Abstract
Up-to-date information regarding the antibiotic susceptibility of Neisseria meningitidis strains from African countries is highly limited. Our aim was to comprehensively describe the antibiotic susceptibilities of a selection of N. meningitidis isolates recovered between 2000 and 2006 from 18 African countries, mainly those within the meningitis belt. Susceptibilities to 11 antibiotics were determined using Etest for 137 N. meningitidis isolates (stringently selected from 693 available isolates). The isolates were also characterized by serogrouping, multilocus sequence typing, genosubtyping, and penA allele identification. All N. meningitidis isolates were susceptible to ceftriaxone, chloramphenicol, and ciprofloxacin. No isolate produced β-lactamase. Only three isolates (2%) displayed reduced susceptibility to penicillin G. The two isolates with the highest penicillin G MICs were the only isolates showing reduced susceptibility to ampicillin and cefuroxime. One of these isolates was also resistant to penicillin V. One percent of isolates displayed reduced susceptibility to rifampin, while 52% of the isolates were resistant to tetracycline, 74% were resistant to erythromycin, and 94% were resistant to sulfadiazine. The MICs of rifampin and tetracycline seemed to be associated with the serogroup of the isolates. In total, 18 sequence types (STs), 10 genosubtypes, and 8 different penA alleles were identified; the most common were ST-7, P1.20,9,35-1, and penA4, respectively. A high level of correlation was found between ST, genosubtype, and penA allele. In conclusion, N. meningitidis isolates from the African meningitis belt remain highly susceptible to the antibiotics used. Regarding β-lactam antibiotics, rare isolates showed a reduced susceptibility to penicillins, but the expanded-spectrum cephalosporins are not affected at present.
Invasive meningococcal disease is a significant health problem worldwide. In Africa, particularly in the sub-Saharan, so-called meningitis belt, epidemics of acute meningitis can reach incidence rates of 1,000 cases per 100,000 inhabitants, and in individual communities, attack rates as high as 1:10 for the population have been reported. During these epidemics, a mortality rate of about 10% is usually reported, which most probably is an underestimation (14). These epidemics in the African meningitis belt have historically been caused mostly by a limited number of Neisseria meningitidis serogroup A clones. During recent years, strains of other serogroups, such as serogroups C, W-135, and X, have also been involved (13).
Early antibiotic treatment of meningococcal disease is crucial for keeping the case fatality rate and risk of sequelae as low as possible. In Africa, the general recommendation for treatment during endemic periods is (i) ceftriaxone in multiple doses to also provide effective treatment of other presumptive etiological agents of bacterial meningitis, such as Streptococcus pneumoniae and Haemophilus influenzae; or (ii) multiple doses of penicillins. During meningococcal epidemics, the recommended treatment is (i) a single dose of chloramphenicol in oil or (ii) a single dose of ceftriaxone (13, 30, 43). However, increased levels of reduced susceptibility to penicillins have been reported worldwide (22, 28, 32, 39, 42). This reduced susceptibility has been due mainly to alterations in penicillin-binding protein 2, encoded by the penA gene (3, 27, 32, 35). Furthermore, though still rare, resistance to chloramphenicol has been reported from Australia, France, and Vietnam (12, 25). This resistance is considered to be due mainly to the presence of the catP gene, encoding the enzyme chloramphenicol acetyltransferase. Resistance to ceftriaxone has been reported from India (18). However, these strains certainly need to be examined further, comprehensively characterized phenotypically and genetically, and confirmed/disconfirmed by an independent laboratory (20).
In the African meningitis belt, chemoprophylaxis of close contacts of patients is rarely used and is not recommended by the WHO (43). In the Western world, on the other hand, chemoprophylaxis is often used, and ciprofloxacin and rifampin are recommended antibiotics (30). However, in recent years, there have been reports of reduced susceptibility to ciprofloxacin (1, 6-8, 26, 31) as well as resistance to rifampin from several countries worldwide (23, 29, 33).
Comprehensive data regarding the antibiotic susceptibility of N. meningitidis in many African countries are limited, and no up-to-date extensive study of the antibiotic susceptibility of N. meningitidis is at hand. Susceptibility testing, performed mainly using the disc diffusion method and the most commonly used antibiotics for treatment, is carried out mainly in hospital laboratories in the meningitis belt. Disc diffusion methodology, however, is hard to optimize, standardize, and quality assure, which makes the results from different laboratories and countries difficult to compare.
The aims of the present study were to comprehensively describe the antibiotic susceptibilities (to 11 different antibiotics) of invasive N. meningitidis isolates, strictly selected from 18 different African countries, mainly those within the meningitis belt, during the period 2000-2006 and to genetically characterize them.
MATERIALS AND METHODS
Bacterial isolates.
The N. meningitidis isolates used for this study were from collections available at the WHO Collaborating Centers in Marseille, France, and Oslo, Norway, and from the National Reference Laboratory for Pathogenic Neisseria in Örebro, Sweden. In total, between 2000 and 2006, 693 invasive isolates were recovered from patients, using routine bacteriological procedures, in 18 different African countries, mainly within the meningitis belt (Table 1). From this collection, all isolates with suspected reduced susceptibility according to initial testing, as well as isolates representing the prevalent phenotypes (serogroup and antibiogram) from each country, were included. When several isolates were related to the same epidemic outbreak, only one representative isolate was selected. One hundred thirty-seven invasive isolates were subsequently examined, comprising serogroups A (n = 82), W-135 (n = 38), X (n = 8), Y (n = 7), and C (n = 1), with one nongroupable isolate (Table 1). For quality control, one serogroup A reference strain, OR173/87 (15, 19), was included. The reference strain was susceptible to all antibiotics tested, except for erythromycin (MIC = 0.75 μg/ml) and sulfadiazine (MIC > 256 μg/ml).
TABLE 1.
Numbers and serogroups of N. meningitidis isolates recovered in 18 different African countries, mainly those in the meningitis belt, during the period 2000-2006, according to year and country (n = 137)b
| Country | Yr of isolation | No. of isolates (serogroup distribution)a |
|---|---|---|
| Angola | 2000 | 2 (A) |
| 2004 | 1 (A) | |
| Benin | 2003 | 3 (2 A, 1 W-135) |
| 2004 | 3 (1 A, 1 Y, 1 W-135) | |
| 2006 | 3 (2 W-135, 1 Y) | |
| Burkina Faso | 2001 | 5 (3 A, 2 W-135) |
| 2002 | 2 (W-135) | |
| 2003 | 6 (3 A, 2 W-135, 1 Y) | |
| 2004 | 12 (7 A, 3 W-135, 1 Y, 1 NG) | |
| Burundi | 2002 | 2 (A) |
| Cameroon | 2000 | 4 (A) |
| 2001 | 5 (4 A, 1 W-135) | |
| Central African Republic | 2001 | 2 (W-135) |
| Chad | 2001 | 7 (6 A, 1 W-135) |
| 2003 | 1 (W-135) | |
| 2004 | 1 (W-135) | |
| 2005 | 2 (W-135) | |
| Djibouti | 2001 | 1 (A) |
| Ethiopia | 2000 | 1 (A) |
| 2001 | 1 (A) | |
| 2002 | 2 (A) | |
| 2003 | 2 (A) | |
| Ghana | 2004 | 3 (2 A, 1 W-135) |
| Kenya | 2005 | 2 (W-135) |
| Niger | 2000 | 8 (A) |
| 2002 | 5 (3 A, 1 X, 1 W-135) | |
| 2003 | 11 (4 A, 3 Y, 4 W-135) | |
| 2004 | 4 (3 A, 1 X) | |
| 2005 | 5 (3 X, 2 W-135) | |
| Nigeria | 2003 | 2 (1 A, 1 W-135) |
| 2004 | 2 (1 A, 1 W-135) | |
| Republic of the Congo | 2001 | 4 (2 A, 1 C, 1 W-135) |
| Senegal | 2000 | 2 (1 A, 1 W-135) |
| 2001 | 3 (2 A, 1 W-135) | |
| 2002 | 2 (1 Y, 1 W-135) | |
| Somalia | 2002 | 2 (A) |
| Sudan | 2000 | 6 (5 A, 1 W-135) |
| 2001 | 6 (A) | |
| Uganda | 2006 | 2 (X) |
NG, nongroupable.
All isolates were from cerebrospinal fluid.
Phenotypic antibiotic susceptibility testing.
The MICs of penicillin G, ampicillin, penicillin V, ceftriaxone, cefuroxime, chloramphenicol, ciprofloxacin, rifampin, tetracycline, erythromycin, and sulfadiazine were determined for all isolates, using the Etest method (AB Biodisk, Solna, Sweden) on Mueller-Hinton agar (Becton Dickinson and Company, Sparks, MD) supplemented with 5% sheep blood at 37°C in 5% CO2 for 16 to 18 h (40). The present Etest methodology was previously evaluated in comparison to a reference method, i.e., agar dilution (41). The breakpoints used are shown in Table 2. The breakpoints used were from several different organizations, since no organization describes breakpoints for all examined antibiotics. All isolates were tested for β-lactamase production by use of nitrocefin discs (AB Biodisk, Solna, Sweden).
TABLE 2.
Susceptibilities of N. meningitidis isolates (n = 137) recovered during the period 2000-2006 in 18 different African countries, mainly those within the meningitis belt, to 11 different antibiotics
| Antibiotic | Breakpoints (susceptible/resistant) | MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | % of isolates
|
||
|---|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | |||||
| Penicillin G | ≤0.094/>1a | 0.006-0.38 | 0.047 | 0.094 | 98 | 2 | 0 |
| Ampicillin | ≤0.12/>1b | 0.016-0.75 | 0.064 | 0.094 | 99 | 1 | 0 |
| Penicillin V | ≤1/>1c | 0.016-1.5 | 0.19 | 0.38 | 99 | 0 | 1 |
| Ceftriaxone | ≤0.12/b | <0.002-0.002 | <0.002 | <0.002 | 100 | 0 | 0 |
| Cefuroxime | ≤0.25/>1c | <0.016-0.5 | 0.047 | 0.094 | 99 | 1 | 0 |
| Chloramphenicol | ≤2/>4b | 0.38-1.5 | 0.75 | 1 | 100 | 0 | 0 |
| Ciprofloxacin | ≤0.03/>0.25b | 0.002-0.012 | 0.004 | 0.004 | 100 | 0 | 0 |
| Rifampin | ≤0.25/>1b | 0.002-0.38 | 0.064 | 0.125 | 99 | 1 | 0 |
| Tetracycline | ≤1/>1d | 0.064-6 | 2 | 4 | 48 | 0 | 52 |
| Erythromycin | ≤0.5/>0.5d | 0.032-3 | 0.75 | 1 | 26 | 0 | 74 |
| Sulfadiazinee | ≤1/>4b | 1.5->256 | >256 | >256 | 0 | 6 | 94 |
Breakpoints previously described by Taha et al., based on a combination of identified penicillin G MICs and the presence/absence of a penA mosaic allele (32).
Breakpoints proposed by the European Meningococcal Disease Society (40).
Breakpoints in accordance with the Swedish Reference Group for Antibiotics (www.srga.org).
Breakpoints in accordance with the British Society for Antimicrobial Chemotherapy (4a).
Breakpoints for sulfisoxazole were used, which displayed a similar MIC distribution to that of sulfadiazine by Etest (data not shown).
Isolation of genomic DNA.
Isolation of genomic DNA from the N. meningitidis isolates was performed using the MagNA Pure system (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's instructions. The DNA preparations were stored at 4°C prior to PCR.
MLST.
Multilocus sequence typing (MLST) was performed mainly as previously described (15, 17). The isolates were assigned a sequence type (ST) according to the Neisseria MLST website (http://pubmlst.org/neisseria/).
Genosubtyping.
The porA gene was amplified by real-time PCR as previously described (19). The porA amplicons were purified using a Multiscreen PCRμ96 plate (Millipore, Bedford, MA) and vacuum filtration according to the manufacturer's instructions. The purified products were resolved in 50 μl of 10 mM Tris-HCl, and cycle sequencing PCR was performed as previously described (19). Subsequently, the products were purified using ethanol-sodium acetate precipitation and resuspended in 10 μl formamide (Applied Biosystems, Warrington, United Kingdom) according to the manufacturer's instructions. The nucleotide sequences were determined using an ABI PRISM 3100 genetic analyzer (Applied Biosystems, Foster City, CA). The deduced amino acid sequences of variable region 1 (VR1) and VR2 were assigned genosubtype numbers according to the Neisseria meningitidis PorA variable regions database (http://neisseria.org/perl/agdbnet/agdbnet.pl?file=poravr.xml), and the sequences of VR3 were assigned numbers according to the PorA VR3 database (http://www.smprl.scot.nhs.uk/PorA_VR3.htm).
penA gene sequencing.
The penA gene was amplified and sequenced as previously described (4, 35). The isolates were assigned a penA allele number by using the N. meningitidis penA database (http://neisseria.org/perl/agdbnet/agdbnet.pl?file=penA.xml). Based on the different penA alleles, a phylogenetic tree was constructed with TREECON (version 1.3b) software by using the Jin and Nei substitution model, the Kimura evolutionary model, an α value of 0.5, and the neighbor-joining method (38), as previously described (37).
RESULTS
Antibiotic susceptibility.
The results of antibiotic susceptibility testing are summarized in Table 2. Briefly, all isolates were β-lactamase negative and susceptible to ceftriaxone (MIC ≤ 0.002 μg/ml), chloramphenicol (MIC, 0.38 to 1.5 μg/ml), and ciprofloxacin (MIC, 0.002 to 0.012 μg/ml). Three of the isolates (2%) displayed reduced susceptibility to penicillin G; two of these isolates were in serogroup A (isolated in Ethiopia and Somalia), and one was in serogroup Y (isolated in Senegal). The two isolates with the highest penicillin G MICs (MIC = 0.25 μg/ml and 0.38 μg/ml for isolates in serogroups A and Y, respectively) were the sole isolates displaying reduced susceptibility to ampicillin and cefuroxime. The serogroup Y isolate was also resistant to penicillin V (MIC = 1.5 μg/ml). Overall, 1 of 82 serogroup A isolates (<1%) displayed reduced susceptibility to rifampin (MIC = 0.38 μg/ml). Fifty-two percent of the isolates were resistant to tetracycline, 74% were resistant to erythromycin, and 94% were resistant to sulfadiazine.
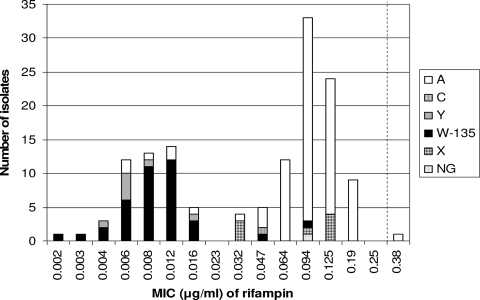
For rifampin, two susceptible populations could be observed, one comprising mainly serogroup W-135 and Y isolates, displaying very low MICs, and one with higher MICs, comprising mainly serogroup A and X isolates (Fig. 1). For tetracycline, a similar pattern was observed, with a resistant population (MIC, 2 to 6 μg/ml) of mainly serogroup A isolates, belonging to ST-7 or ST-2859, and a susceptible population (MIC, 0.064 to 0.38 μg/ml) consisting of isolates representing other serogroups and STs.
FIG. 1.
Serogroup distribution and MICs of rifampin for N. meningitidis isolates (n = 137) collected in 18 different African countries, mainly those from the meningitis belt, from 2000 to 2006. The broken line indicates the breakpoint for susceptible isolates (MIC ≤ 0.25 μg/ml).
MLST, genosubtyping, and penA gene sequencing.
Eighteen different STs (comprising five clonal complexes and six STs not belonging to any clonal complex) were identified. ST-7 (47%), ST-11 (20%), and ST-2881 (9%) were the most prevalent STs, and clonal complex ST-5 (cc5) was the most common clonal complex (58% of the isolates).
A total of 10 genosubtypes were identified, with P1.20,9,35-1 (58%), P1.5,2,36-2 (20%), and P1.5-1,2-36,36-2 (9%) being the most prevalent.
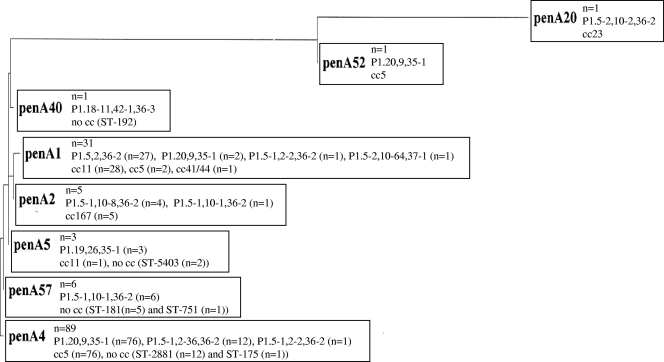
Eight penA alleles were identified, with penA4 being the most common (65%) (Fig. 2). penA4 was also found to be associated with serogroup A and cc5 (Fig. 2). Accordingly, 93% of all serogroup A and 97% of all cc5 isolates had the penA4 allele.
FIG. 2.
Phylogenetic tree based on a 402-bp alignment of penA gene sequences in N. meningitidis isolates (n = 137) collected in 18 different African countries, mainly those in the meningitis belt, from 2000 to 2006. The genosubtypes and clonal complexes (cc) associated with the different penA alleles are included in the boxes.
The two isolates with the highest MICs of penicillin G (MIC = 0.25 μg/ml and 0.38 μg/ml) comprised mosaic alleles (penA52 and penA20, respectively), while all other isolates displayed wild-type penA alleles. Consequently, there was an association between reduced susceptibility to penicillins and the presence of penA mosaic alleles in African meningitis belt N. meningitidis isolates.
There was also an association between penA alleles and genosubtypes (Fig. 2). Ninety-six percent of the isolates with genosubtype P1.20,9,35-1 carried penA4, and 100% of the genosubtype P1.5,2,36-2 isolates contained penA1. Genosubtypes and STs were also associated, especially in studying the clonal complexes. The most common genosubtype, P1.20,9,35-1 (n = 79 isolates), consisted of only isolates belonging to cc5, and the second most common genosubtype, P1.5,2,36-2 (n = 27), consisted of only cc11 isolates.
DISCUSSION
In the present study, N. meningitidis isolates recovered in 2000 to 2006, mainly from countries constituting the African meningitis belt, were found to remain highly susceptible to the antibiotics used for treatment and prophylaxis. However, isolates from northern and southern Africa were not included and may differ substantially (10, 44).
Only a few isolates included in the study displayed reduced susceptibility to penicillin G (2%) and other β-lactam antibiotics (1% had reduced susceptibility to ampicillin, penicillin V, and cefuroxime). Since the study was based partly on isolates selected for reduced antibiotic susceptibility, this proportion is most likely lower among all clinical N. meningitidis isolates in the African meningitis belt, where numerous isolates representing identical strains are recovered in outbreak situations.
All isolates were susceptible to ceftriaxone. In spite of the fact that there have been reports of rare chloramphenicol-resistant N. meningitidis isolates in Australia, France, and Vietnam (12, 25), all of the presently examined African meningococci were fully susceptible to chloramphenicol. Previous studies of African N. meningitidis isolates have displayed identical results, i.e., all isolates were fully susceptible to chloramphenicol (11, 36, 44). Furthermore, all previously described chloramphenicol-resistant isolates have been serogroup B isolates (12, 25), which is extremely rare in Africa. Serogroup B N. meningitidis strains are highly transformable, unlike serogroup A strains (25), and hence the lower transformation rate for serogroup A could be a possible explanation for the lack of chloramphenicol resistance in the examined African N. meningitidis isolates. However, serogroup A accounted for only 60% of the isolates in the present study, so this is not the only explanation.
For continents other than Africa, there have been reports of reduced susceptibility or resistance to ciprofloxacin (1, 6-8, 26, 31) and rifampin (23, 29, 33), which are commonly used antibiotics for prophylaxis. In the present study, however, the examined N. meningitidis isolates, mainly from the African meningitis belt, were highly susceptible to both antibiotics, with only one serogroup A isolate from Ethiopia displaying reduced susceptibility to rifampin (MIC = 0.38 μg/ml). This finding may be due to the infrequent use of prophylaxis in Africa.
Tetracycline and erythromycin are not used for treatment of meningococcal disease. Since these antibiotics are used for treatment of many other infections, their susceptibility patterns may reflect the overall antibiotic pressure on African N. meningitidis isolates. A large number of the presently examined African N. meningitidis isolates were resistant to tetracycline (52%) and erythromycin (74%). A high level of resistance to tetracycline (85%) has also been described in a previous study including a limited number of African N. meningitidis isolates (n = 20) (24). According to other previous reports (9, 16), among 441 examined N. meningitidis isolates, including 22 serogroup A isolates, resistance to tetracycline was found only in the serogroup A isolates, all belonging to cc5. This resistance was associated with the drug efflux mechanism encoded by the gene tet(B) (9). The close association between serogroup A isolates belonging to ST-7 or ST-2859 and tetracycline resistance observed both in the present study and in the previous one (9) may explain the low resistance to tetracycline (0.3%) observed in a previous French study including 2,167 N. meningitidis isolates, with only nine serogroup A isolates (2). In that study, 9.1% of all invasive isolates in France between 1999 and 2002 also displayed reduced susceptibility to erythromycin (2).
In the present study, almost all examined African isolates displayed wild-type penA alleles. The most common allele was penA4, which was present in 65% of the isolates. This can be compared to a recent extensive study where the penA genes of N. meningitidis isolates (n = 1,670) from 22 mainly European countries were sequenced (32). In this study, penA4 was found in only 10% of the isolates, while penA1 and penA3 together accounted for 46% of the isolates (32). Accordingly, during recent years, i.e., 2000 to 2006, penA4 may seem strongly associated with cc5, and hence serogroup A (Fig. 2). This may be due to the clonal expansion of cc5 in the meningitis belt since the 1980s (5, 34). The two African isolates with the highest MICs for the different penicillins displayed the penA mosaic alleles 52 and 20. penA52 was found in a serogroup A, ST-7 isolate from Somalia. In the previous European study (32), penA52 was found in 19 serogroup B isolates from six different European countries and in 1 serogroup B isolate from the United States. In the present study, penA20 was found in a serogroup Y, ST-23 isolate cultured in Senegal. For comparison, in the previous European study (32), penA20 was found in six serogroup Y isolates and one serogroup C isolate, recovered in France and Italy.
In the present study, the most common ST was ST-7 (n = 64 isolates) (in cc5 [n = 79]), followed by ST-11 (n = 28) (in cc11 [n = 29]). These findings are in congruence with earlier studies regarding N. meningitidis isolates from the meningitis belt (5, 15, 21, 34). In Africa, cc5 and cc11 have been predominant, and only a few other sequence types, such as ST-2881 and ST-181, have been detected sporadically (5). In the present study, cc5 consisted of two subgroups of serogroup A isolates, all of which were resistant to sulfadiazine, but ST-5 and ST-2207 isolates were susceptible to tetracycline and ST-7 and ST-2859 isolates were resistant. Both ST-7 and ST-2859 are relatively new variants of cc5 and have recently expanded in the meningitis belt to replace ST-5 (5). cc11 and ST-2881 consisted mainly of sulfadiazine-resistant, tetracycline-susceptible serogroup W-135 isolates. In ST-181, only sulfadiazine- and tetracycline-susceptible serogroup X isolates were found. All of these isolates were cultured in Niger during 2002 to 2005 and probably represented a single clone, since all phenotypic and genotypic characteristics for the five individual isolates were identical, with the exception of slightly different MICs.
In conclusion, N. meningitidis isolates from the African meningitis belt remain highly susceptible to the antibiotics used for treatment and prophylaxis, and hence, the present results support the WHO recommendations (43). Regarding β-lactam antibiotics, a few isolates displayed reduced susceptibility to penicillins, but at present the expanded-spectrum cephalosporins, such as ceftriaxone, are not affected. However, it is crucial to continuously monitor the antibiotic susceptibility of N. meningitidis to avoid future treatment failures in Africa during both endemic and epidemic periods.
Acknowledgments
This study was supported by grants from the Örebro County Council Research Committee and the Foundation for Medical Research at Örebro University Hospital, Örebro, Sweden.
Footnotes
Published ahead of print on 2 February 2009.
REFERENCES
- 1.Alcalá, B., C. Salcedo, L. de la Fuente, L. Arreaza, M. J. Uría, R. Abad, R. Enríquez, J. A. Vázquez, M. Motgé, and J. de Batlle. 2004. Neisseria meningitidis showing decreased susceptibility to ciprofloxacin: first report in Spain. J. Antimicrob. Chemother. 53:409. [DOI] [PubMed] [Google Scholar]
- 2.Antignac, A., M. Ducos-Galand, A. Guiyoule, R. Pirès, J. M. Alonso, and M. K. Taha. 2003. Neisseria meningitidis strains isolated from invasive infections in France (1999-2002): phenotypes and antibiotic susceptibility patterns. Clin. Infect. Dis. 37:912-920. [DOI] [PubMed] [Google Scholar]
- 3.Antignac, A., P. Kriz, G. Tzanakaki, J. M. Alonso, and M. K. Taha. 2001. Polymorphism of Neisseria meningitidis penA gene associated with reduced susceptibility to penicillin. J. Antimicrob. Chemother. 47:285-296. [DOI] [PubMed] [Google Scholar]
- 4.Arreaza, L., and J. A. Vázquez. 2001. Molecular approach for the study of penicillin resistance in Neisseria meningitidis, p. 107-119. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal disease: methods and protocols. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 4a.British Society for Antimicrobial Chemotherapy. February 2008. BSAC methods for antimicrobial susceptibility testing, version 7.1. BSAC, Birmingham, United Kingdom.
- 5.Caugant, D. A., and P. Nicolas. 2007. Molecular surveillance of meningococcal meningitis in Africa. Vaccine 25(Suppl. 1):A8-A11. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC). 2008. Emergence of fluoroquinolone-resistant Neisseria meningitidis—Minnesota and North Dakota, 2007-2008. MMWR Morb. Mortal. Wkly. Rep. 57:173-175. [PubMed] [Google Scholar]
- 7.Chu, Y. W., T. K. Cheung, V. Tung, F. Tiu, J. Lo, R. Lam, R. Lai, and K. K. Wong. 2007. A blood isolate of Neisseria meningitidis showing reduced susceptibility to quinolones in Hong Kong. Int. J. Antimicrob. Agents 30:94-95. [DOI] [PubMed] [Google Scholar]
- 8.Corso, A., D. Faccone, M. Miranda, M. Rodriguez, M. Regueira, C. Carranza, C. Vencina, J. A. Vázquez, and M. Galas. 2005. Emergence of Neisseria meningitidis with decreased susceptibility to ciprofloxacin in Argentina. J. Antimicrob. Chemother. 55:596-597. [DOI] [PubMed] [Google Scholar]
- 9.Crawford, S. A., K. R. Fiebelkorn, J. E. Patterson, and J. H. Jorgensen. 2005. International clone of Neisseria meningitidis serogroup A with tetracycline resistance due to tet(B). Antimicrob. Agents Chemother. 49:1198-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.du Plessis, M., A. von Gottberg, C. Cohen, L. de Gouveia, and K. P. Klugman. 2008. Neisseria meningitidis intermediately resistant to penicillin and causing invasive disease in South Africa in 2001 to 2005. J. Clin. Microbiol. 46:3208-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagneux, S., A. Hodgson, I. Ehrhard, G. Morelli, B. Genton, T. Smith, M. Tanner, F. Binka, M. Achtman, and G. Pluschke. 2000. Microheterogeneity of serogroup A (subgroup III) Neisseria meningitidis during an outbreak in northern Ghana. Trop. Med. Int. Health 5:280-287. [PubMed] [Google Scholar]
- 12.Galimand, M., G. Gerbaud, M. Guibourdenche, J. Y. Riou, and P. Courvalin. 1998. High-level chloramphenicol resistance in Neisseria meningitidis. N. Engl. J. Med. 339:868-874. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood, B. 2006. Editorial: 100 years of epidemic meningitis in West Africa—has anything changed? Trop. Med. Int. Health 11:773-780. [DOI] [PubMed] [Google Scholar]
- 14.Greenwood, B. 1999. Manson lecture. Meningococcal meningitis in Africa. Trans. R. Soc. Trop. Med. Hyg. 93:341-353. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsson, S., M. Issa, M. Unemo, A. Bäckman, P. Mölling, N. Sulaiman, and P. Olcén. 2003. Molecular characterisation of group A Neisseria meningitidis isolated in Sudan 1985-2001. APMIS 111:1060-1066. [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen, J. H., S. A. Crawford, and K. R. Fiebelkorn. 2005. Susceptibility of Neisseria meningitidis to 16 antimicrobial agents and characterization of resistance mechanisms affecting some agents. J. Clin. Microbiol. 43:3162-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manchanda, V., and P. Bhalla. 2006. Emergence of non-ceftriaxone-susceptible Neisseria meningitidis in India. J. Clin. Microbiol. 44:4290-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mölling, P., S. Jacobsson, A. Bäckman, and P. Olcén. 2002. Direct and rapid identification and genogrouping of meningococci and porA amplification by LightCycler PCR. J. Clin. Microbiol. 40:4531-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicolas, P., V. Manchanda, and P. Bhalla. 2007. Emergence of non-ceftriaxone-susceptible Neisseria meningitidis in India. J. Clin. Microbiol. 45:1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicolas, P., G. Norheim, E. Garnotel, S. Djibo, and D. A. Caugant. 2005. Molecular epidemiology of Neisseria meningitidis isolated in the African meningitis belt between 1988 and 2003 shows dominance of sequence type 5 (ST-5) and ST-11 complexes. J. Clin. Microbiol. 43:5129-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oppenheim, B. A. 1997. Antibiotic resistance in Neisseria meningitidis. Clin. Infect. Dis. 24(Suppl. 1):S98-S101. [DOI] [PubMed] [Google Scholar]
- 23.Rainbow, J., E. Cebelinski, J. Bartkus, A. Glennen, D. Boxrud, and R. Lynfield. 2005. Rifampin-resistant meningococcal disease. Emerg. Infect. Dis. 11:977-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scarborough, M., S. B. Gordon, C. J. Whitty, N. French, Y. Njalale, A. Chitani, T. E. Peto, D. G. Lalloo, and E. E. Zijlstra. 2007. Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N. Engl. J. Med. 357:2441-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shultz, T. R., J. W. Tapsall, P. A. White, C. S. Ryan, D. Lyras, J. I. Rood, E. Binotto, and C. J. Richardson. 2003. Chloramphenicol-resistant Neisseria meningitidis containing catP isolated in Australia. J. Antimicrob. Chemother. 52:856-859. [DOI] [PubMed] [Google Scholar]
- 26.Skoczynska, A., J. M. Alonso, and M. K. Taha. 2008. Ciprofloxacin resistance in Neisseria meningitidis, France. Emerg. Infect. Dis. 14:1322-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spratt, B. G. 1994. Resistance to antibiotics mediated by target alterations. Science 264:388-393. [DOI] [PubMed] [Google Scholar]
- 28.Stefanelli, P., A. Carattoli, A. Neri, C. Fazio, and P. Mastrantonio. 2003. Prediction of decreased susceptibility to penicillin of Neisseria meningitidis strains by real-time PCR. J. Clin. Microbiol. 41:4666-4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefanelli, P., C. Fazio, G. La Rosa, C. Marianelli, M. Muscillo, and P. Mastrantonio. 2001. Rifampicin-resistant meningococci causing invasive disease: detection of point mutations in the rpoB gene and molecular characterization of the strains. J. Antimicrob. Chemother. 47:219-222. [DOI] [PubMed] [Google Scholar]
- 30.Stephens, D. S., B. Greenwood, and P. Brandtzaeg. 2007. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 369:2196-2210. [DOI] [PubMed] [Google Scholar]
- 31.Strahilevitz, J., A. Adler, G. Smollan, V. Temper, N. Keller, and C. Block. 2008. Serogroup A Neisseria meningitidis with reduced susceptibility to ciprofloxacin. Emerg. Infect. Dis. 14:1667-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taha, M. K., J. A. Vàzquez, E. Hong, D. E. Bennett, S. Bertrand, S. Bukovski, M. T. Cafferkey, F. Carion, J. J. Christensen, M. Diggle, G. Edwards, R. Enríquez, C. Fazio, M. Frosch, S. Heuberger, S. Hoffmann, K. A. Jolley, M. Kadlubowski, A. Kechrid, K. Kesanopoulos, P. Kriz, L. Lambertsen, I. Levenet, M. Musilek, M. Paragi, A. Saguer, A. Skoczynska, P. Stefanelli, S. Thulin, G. Tzanakaki, M. Unemo, U. Vogel, and M. L. Zarantonelli. 2007. Target gene sequencing to characterize the penicillin G susceptibility of Neisseria meningitidis. Antimicrob. Agents Chemother. 51:2784-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taha, M. K., M. L. Zarantonelli, C. Ruckly, D. Giorgini, and J. M. Alonso. 2006. Rifampin-resistant Neisseria meningitidis. Emerg. Infect. Dis. 12:859-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teyssou, R., and E. Muros-Le Rouzic. 2007. Meningitis epidemics in Africa: a brief overview. Vaccine 25(Suppl. 1):A3-A7. [DOI] [PubMed] [Google Scholar]
- 35.Thulin, S., P. Olcén, H. Fredlund, and M. Unemo. 2006. Total variation in the penA gene of Neisseria meningitidis: correlation between susceptibility to β-lactam antibiotics and penA gene heterogeneity. Antimicrob. Agents Chemother. 50:3317-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tondella, M. L., N. E. Rosenstein, L. W. Mayer, F. C. Tenover, S. A. Stocker, M. W. Reeves, and T. Popovic. 2001. Lack of evidence for chloramphenicol resistance in Neisseria meningitidis, Africa. Emerg. Infect. Dis. 7:163-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unemo, M., P. Olcén, J. Albert, and H. Fredlund. 2003. Comparison of serologic and genetic porB-based typing of Neisseria gonorrhoeae: consequences for future characterization. J. Clin. Microbiol. 41:4141-4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van de Peer, Y., and R. De Wachter. 1994. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Appl. Biosci. 10:569-570. [DOI] [PubMed] [Google Scholar]
- 39.Vázquez, J. A. 2001. The resistance of Neisseria meningitidis to the antimicrobial agents: an issue still in evolution. Rev. Med. Microbiol. 12:39-45. [Google Scholar]
- 40.Vázquez, J. A. 2007. Resistance testing of meningococci: the recommendations of the European Monitoring Group on Meningococci. FEMS Microbiol. Rev. 31:97-100. [DOI] [PubMed] [Google Scholar]
- 41.Vázquez, J. A., L. Arreaza, C. Block, I. Ehrhard, S. J. Gray, S. Heuberger, S. Hoffmann, P. Kriz, P. Nicolas, P. Olcén, A. Skoczynska, L. Spanjaard, P. Stefanelli, M. K. Taha, and G. Tzanakaki. 2003. Interlaboratory comparison of agar dilution and Etest methods for determining the MICs of antibiotics used in management of Neisseria meningitidis infections. Antimicrob. Agents Chemother. 47:3430-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vázquez, J. A., R. Enriquez, R. Abad, B. Alcalá, C. Salcedo, and L. Arreaza. 2007. Antibiotic resistant meningococci in Europe: any need to act? FEMS Microbiol. Rev. 31:64-70. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. 2007. Standardized treatment of bacterial meningitidis in Africa in epidemic and non epidemic situations. Epidemic and pandemic alert and response 3. World Health Organization, Geneva, Switzerland.
- 44.Zerouali, K., N. Elmdaghri, M. Boudouma, and M. Benbachir. 2002. Serogroups, serotypes, serosubtypes and antimicrobial susceptibility of Neisseria meningitidis isolates in Casablanca, Morocco. Eur. J. Clin. Microbiol. Infect. Dis. 21:483-485. [DOI] [PubMed] [Google Scholar]