VA, Castellino FJ. Afibrinogen deficiency accelerates the initiation of LDL cholesterol-driven atherosclerosis via thrombin generation and platelet activation in genetically predisposed mice. Blood. 2006;107:3883–3891. doi: 10.1182/blood-2005-09-3780. [DOI] [PubMed] [Google Scholar]
- 38.Barazzoni RB, et al. Increased fibrinogen production in type 2 diabetic patients without detectable vascular complications: correlation with plasma glucagon concentrations. J. Clin. Endocrinol. 2000;85:3121–3125. doi: 10.1210/jcem.85.9.6779. [DOI] [PubMed] [Google Scholar]
- 39.Adler AI, Gunn E, Haworth CS, Bilton D. Characteristics of adults with and without cystic fibrosis-related diabetes. Diabet. Med. 2007;24:1143–1148. doi: 10.1111/j.1464-5491.2007.02252.x. [DOI] [PubMed] [Google Scholar]
- 40.Hoppener JWM, Ahpen B, Lips CJM. Islet amyloid and type 2 diabetes mellitus. N. Engl. J. Med. 2008;343:411–419. doi: 10.1056/NEJM200008103430607. [DOI] [PubMed] [Google Scholar]
- 41.Oijen MV, Witteman JC, Hofman A, Koudstaal PJ, Breteler MMB. Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke. 2005;36:2637–2641. doi: 10.1161/01.STR.0000189721.31432.26. [DOI] [PubMed] [Google Scholar]
- 42.Herrick S, Blanc-Brude O, Gray A, Laurent G. Fibrinogen. Int. J. Biochem. Cell Biol. 1999;31:741–746. doi: 10.1016/s1357-2725(99)00032-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbrc.2008.11.112.