Abstract
We report a case of accelerated metabolism of voriconazole during therapy for invasive pulmonary aspergillosis, resulting in subtherapeutic levels. Target voriconazole levels were restored with high dosages of voriconazole (up to 40 mg/kg of body weight/day) and the addition of cimetidine as a cytochrome P450 enzyme inhibitor.
Autoinduction of voriconazole metabolism has been found in animals but not in humans (18). While voriconazole autoinduction occurred in animals at high dosages for extended durations, such dosages are seldom employed for most patients receiving voriconazole. We describe herein a patient receiving high-dose voriconazole who demonstrated possible autoinduction.
Case description.
A 56-year-old Caucasian male developed graft-versus-host disease and invasive pulmonary aspergillosis of the right upper and lower lobes after an allogeneic hematopoietic stem cell transplantation for treatment of mantle cell lymphoma. The patient's intensive-care-unit course was complicated by mechanical-ventilation-dependent respiratory failure due to recurrent episodes of pulmonary hemorrhage and acute renal failure requiring continuous venovenous hemofiltration (CVVH). Aspergillus ustus and Aspergillus terreus were recovered from bronchoalveolar lavage samples, and therapy with voriconazole in combination with echinocandin and liposomal amphotericin B was then initiated. The A. ustus MIC for voriconazole was 4 μg/ml, and therefore, the dose of voriconazole was increased and guided by therapeutic drug monitoring. After voriconazole dosages were substantially increased in response to low levels, the CVVH prescription was intensified to deliver small solute clearances of >40 ml/min to enhance removal of sulfobutyl-ether-β-cyclodextrin. Voriconazole levels were determined by a previously validated high-performance liquid chromatography assay at the Fungus Testing Laboratory, University of Texas Health Science Center (San Antonio, TX) (13).
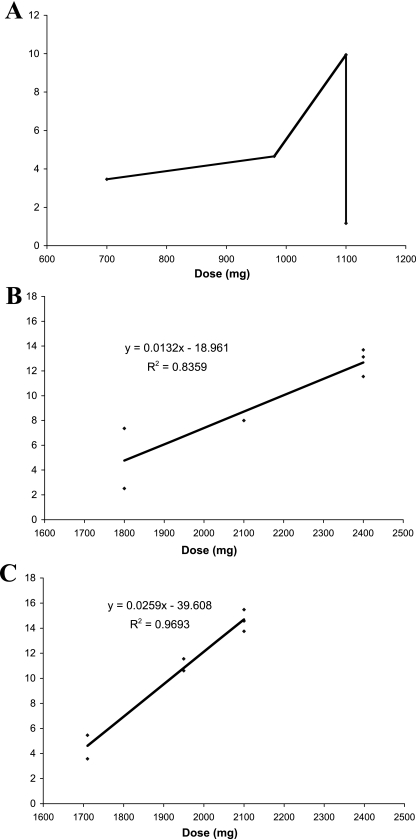
Intravenous (i.v.) voriconazole was initiated for invasive pulmonary aspergillosis on 8 February 2008. Trough levels measured from 18 March to 1 April 2008 indicated nonlinear pharmacokinetics (Fig. 1A). The patient's voriconazole trough level with 9.2 mg/kg of body weight given i.v. every 12 h (1,100 mg per day) was 9.94 μg/ml on 1 April 2008. However, the trough level decreased to 1.17 μg/ml on 8 April 2008 on the same dosage. Confirming this low trough level, random postinfusion levels, which were 11.85 μg/ml and 10.73 μg/ml on 1 April 2008, were now 2.84 μg/ml and 2.26 μg/ml on 8 April 2008. The evolving status of voriconazole therapeutic monitoring has been recently reviewed (2, 4).
FIG. 1.
Voriconazole trough-serum-concentration-versus-dose relationships in a patient with possible autoinduction. Doses represent total amounts of voriconazole administered over 24 h. (A) Voriconazole concentrations from 18 March 2008 to 8 April 2008 (pre-administration of cimetidine). (B) Voriconazole concentrations from 15 April 2008 to 25 April 2008 (postcimetidine). (C) Voriconazole concentrations from 28 April 2008 to 11 May 2008 (postcimetidine). After continued administration of cimetidine, the concentration-dosage curve changed further with a steeper slope. Panels B and C are graphed on the same scale to depict the change in slope. The y axis is expressed in micrograms per ml of voriconazole.
We then investigated several possible reasons for the patient's unexpectedly low trough levels. A thorough review of the patient's medications revealed no medically important cytochrome P450 enzyme inducers. We then explored whether lot variability could account for these low levels. The NIH Pharmaceutical Development Section assayed the potency of voriconazole lots prepared from 2 April to 6 May 2008 using high-performance liquid chromatography and infrared spectroscopy. Lot A30756 was found to be 97% potent and lot A29382 was found to be 99 to 100% potent compared to two reference standards. Furthermore, the NIH pharmacy's i.v. additive service preparation records were also accurate, and proper voriconazole administration was documented.
The voriconazole dosage was increased and administered over a range of 10.8 mg/kg given i.v. every 8 h to 10 mg/kg given i.v. every 6 h (1,950 to 2,400 mg per day), and cimetidine at 300 mg given i.v. every 12 h was added as an enzyme inhibitor (14 April to 13 May 2008) to achieve persistent trough levels of ≥8 μg/ml (Fig. 1B and C). Seventy-five days after initiating treatment with voriconazole, the patient underwent a right upper lobectomy and a right lower lobe superior segmentectomy for recurrent hemoptysis. Cultures failed to grow Aspergillus spp., but Gomori methenamine silver staining revealed nonseptate hyphae.
Discussion.
Possible causes of this patient's decline in serum voriconazole levels were clearance by CVVH, drug induction of cytochrome P450 enzymes, and autoinduction. Clearance by CVVH was eliminated as a possibility because the pharmacokinetic properties of voriconazole (volume of distribution, 4.6 liters/kg; liver metabolism) suggest that extracorporeal removal by continuous renal replacement therapy would not be clinically significant. In support of this conclusion, two studies have shown that voriconazole pharmacokinetics are not significantly affected by continuous venovenous hemodiafiltration (6, 17).
In evaluating the possibility of drug induction of cytochrome P450 enzymes, we excluded all known documented inducers of metabolism by an exhaustive review of the patient's medication records. Our patient received pantoprazole, which has not been found in clinical trials to be an inducer of cytochrome P450 enzymes (3). Although dexamethasone has been reported to be a CYP3A4 enzyme inducer, our patient did not receive this corticosteroid (5, 14). While oral budesonide was administered for the treatment of graft-versus-host disease, clinical studies have not indicated that it is a medically significant enzyme inducer (19). Therefore, it seems unlikely that budesonide accelerated metabolism of voriconazole, resulting in subtherapeutic levels, but this possibility cannot be completely eliminated. Finally, inflammation and infection have also been demonstrated to alter cytochrome P450 enzymes, but the net effect of this mechanism appears to be one of inhibition (1, 7, 11, 16).
After this process of exclusion, voriconazole autoinduction remains a plausible explanation for the low drug levels in serum and high dosage requirements encountered in our patient. Autoinduction has been described with voriconazole in mice, rats, and dogs (18). Compared with human pharmacokinetic studies, these animal toxicology studies have used high dosages and a long duration of therapy. Likewise, a case series by Mulanovich et al. suggested that voriconazole levels were affected by the duration of therapy in humans (12). Patients who received voriconazole for >2 months had lower plasma concentrations than patients who received voriconazole for <2 months. Further supporting the hypothesis of autoinduction are the observations that clotrimazole serves in vitro as a ligand for the human pregnane X receptor, which may then induce CYP3A4, even though it is a deactivator or inverse agonist of the constitutive androstane receptor (8-10).
Cimetidine is a nonspecific inhibitor of CYP1A2, CYP2C19, CYP2D6, and CYP3A4. Since voriconazole is metabolized by CYP2C19, CYP2C9, and CYP3A4, cimetidine was initiated to inhibit metabolism of voriconazole by CYP2C19 and CYP3A4. In healthy volunteers, the maximum concentration of drug in serum and area under the concentration-time curve of voriconazole were increased by 18.3% and 22.5%, respectively, with the concomitant administration of cimetidine (15). Inhibition of voriconazole metabolism by cimetidine may be especially important when higher levels are needed.
In conclusion, accelerated metabolism of voriconazole may occur in humans. This accelerated metabolism may be secondary to autoinduction and drug interactions. Awareness of this phenomenon and the use of judicious therapeutic drug level monitoring may be lifesaving for severely ill patients with relatively resistant fungal infections. Cimetidine may be a useful adjunct to restore therapeutic levels of voriconazole in this setting.
Acknowledgments
We thank Scott Penzak for his thoughtful comments and review of the manuscript, Peng Yuan for analysis of the voriconazole samples, and Nancy Ames for assisting with therapeutic drug monitoring.
This work was supported in part by the intramural research program of the National Institutes of Health.
Footnotes
Published ahead of print on 26 January 2009.
REFERENCES
- 1.Aitken, A. E., T. A. Richardson, and E. T. Morgan. 2006. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu. Rev. Pharmacol. Toxicol. 46:123-149. [DOI] [PubMed] [Google Scholar]
- 2.Andes, D., A. Pascual, and O. Marchetti. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob. Agents Chemother. 53:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blume, H., F. Donath, A. Warnke, and B. S. Schug. 2006. Pharmacokinetic drug interaction profiles of proton pump inhibitors. Drug Saf. 29:769-784. [DOI] [PubMed] [Google Scholar]
- 4.Bruggemann, R. J. M., J. P. Donnelly, R. E. Aarnoutse, A. Warris, N. M. A. Blijlevens, J. W. Mouton, P. E. Verweij, and D. M. Burger. 2008. Therapeutic drug monitoring of voriconazole. Ther. Drug Monit. 30:403-411. [DOI] [PubMed] [Google Scholar]
- 5.Czock, D., F. Keller, F. M. Rasche, and U. Haussler. 2005. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin. Pharmacokinet. 44:61-98. [DOI] [PubMed] [Google Scholar]
- 6.Fuhrmann, V., P. Schenk, W. Jaeger, M. Miksits, N. Kneidinger, J. Warszawska, U. Holzinger, R. Kitzberger, and F. Thalhammer. 2007. Pharmacokinetics of voriconazole during continuous venovenous haemodiafiltration. J. Antimicrob. Chemother. 60:1085-1090. [DOI] [PubMed] [Google Scholar]
- 7.Iber, H., M. B. Sewer, T. B. Barclay, S. R. Mitchell, T. Li, and E. T. Morgan. 1999. Modulation of drug metabolism in infectious and inflammatory diseases. Drug Metab. Rev. 31:29-41. [DOI] [PubMed] [Google Scholar]
- 8.Luo, G., M. Cunningham, S. Kim, T. Burn, J. Lin, M. Sinz, G. Hamilton, C. Rizzo, S. Jolley, D. Gilbert, A. Downey, D. Mudra, R. Graham, K. Carroll, J. Xie, A. Madan, A. Parkinson, D. Christ, B. Selling, E. LeCluyse, and L. S. Gan. 2002. CYP3A4 induction by drugs: correlation between a pregnane X receptor reporter gene assay and CYP3A4 expression in human hepatocytes. Drug Metab. Dispos. 30:795-804. [DOI] [PubMed] [Google Scholar]
- 9.Luo, G., T. Guenthner, L. S. Gan, and W. G. Humphreys. 2004. CYP3A4 induction by xenobiotics: biochemistry, experimental methods and impact on drug discovery and development. Curr. Drug Metab. 5:483-505. [DOI] [PubMed] [Google Scholar]
- 10.Moore, L. B., D. J. Parks, S. A. Jones, R. K. Bledsoe, T. G. Consler, J. B. Stimmel, B. Goodwin, C. Liddle, S. G. Blanchard, T. M. Willson, J. L. Collins, and S. A. Kliewer. 2000. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J. Biol. Chem. 275:15122-15127. [DOI] [PubMed] [Google Scholar]
- 11.Morgan, E. T., K. B. Goralski, M. Piquette-Miller, K. W. Renton, G. R. Robertson, M. R. Chaluvadi, K. A. Charles, S. J. Clarke, M. Kacevska, C. Liddle, T. A. Richardson, R. Sharma, and C. J. Sinal. 2008. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab. Dispos. 36:205-216. [DOI] [PubMed] [Google Scholar]
- 12.Mulanovich, V., R. E. Lewis, I. I. Raad, and D. P. Kontoyiannis. 2007. Random plasma concentrations of voriconazole decline over time. J. Infect. 55:e129-e1130. [DOI] [PubMed] [Google Scholar]
- 13.Pennick, G. J., M. Clark, D. A. Sutton, and M. G. Rinaldi. 2003. Development and validation of a high-performance liquid chromatography assay for voriconazole. Antimicrob. Agents Chemother. 47:2348-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pichard, L., I. Fabre, M. Daujat, J. Domergue, H. Joyeux, and P. Maurel. 1992. Effect of corticosteroids on the expression of cytochromes P450 and on cyclosporin A oxidase activity in primary cultures of human hepatocytes. Mol. Pharmacol. 41:1047-1055. [PubMed] [Google Scholar]
- 15.Purkins, L., N. Wood, D. Kleinermans, and D. Nichols. 2003. Histamine H2-receptor antagonists have no clinically significant effect on the steady-state pharmacokinetics of voriconazole. Br. J. Clin. Pharmacol. 56(Suppl. 1):51-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renton, K. W. 2005. Regulation of drug metabolism and disposition during inflammation and infection. Expert. Opin. Drug Metab. Toxicol. 1:629-640. [DOI] [PubMed] [Google Scholar]
- 17.Robatel, C., M. Rusca, C. Padoin, O. Marchetti, L. Liaudet, and T. Buclin. 2004. Disposition of voriconazole during continuous veno-venous haemodiafiltration (CVVHDF) in a single patient. J. Antimicrob. Chemother. 54:269-270. [DOI] [PubMed] [Google Scholar]
- 18.Roffey, S. J., S. Cole, P. Comby, D. Gibson, S. G. Jezequel, A. N. R. Nedderman, D. A. Smith, D. K. Walker, and N. Wood. 2003. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab. Dispos. 31:731-741. [DOI] [PubMed] [Google Scholar]
- 19.Seidegard, J., M. Simonsson, and S. Edsbacker. 2000. Effect of an oral contraceptive on the plasma levels of budesonide and prednisolone and the influence on plasma cortisol. Clin. Pharmacol. Ther. 67:373-381. [DOI] [PubMed] [Google Scholar]