Abstract
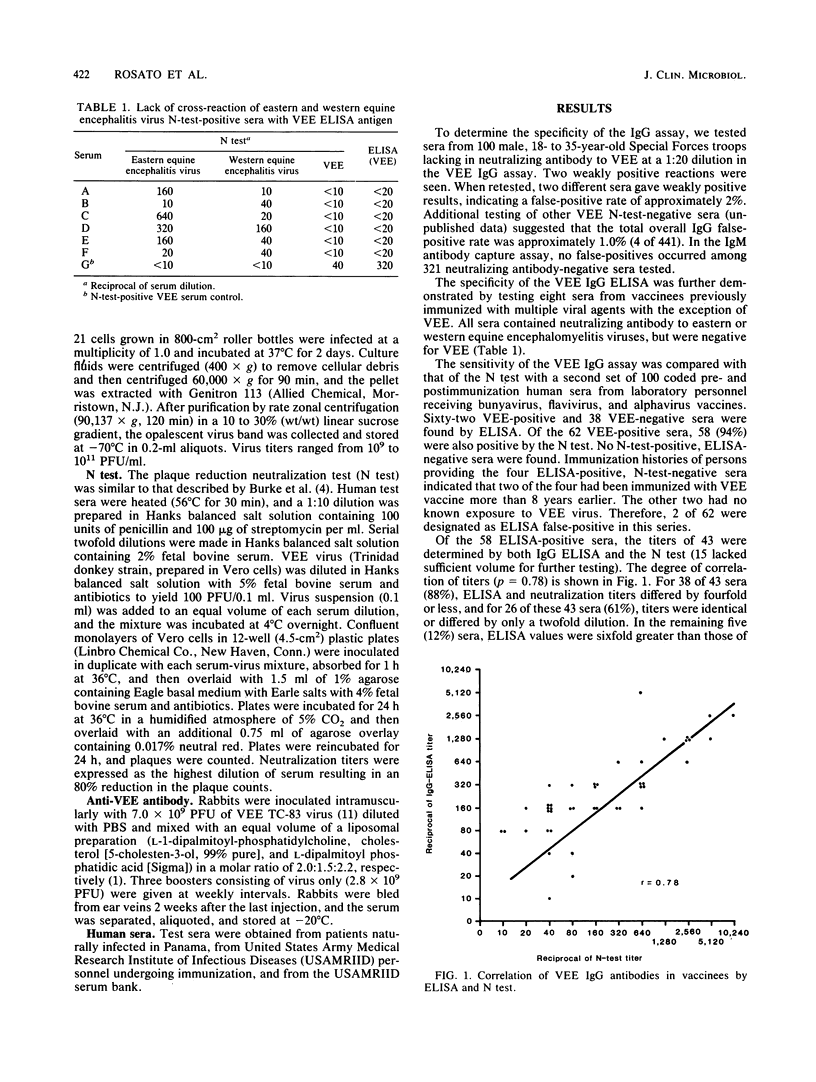
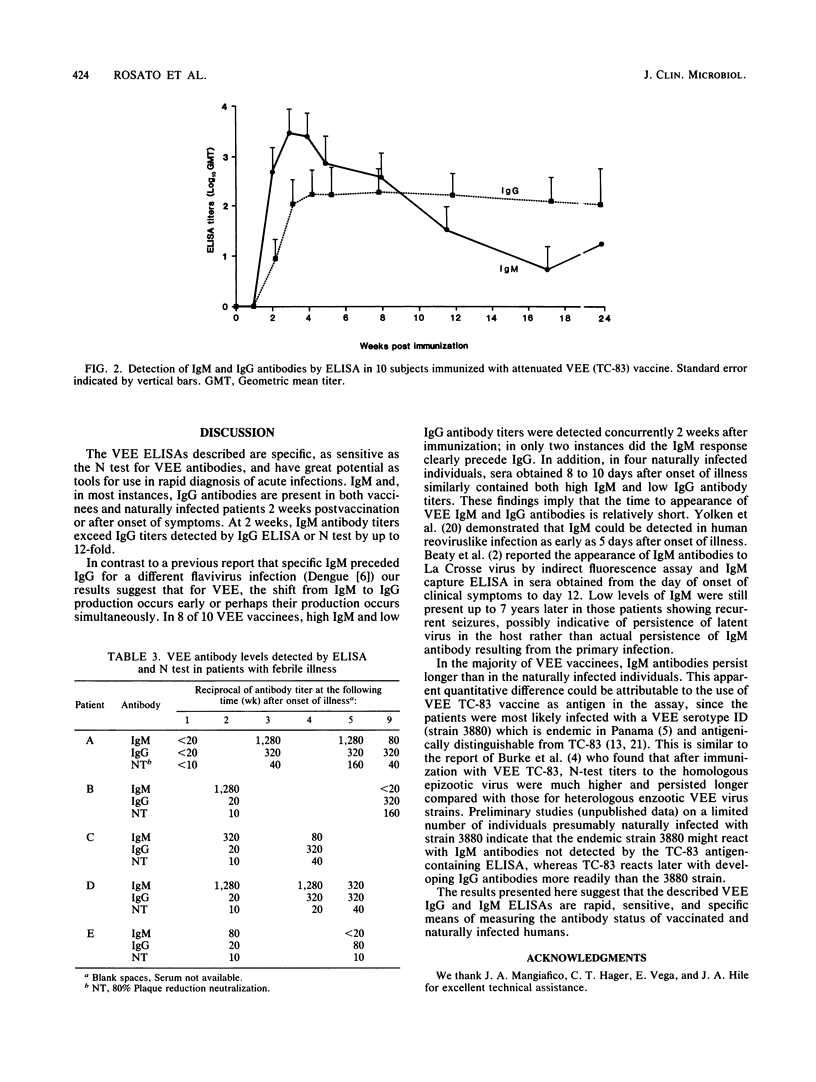
Enzyme-linked immunosorbent assays (ELISAs) were developed for detection of immunoglobulin G (IgG) and IgM antibodies to Venezuelan equine encephalomyelitis (VEE) virus in vaccinated and naturally infected humans. A total of 441 sera found negative for VEE antibodies by plaque reduction neutralization were examined by IgG ELISA and gave a 1.0% false-positive rate; no false-positives were found in the IgM ELISA. Sera with neutralizing antibody to western or eastern equine encephalomyelitis virus did not react with VEE antigen in the IgG ELISA. Sensitivity of the IgG ELISA was determined by testing 100 coded pre- and postvaccination human sera. Sixty-two were positive by ELISA; 58 of these 62 were also positive by neutralization tests, and 38 were negative by both tests. No neutralization-positive, ELISA-negative sera were found. Comparison of titers obtained by ELISA and neutralization tests indicated that 88% varied randomly by a fourfold dilution factor or less, while 61% were identical or varied only twofold. In sera obtained sequentially from 10 vaccinees and 5 naturally infected patients, both IgG and IgM antibodies appeared between 2 and 3 weeks after vaccination or onset of symptoms. The IgG and IgM antibody ELISAs described are rapid, specific, and sensitive indicators of VEE antibody status in vaccinated and naturally infected individuals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaty B. J., Jamnback T. L., Hildreth S. W., Brown K. L. Rapid diagnosis of La Crosse virus infections: evaluation of serologic and antigen detection techniques for the clinically relevant diagnosis of La Crosse encephalitis. Prog Clin Biol Res. 1983;123:293–302. [PubMed] [Google Scholar]
- Bowen G. S., Fashinell T. R., Dean P. B., Gregg M. B. Clinical aspects of human Venezuelan equine encephalitis in Texas. Bull Pan Am Health Organ. 1976;10(1):46–57. [PubMed] [Google Scholar]
- Burke D. S., Ramsburg H. H., Edelman R. Persistence in humans of antibody to subtypes of Venezuelan equine encephalomyelitis (VEE) virus after immunization with attenuated (TC-83) VEE virus vaccine. J Infect Dis. 1977 Sep;136(3):354–359. doi: 10.1093/infdis/136.3.354. [DOI] [PubMed] [Google Scholar]
- Dietz W. H., Jr, Peralta P. H., Johnson K. M. Ten clinical cases of human infection with venezuelan equine encephalomyelitis virus, subtype I-D. Am J Trop Med Hyg. 1979 Mar;28(2):329–334. doi: 10.4269/ajtmh.1979.28.329. [DOI] [PubMed] [Google Scholar]
- Dittmar D., Cleary T. J., Castro A. Immunoglobulin G- and M-specific enzyme-linked immunosorbent assay for detection of dengue antibodies. J Clin Microbiol. 1979 Apr;9(4):498–502. doi: 10.1128/jcm.9.4.498-502.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenkranz N. J., Sinclair M. C., Buff E., Lyman D. O. The natural occurrence of Venezuelan equine encephalitis in the United States. N Engl J Med. 1970 Feb 5;282(6):298–302. doi: 10.1056/NEJM197002052820603. [DOI] [PubMed] [Google Scholar]
- Franck P. T., Johnson K. M. An outbreak of Venezuelan encephalitis in man in the Panamá Canal Zone. Am J Trop Med Hyg. 1970 Sep;19(5):860–865. doi: 10.4269/ajtmh.1970.19.860. [DOI] [PubMed] [Google Scholar]
- Frazier C. L., Shope R. E. Detection of antibodies to alphaviruses by enzyme-linked immunosorbent assay. J Clin Microbiol. 1979 Oct;10(4):583–585. doi: 10.1128/jcm.10.4.583-585.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Frisch-Niggemeyer W., Heinz F. Rapid diagnosis of tick-borne encephalitis by means of enzyme linked immunosorbent assay. J Gen Virol. 1979 Mar;42(3):505–511. doi: 10.1099/0022-1317-42-3-505. [DOI] [PubMed] [Google Scholar]
- Jahrling P. B., Gorelkin L. Selective clearance of a benign clone of Venezuelan equine encephalitis virus from hamster plasma by hepatic reticuloendothelial cells. J Infect Dis. 1975 Dec;132(6):667–676. doi: 10.1093/infdis/132.6.667. [DOI] [PubMed] [Google Scholar]
- Jamnback T. L., Beaty B. J., Hildreth S. W., Brown K. L., Gundersen C. B. Capture immunoglobulin M system for rapid diagnosis of La Crosse (California encephalitis) virus infections. J Clin Microbiol. 1982 Sep;16(3):577–580. doi: 10.1128/jcm.16.3.577-580.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggendorf M., Heinz F., Deinhardt F., Kunz C. Serological diagnosis of acute tick-borne encephalitis by demonstration of antibodies of the IgM class. J Med Virol. 1981;7(1):41–50. doi: 10.1002/jmv.1890070105. [DOI] [PubMed] [Google Scholar]
- Shope R. E. Arbovirus-related encephalitis. Yale J Biol Med. 1980 Jan-Feb;53(1):93–99. [PMC free article] [PubMed] [Google Scholar]
- Walton T. E., Alvarez O., Jr, Buckwalter R. M., Johnson K. M. Experimental infection of horses with enzootic and epizootic strains of Venezuelan equine encephalomyelitis virus. J Infect Dis. 1973 Sep;128(3):271–282. doi: 10.1093/infdis/128.3.271. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Leister F. J. Comparison of fluorescent and colorigenic substrates for enzyme immunoassays. J Clin Microbiol. 1982 May;15(5):757–760. doi: 10.1128/jcm.15.5.757-760.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Leister F. J. Enzyme immunoassays for measurement of cytomegalovirus immunoglobulin M antibody. J Clin Microbiol. 1981 Oct;14(4):427–432. doi: 10.1128/jcm.14.4.427-432.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Wyatt R. G., Kim H. W., Kapikian A. Z., Chanock R. M. Immunological response to infection with human reovirus-like agent: measurement of anti-human reovirus-like agent immunoglobulin G and M levels by the method of enzyme-linked immunosorbent assay. Infect Immun. 1978 Feb;19(2):540–546. doi: 10.1128/iai.19.2.540-546.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N. A., Johnson K. M. Antigenic variants of Venezuelan equine encephalitis virus: their geographic distribution and epidemiologic significance. Am J Epidemiol. 1969 Mar;89(3):286–307. doi: 10.1093/oxfordjournals.aje.a120942. [DOI] [PubMed] [Google Scholar]


