Abstract
Leishmaniasis represents a serious public health problem worldwide. The first line of treatment is based on glucantime and pentostan, which generate toxic effects in treated patients. We have recently shown that amiodarone, frequently used as an antiarrhythmic, possesses activity against Trypanosoma cruzi through the disruption of mitochondrial Ca2+ homeostasis and the inhibition of parasite ergosterol biosynthesis, specifically at the level of oxidosqualene cyclase activity (G. Benaim, J. Sanders, Y. Garcia-Marchan, C. Colina, R. Lira, A. Caldera, G. Payares, C. Sanoja, J. Burgos, A. Leon-Rossell, J. Concepcion, A. Schijman, M. Levin, E. Oldfield, and J. Urbina, J. Med. Chem. 49:892-899, 2006). Here we show that at therapeutic concentrations, amiodarone has a profound effect on the viability of Leishmania mexicana promastigotes. Additionally, its effect on the viability of the parasite was greater against intracellular amastigotes than against promastigotes, and it did not affect the host cell. Using fluorimetric and confocal microscopy techniques, we also demonstrated that the mechanism of action of amiodarone was related to the disruption of intracellular Ca2+ homeostasis through a direct action not only on the mitochondria but also on the acidocalcisomes. On the other hand, analysis of the free sterols in promastigotes incubated with amiodarone showed that this drug also affected the biosynthesis of 5-dehydroepisterol, which results in squalene accumulation, thus suggesting that amiodarone inhibits the squalene epoxidase activity of the parasite. Taken together, the results obtained in the present work point to a more general effect of amiodarone in trypanosomatids, opening potential therapeutic possibilities for this infectious disease.
Chemotherapy is the most effective treatment against leishmaniasis, due to the lack of an effective vaccine (14, 20). The recommended first-line therapy for different forms of leishmaniasis includes pentavalent antimonials like glucantime and pentostan (8, 25). However, these drugs have secondary effects on the renal, cardiac, and hepatic systems (25). Resistance to pentavalent antimonial drugs is also an important factor that limits the treatments available in several countries (1, 13). Another disadvantage of antimonial therapy is the requirement for long-term parenteral administration in order to obtain optimal results (12, 21). At present, miltefosine appears to represent a major advance in the treatment of visceral and cutaneous leishmaniasis, despite its teratogenic characteristics, which limits its use during pregnancy (11).
Amiodarone (Fig. 1), an antiarrhythmic class III drug commonly used to treat several cardiomyopathies, has been the subject of recent studies as an antimycotic and parasitocidal agent, since this drug possesses excellent pharmacokinetic properties and a relative low cost. Thus, this drug has been shown to have potent effects against the yeast Saccharomyces cerevisiae as well as Candida albicans and other fungi (9). The reported mechanism of action of amiodarone against Saccharomyces cerevisiae (19) and Trypanosoma cruzi (7) is, at least in part, the disruption of Ca2+ homeostasis.
FIG. 1.

Chemical structure of amiodarone.
The crucial role of Ca2+ in the regulation of many important processes for cellular viability has been demonstrated in different trypanosomatids (6, 23, 24). At the plasma membrane level, it has been reported that a Ca2+ ATPase is stimulated by calmodulin in T. cruzi (3, 5), Trypanosoma brucei (4), and different species of Leishmania (2, 22). In all of these parasites, the regulation of cytoplasmic Ca2+ is carried out by three major organelles: the endoplasmic reticulum; the unique mitochondrion that is present in these parasites; and the acidocalcisome, a special compartment that is devoted to the accumulation of polyphosphates and that is also probably involved in the regulation of Ca2+ (17). The parasite mitochondrion possess a Ca2+ electrophoretic uniporter in its internal membrane which utilizes the difference in the proton electrochemical potential between the intramitochondrial space and the cytoplasm as the driving force for the accumulation of this cation (2, 15). This mitochondrial Ca2+ transport system is characterized by a low affinity for Ca2+ but a high capacity for Ca2+ accumulation. All these attributes are very similar to the mitochondrial Ca2+ transport system in mammals (2, 15). The acidocalcisomes, however, are acidic organelles with high levels of pyrophosphates and polyphosphates which are capable of accumulating large amounts of Ca2+ (16, 17). All these systems, which operate in a concerted fashion, contribute to the maintenance of intracellular Ca2+ homeostasis, keeping the intracellular (cytoplasmic) Ca2+ concentration ([Ca2+]i) under 100 nM, well below the extracellular Ca2+ concentration (which is on the order of mM), thus allowing this cation to perform its essential function as an internal signaling messenger (6).
We have previously shown that amiodarone affects the viability of T. cruzi by disrupting mitochondrial Ca2+ homeostasis in the parasite (7). In the present study, we demonstrate that amiodarone also affects the viability of Leishmania mexicana by destabilizing Ca2+ homeostasis in both the mitochondrion and the acidocalcisome.
Similar to T. cruzi, however, L. mexicana contains ergosterol instead of cholesterol as the main sterol component on its membranes. This property constitutes a notable difference between trypanosomatids and humans and validates the use of the sterol biosynthetic pathway as a potential target for the development of new drugs (31). We have also demonstrated that amiodarone blocks T. cruzi ergosterol biosynthesis at the level of the oxidosqualene cyclase (7). In this context, in this work we also show that amiodarone inhibits the L. mexicana sterol pathway at the level of the squalene epoxidase, which is essential for the synthesis of 5-dehydroepisterol.
MATERIALS AND METHODS
Chemicals.
Amiodarone {(2-butyl-3-benzofuranyl)-[4-[2-(diethylamino)ethoxi] 3,5-diiodophenyl] methanone hydrochloride}, EGTA, digitonin, fluoro-carbonylcyanide P-(trifluoromethoxy) phenylhydrazone (FCCP), bafilomycin A, nigericin, lectin from the coral tree (Eritrina cristagalli[r]), and l-polylysine were from Sigma (St. Louis, MO). Fura 2-acetoxymethyl ester (FURA 2-AM), rhodamine 123, rhod 2-AM, and acridine orange were from Molecular Probes (Eugene, OR).
Culture of promastigotes and determination of cellular proliferation.
Promastigotes of L. mexicana were cultured in RPMI 1640 medium (Gibco) supplemented with 10% inactivated fetal bovine serum (Gibco) under continuous agitation at 29°C. The susceptibility of L. mexicana to amiodarone was evaluated by use of a parasite growth curve obtained in the absence or the presence of the drug and a Neubauer chamber. The drug was added 24 h after the cultures were established (106 parasites/ml). At least three independent experiments were performed for each condition.
Determination of percentage of infected macrophages.
J774G8 macrophages were maintained at 37°C and 5% CO2 in RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum. For parasite infection, a proportion of 10 promastigotes to 1 macrophage was employed, and the infected cells were incubated for 24 h at 37°C in 5% CO2. To determine the effect of amiodarone on intracellular amastigotes, growth curve studies were carried out in the presence or the absence of the drug. The percentage of infected macrophages was determined at 72 h posttreatment under a light microscope by the use of Giemsa stain. At least three independent experiments were performed for each condition.
Determination of [Ca2+]i.
To measure [Ca2+]i, we used the fluorescent ratiometric Ca2+ indicator Fura 2, whose excitation spectrum depends on the concentration of the cation, while its emission peak does not vary (30). Parasites were loaded with Fura 2-AM as described previously (7), with slight modifications. Briefly, parasites (4 × 106 promastigotes/1.5 ml of culture medium) were centrifuged at 600 × g for 2.5 min and were then washed twice with loading buffer (137 mM NaCl, 4 mM KCl, 1.5 mM KH2PO4, 8.5 mM Na2HPO4, 11 mM glucose, 1 mM CaCl2 0.8 mM, MgSO4, 20 mM HEPES-NaOH, pH 7.4) (32). The parasite pellet was resuspended in the same buffer, and a mixture of 6 μM Fura 2-AM and 2.4 mM probenecid was applied. The parasites were then incubated at 29°C for 45 min under continuous agitation. Following two washes with this buffer, fluorescence measurements were performed in a Hitachi 2000 spectrofluorimeter at 29°C with continuous agitation by stirring in a cuvette; the spectrofluorimeter was coupled to a computer with an appropriate data acquisition system. [Ca2+]i was evaluated as described by Grynkiewicz et al. (18) by applying the following equation: Kd × [(R − Rmin)/(Rmax − R)] × [Fmin (380)/Fmax (380)], were Kd is the dissociation constant of Fura 2 (244 nM); R is the ratio of the fluorescence emission obtained after excitation at 340 nm/after excitation at 380 nm; Rmax and Fmax are the ratio of excitation fluorescence at 340 nm/excitation fluorescence at 380 nm and the fluorescence of Fura 2 at 380 nm, respectively, under saturated Ca2+ concentrations; and Rmin and Fmin are the ratio of excitation fluorescence at 340 nm/excitation fluorescence at 380 nm and the fluorescence of Fura 2 at 380 nm, respectively, in the absence of Ca2+. Maximum and minimum values were obtained after the addition of 30 μM digitonin, which allows the flow of Ca2+ to the interior of the cell. Then, 8 mM EGTA was added to chelate all the remaining Ca2+.
Determination of the mitochondrial membrane potential.
The mitochondrial membrane potential estimations were carried out with the fluorescent dye rhodamine 123, which presents maximum peaks in its excitation and emission spectra at 488 and 530 nm, respectively. Rhodamine 123, is a mitochondrion-specific cationic dye which allows visualization of the state of the electrochemical potential of this organelle since this dye is distributed between the internal and the external mitochondrial membrane according to the electrochemical potential. The experimental conditions used to load the parasites with this dye were essentially similar to the conditions described above for Fura 2-AM. Briefly, the parasites were loaded with 10 μg/ml of rhodamine 123 for 30 min at 29°C. All measurements were performed in a Hitachi 2000 spectrofluorimeter at 29°C and under continuous agitation; the spectrofluorimeter was coupled to a computer with an appropriate data acquisition system.
Determination of acidocalcisome alkalinization level.
The accumulation of acridine orange in the acidocalcisomes was used as a probe for alkalinization, as reported by Docampo et al. (16). Initially, promastigotes were washed twice with 130 mM KCl-1 mM MgCl2-2 mM KH2PO4-20 mM Tris-HCl, pH 7.4. The promastigotes (109 cells/ml) were then loaded with 2 μM acridine orange for 5 min at 29°C and under constant agitation. Measurements were performed at an excitation λ of 488 nm and an emission λ of 530 nm in a Hitachi 2000 spectrofluorimeter under continuous agitation in a stirred cuvette at 29°C.
Confocal microscopy determinations.
Promastigotes of L. mexicana were immobilized on coverslips on four- to eight-well plates coated with lectin from the coral tree (Erythrina cristagalli), which interacts with glycoproteins on the parasite surface membrane that contain oligosaccharides with galactosyl (β-1,4)-N-acetylglucosamine, as described by Rohloff et al. (28). This lectin was demonstrated to be able to immobilize T. cruzi (28) and also L. mexicana (this work) in confocal microscopy experiments while maintaining their viability. The lectin was dissolved in Dulbecco's phosphate-buffered saline (PBS) at a concentration of 0.5 mg/ml. The glass surfaces coated with poly-l-lysine were treated with the lectin solution for 30 min and air dried under a laminar-flow hood. Promastigotes in exponential phase were collected by centrifugation, washed three times with PBS, and resuspended in buffer A (116 mM NaCl, 5.4 mM KCl, 0.8 mM, MgSO4, 50 mM HEPES-NaOH, pH 7.4) without glucose. The cells were allowed to attach for 30 min at room temperature, and the unattached cells were removed by rinsing the coverslips three times with buffer A (28). Then, the fixed promastigotes were loaded with rhod 2-AM (10 μM) and rhodamine 123 (16 μg/ml) in Tyrode medium for 45 min at 29°C. Similarly, macrophages infected with promastigotes of L. mexicana (10 promastigotes to 1 macrophage) were loaded with rhod 2-AM (10 μM) and rhodamine 123 (16 μg/ml) in Tyrode medium for 45 min at 29°C and 5% CO2. Under these conditions, the fluorescence of rhod 2-AM is mainly produced in intracellular Ca2+-rich compartments due to its low affinity for Ca2+, thus allowing monitoring of the changes in the mitochondrial Ca2+ concentration simultaneously with the changes in the electrochemical potential of this organelle with rhodamine 123, as described above.
Free sterol content determinations.
Free sterol contents were determined by high-resolution gas-liquid chromatography coupled with mass spectrometry, as described previously (33). Briefly, for the extraction and the separation of neutral lipids, L. mexicana was cultured in the presence of amiodarone, as described above (see Table 2). Lipids were extracted with chloroform-methanol (2:1, vol/vol). The extract was dried and suspended in a minimum volume of chloroform. The chloroform suspension was applied to a silicic acid column (1.5 by 4 cm) and washed with 5 column volumes of chloroform to separate the neutral lipids from the other lipid fractions.
TABLE 2.
Effect of amiodarone on the biosynthesis of free sterols in L. mexicana promastigotes
| Sterol | Retention time (min) | Mass % after treatment with:
|
|
|---|---|---|---|
| Control | Amiodarone | ||
| Exogenous cholesterol | 24.9 | 13.8 | 11.54 |
| Endogenous 14-desmethyl ergosta-5,7,24(241)-trien-3β-ol (5-dehydroepisterol) | 28.9 | 67.0 | NDa |
| Ergosta-7,24(241)-dien-3β-ol (episterol) | 29.2 | 12.2 | ND |
| Cholesta-8,24-dien-3β-ol (zymosterol) | 25.9 | ND | ND |
| Endogenous 14-methyl lanosterol | 30.8 | 3.0 | ND |
| Squalene epoxide | 22.6 | 4.0 | ND |
| Squalene | 20.5 | 19.2 | 88.46 |
ND, not detected.
For the quantitative analysis of free sterols and structural assignment, the neutral lipids were then separated in a capillary high-resolution column (25 m by 0.20 mm [inner diameter]; Ultra-2; 5% phenyl-methyl-siloxane; film thickness, 0.33 μm) in a Hewlett-Packard HP-5890 series II gas chromatograph equipped with mass-sensitive detector HP-5971. The lipids were dissolved in chloroform and injected into the column at an initial temperature of 50°C (1 min), followed by a temperature increase to 280°C at a rate of 25°C/min and a further rise to 300°C at a rate of 1°C/min. The flow rate of the carrier gas (He) was kept constant at 0.6 ml/min. The injector temperature was 250°C; the detector was kept at 280°C.
RESULTS
Susceptibility of L. mexicana promastigotes to amiodarone.
L. mexicana promastigotes were exposed to different concentrations of amiodarone, and their viability was determined for 5 days. As can be seen in Fig. 2, amiodarone produced a dose-dependent inhibition of growth. Inhibition of 100% was obtained with a concentration of 5 μM amiodarone, and the 50% inhibitory concentration (EC50) was 900 nM (Fig. 2). This result indicates that amiodarone is able to affect the viability of these parasites with an even greater efficacy than it affects the viability of Trypanosoma cruzi epimastigotes, for which the drug concentration needed for 100% inhibition was 1 order of magnitude greater (EC50, 9 μM) (7).
FIG. 2.
Susceptibility of L. mexicana promastigotes to amiodarone. Populations of L. mexicana promastigotes were exposed to different concentrations of amiodarone. Experiments were performed at least in triplicate for each condition.
Effect of amiodarone on L. mexicana intracellular amastigotes.
Macrophages were infected with promastigotes in order to evaluate the effects of different concentrations of amiodarone on the number of infected macrophages. Figure 3A shows that as the amiodarone concentration increased, a concomitant decrease in the proportion of infected macrophages was observed. This effect was more pronounced than the inhibition of the promastigotes that was observed, since the EC50 was 8 nM. This fact was also reflected in the MIC of 20 nM obtained under these conditions. Figure 3B also shows that amiodarone did not affect the viability of macrophages in culture, as expected.
FIG. 3.

Effect of amiodarone against intracellular amastigotes of L. mexicana. Macrophages infected with L. mexicana amastigotes were exposed to different concentrations of amiodarone. The percentage of infected macrophages (A) and the effect on noninfected macrophages (B) were determined at 72 h after the addition of the drug. Experiments were performed at least in triplicate for each condition.
Effect of amiodarone on the [Ca2+]i of promastigote from of L. mexicana.
The promastigotes were initially loaded with the Ca2+ indicator Fura 2. Figure 4 and Table 1 show that 5 μM amiodarone induced an increase in the [Ca2+]i in promastigote populations when an extracellular medium containing 2 mM Ca2+ was employed. In order to determine if the observed increase in the [Ca2+]i was due to the entrance of this cation from the extracellular milieu through the opening of a Ca2+ channel in the plasma membrane or, instead, the cytoplasmic Ca2+ concentration was elevated as a result of the release of the cation from intracellular compartments, we performed the same experiment but in the absence of extracellular Ca2+ (and in the presence of EGTA). Since a similar response was obtained (Fig. 4B and Table 1), the results demonstrate that the increase in the parasite [Ca2+]i is produced by the Ca2+ released from intracellular organelles.
FIG. 4.
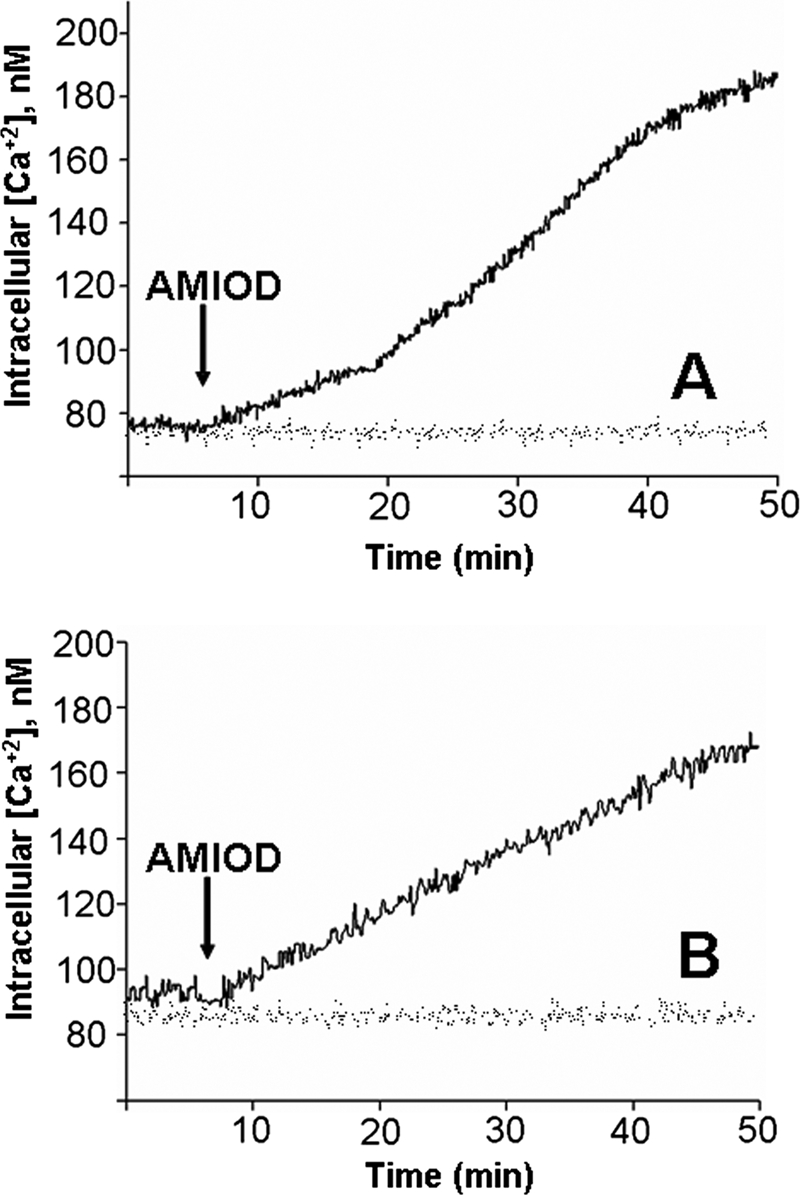
Effect of amiodarone (AMIOD) on the [Ca2+]i of L. mexicana promastigotes. Populations of promastigotes (4 × 106 cells in 1.5 ml) of L. mexicana were loaded with Fura 2-AM (6 μM) in a loading buffer (137 mM NaCl, 4 mM KCl, 1.5 mM KH2PO4, 8.5 mM Na2HPO4, 11 mM glucose, 1 mM CaCl2, 0.8 mM MgSO4, and 20 mM HEPES-NaOH, pH 7.4, containing 2.4 mM probenecid), and the [Ca2+]i was calculated as described in Materials and Methods. (A) Effect of 5 μM amiodarone on the parasite [Ca2+]i in the presence of 2 mM Ca2+; (B) effect of 5 μM amiodarone on promastigotes loaded with Fura 2 in the absence of external Ca2+.
TABLE 1.
Effect of amiodarone on free cytoplasmic Ca2+ concentration of L. mexicana promastigotes
| Ca2+ concn in extracellular medium | Free cytoplasmic Ca2+ concn (nM)a
|
|
|---|---|---|
| Control | Amiodaroneb | |
| 2 mM | 98 ± 14 (n = 10) | 185 ± 11 (n = 12) |
| 0 mM | 94 ± 18 (n = 8) | 172 ± 11 (n = 9) |
Free cytoplasmic Ca2+ concentrations were determined with Fura 2, as described in Materials and Methods.
Amiodarone concentration, 5 μM.
Effect of amiodarone on the mitochondrial electrochemical potential of L. mexicana.
Promastigotes were loaded with rhodamine 123, a reagent that senses the electrochemical potential of the mitochondria. Figure 5A shows that amiodarone affected the mitochondrial electrochemical potential in the promastigotes. This result was corroborated when the parasites were exposed to FCCP, a classical protonophore uncoupler that dissipates the mitochondrial H+ gradient. Accordingly, FCCP generated the same effect as amiodarone (Fig. 5B). These results suggest that, similar to its effect in T. cruzi, amiodarone affects the parasite's mitochondria.
FIG. 5.
Action of amiodarone (AMIOD) on the mitochondrial electrochemical potential of L. mexicana promastigotes. Parasites (4 × 106 cells in 1.5 ml) were incubated in the presence of rhodamine 123 (10 μg/ml) for 30 min at room temperature in the same loading buffer (137 mM NaCl, 4 mM KCl, 1.5 mM KH2PO4, 8.5 mM Na2HPO4, 11 mM glucose, 1 mM CaCl2, 0.8 mM MgSO4, 20 mM HEPES-NaOH, pH 7.4). The cells were permeabilized with 1 μM digitonin (DIG). (A) Effect of amiodarone (5 μM), followed by the addition of FCCP (1 μM), on the mitochondrial electrochemical potential; (B) effect of FCCP (1 μM), followed by the addition of amiodarone (5 μM), on the mitochondrial electrochemical potential.
Effect of amiodarone on acidocalcisomes from L. mexicana.
When the promastigotes were loaded with acridine orange (Fig. 6), it was observed that amiodarone generated a rapid alkalinization of the parasite acidocalcisomes. This effect was similar to the effect generated by the vacuolar H+ ATPase inhibitor bafilomycin A (Fig. 6A). It was seen that only a partial alkalinization was generated by amiodarone and/or bafilomycin A, since nigericin, an electroneutral K+-H+ exchanger known to alkalinize the acidocalcisomes from these parasites (17), produced a second alkalinization response of a greater magnitude (Fig. 6A and 6B). These results suggest that the acidocalcisomes also participate in the increase in the parasite's [Ca2+]i produced by amiodarone.
FIG. 6.
Effect of amiodarone (AMIOD) on acidocalcisomes from L. mexicana promastigotes. Parasites (109 cells/ml) were loaded with acridine orange (2 μM) in a buffer containing 130 mM KCl, 1 mM MgCl2, 2 mM KH2PO4, and 20 mM Tris-HCl, pH 7.4, as described in Materials and Methods. (A) Effect of amiodarone (5 μM), followed by the addition of bafilomycin A (Baf; 5 μM) and then nigericin (Nig; 2 μM), on the acid level in acidocalcisomes; (B) effect of bafilomycin A (5 μM), followed by the addition of amiodarone (5 μM) and then nigericin (2 μM), on the acid level in acidocalcisomes. arb., arbitrary.
Evaluation of effect of amiodarone on L. mexicana promastigotes and amastigotes by confocal microscopy.
The [Ca2+]i and the membrane electrochemical potentials of promastigotes (Fig. 7A) and of intracellular amastigotes from an infected macrophage (Fig. 7B) were also evaluated by confocal microscopy in order to identify the intracellular locus of action involved in the effect of amiodarone in these parasites.
FIG. 7.
Determination of the intracellular site of action of amiodarone in promastigotes and amastigote-infected macrophages from L. mexicana by confocal microscopy. (A) L. mexicana promastigotes were loaded with rhod 2 (red) and rhodamine 123 (green), as described in Materials and Methods. The top row shows control cells not exposed to amiodarone, while the bottom row shows cells treated with 5 μM amiodarone for 20 min. (B) Amastigotes inside an infected macrophage loaded with rhod 2 (red) and rhodamine 123 (green), as described in Materials and Methods. The top row shows a control cell not exposed to amiodarone, while the bottom row shows the same cell treated with 20 nM amiodarone after 20 min. Ψ, electrochemical potential.
The rhod 2 fluorescence level allowed the measurement of the amount of Ca2+ released from the organelles. The use of this indicator is based on its relatively low affinity for calcium, so that the fluorescence observed is visible, in principle, only in compartments with relatively high calcium concentrations. An increase in the fluorescence is obtained only when calcium is released from intracellular compartments and reaches a concentration high enough to be detected by the indicator, which is also present in the cytoplasm. On the other hand, rhodamine 123 measures energized mitochondria (Fig. 7A and B, top panels). The bottom panel of Fig. 7A shows that amiodarone generated the release of Ca2+ from the mitochondrion toward the parasite cytoplasm (red). It can be also observed that rhodamine 123 was released from the parasite mitochondrion as a product of the collapse of the electrochemical potential of the mitochondrion (green). The merged image (yellow) indicates that the mitochondrion is at least one of the organelles involved in the release of Ca2+ caused by amiodarone. In fact, acidocalcisomes were hardly seen under these experimental conditions because of their much smaller size. A similar effect was observed with intracellular amastigotes of L. mexicana that were loaded under similar conditions and treated with 20 nM amiodarone (Fig. 7B, arrows). Thus, the cytoplasmic Ca2+ concentration is elevated at the expense of the collapse of the electrochemical potential of the mitochondrion and the concomitant release of Ca2+. Figure 7 also shows that amiodarone does not seem to affect the Ca2+ homeostasis in the host cell, since the cytoplasm remains black, which suggests that [Ca2+]i was not apparently altered (Fig. 7B).
Effect of amiodarone on synthesis of free sterols in L. mexicana promastigotes.
After quantification of the neutral lipid fraction in a gas chromatograph equipped with a mass-sensitive detector, it was possible to determine that control promastigotes contained 67% 5-dehydroepisterol, similar to the values reported by others (27). On the other hand, we also demonstrated in this experiment that these parasites contain 19.2% squalene at the basal level. Interestingly, when promastigotes were exposed to 900 nM amiodarone, which corresponded to the EC50 of this drug, a drastic reduction (Table 2) in the amount of the main sterol, 5-dehydroepisterol, was seen and the accumulation of a significant amount of squalene (88.46%) was seen. These results strongly suggest that amiodarone affects the squalene epoxidase activity, which is essential for overall sterol biosynthesis (Table 2 and Fig. 8).
FIG. 8.
Part of the sterol biosynthetic pathway in Leishmania mexicana showing the possible site of action of amiodarone. Acetyl-CoA, acetyl coenzyme A; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A.
DISCUSSION
The results obtained in this work clearly demonstrate that amiodarone generates a dose-dependent effect on L. mexicana promastigote populations. It had an EC50 (900 nM) that was 1 order of magnitude lower than that which we found for T. cruzi epimastigotes (EC50, 9 μM) (7) and that was also significantly less than the EC50s of other drugs with inhibitory actions on L. mexicana, such as glibenclamide, an inhibitor of K+ATP[r] channels (EC50, 54.3 μM), as reported by our group (29). To evaluate the effect of this drug on the clinically relevant stage of the disease caused by L. mexicana, we performed experiments with macrophages infected with L. mexicana amastigotes. It was possible to observe that amiodarone was 2 orders of magnitude more potent against parasites at this stage (EC50, 8 nM) than it was against free promastigotes. Interestingly, the effect of amiodarone on L. mexicana amastigotes was more than 2 orders of magnitude less than it was against cells infected with T. cruzi amastigotes (EC50s, 8 nM and 2.7 μM, respectively). It was also interesting to observe that the drug did not affect the viability of the host cells.
Similar to the effect on epimastigotes and amastigotes from T. cruzi (7), amiodarone increased the [Ca2+]i in the promastigotes and amastigotes of L. mexicana at least in part by the same mechanism that it does in T. cruzi epimastigotes and amastigotes, since we could demonstrate that this drug induces the release of Ca2+ from the unique mitochondrion present in these parasites. This was demonstrated in several ways. First, we showed that amiodarone can simulate the effect of the protonophore FCCP on the release of rhodamine 123 from the mitochondrial internal membrane, which indicates the collapse of the electrochemical potential. This was first performed with cell populations (Fig. 5). Second, this was demonstrated with individual cells by the use of confocal microscopy with isolated promastigotes and amastigotes present in infected macrophages. This mechanism of action of amiodarone in L. mexicana (and similar to that in T. cruzi) is different from that in fungi reported by Gupta et al. (19) and Courchesne and Ozturk (10), since those authors observed that even though in these organisms the drug also acted by disrupting Ca2+ homeostasis, the increase in the [Ca2+]i was mediated by an MD-1 caffeine-sensitive Ca2+ channel in the plasma membrane.
Remarkably, in this study we also demonstrated that amiodarone is able to affect another important organelle characteristic of all trypanosomatids, namely, the acidocalcisomes. Our results clearly demonstrated that, besides the mitochondrion, these organelles are also targeted by amiodarone, since the drug induced a rapid alkalinization very similar to that induced by bafilomycin A, a specific inhibitor of the vacuolar H+ ATPase responsible for acidification and subsequent Ca2+ accumulation characteristic of these organelles (16, 17). We did not test for this possibility in our previous study with T. cruzi (7). Taken together, these results strongly support the idea that both mitochondria and acidocalcisomes are involved in the increment of the [Ca2+]i induced by amiodarone in L. mexicana, and this could be an argument for the potent effect of amiodarone on the viability of L. mexicana.
In this study, we also demonstrated that amiodarone inhibits the free sterol biosynthesis pathway in L. mexicana promastigotes. Indeed, we were able to show the accumulation of large amounts of squalene after amiodarone treatment, which suggests the inhibition of squalene epoxidase (Fig. 8), which is essential for the biosynthesis of sterols (31). Accordingly, the levels of 5-dehydroepisterol, the most abundant sterol present in this parasite (27), fell dramatically upon addition of the drug. This result is, in principle, different from that reported in our previous work with T. cruzi (7), in which we found that amiodarone inhibits oxidosqualene cyclase. However, it is perfectly conceivable that amiodarone simultaneously inhibits squalene epoxidase and oxidosqualene cyclase in L. mexicana, since the accumulation of squalene observed would prevent the later accumulation of squalene epoxide. Moreover, these parasites being different, it is possible that squalene epoxidase behaves differently in L. mexicana compared to T. cruzi. Moreover, these two enzymes are contiguous in the sterol biosynthetic pathway in both parasites. For this reason, both substrates, squalene and squalene epoxide, have very similar structures and sizes and differ only by the presence of an epoxide moiety in T. cruzi. Thus, we consider it plausible that amiodarone could inhibit both enzymes. However, by the experimental approach that we have used, it can only be suggested that the first enzyme is inhibited.
In any case, the inhibition of the sterol synthesis pathway could be a second argument that explains the effect of amiodarone on the viability of L. mexicana.
The results obtained in the present work point to a more general effect of amiodarone against trypanosomatids, which was indeed expected, since the mechanisms of action reported in T. cruzi imply that this family of parasites has targets that are common, such as the mitochondria, acidocalcisomes, and sterol biosynthesis. The possible therapeutic use of amiodarone for the treatment of leishmaniasis was addressed in a recently published case report (26). In that report, the authors postulated that a human patient with leishmaniasis had been cured by the use of amiodarone. These results also open the possibility that amiodarone may be used in combination with other drugs, such as miltefosine and/or inhibitors of sterol biosynthesis, and this possibility needs to be investigated.
Acknowledgments
We thank José Bubis for critical reading of the manuscript.
X.S.-M. is the recipient of a fellowship from the Academia de Ciencias, Fisicas, Matematicas y Naturales de Venezuela. This work was supported by grants from Consejo Nacional de Investigaciones Científicas y Tecnológicas (FONACIT), Venezuela G-2001000637, and CDCH-UCV (PI 03-00-7380-2008) to G.B.
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Alvar, J. 1997. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin. Microbiol. Rev. 10:298-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benaim, G., R. Bermudez, and J. A. Urbina. 1990. Ca2+ transport in isolated mitochondrial vesicles from Leishmania braziliensis promastigotes. Mol. Biochem. Parasitol. 39:61-68. [DOI] [PubMed] [Google Scholar]
- 3.Benaim, G., S. Losada, F. R. Gadelha, and R. Docampo. 1991. A calmodulin-activated (Ca2+-Mg2+)-ATPase is involved in Ca2+ transport by plasma membrane vesicles from Trypanosoma cruzi. Biochem. J. 280:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benaim, G., C. Lopez-Estaño, R. Docampo, and S. N. J. Moreno. 1993. A calmodulin-stimulated Ca2+ pump in plasma membrane vesicles from Trypanosoma brucei. Selective inhibition by pentamidine. Biochem. J. 296:759-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benaim, G., S. N. J. Moreno, G. Hutchinson, V. Cervino, T. Hermoso, P. J. Romero, F. Ruiz, W. de Souza, and R. Docampo. 1995. Characterization of the plasma membrane calcium pump from Trypanosoma cruzi. Biochem. J. 306:299-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benaim, G. 1996. Intracellular calcium signaling and regulation in Leishmania, p. 89-106. In F. Tapia, G. Caceres-Dittmar, and M. A. Sanchez (ed.), Molecular and immune mechanism in the pathogenesis of cutaneous Leishmania. R. G. Landes Co. Medical Intelligence Unit, Austin, TX.
- 7.Benaim, G., J. Sanders, Y. Garcia-Marchan, C. Colina, R. Lira, A. Caldera, G. Payares, C. Sanoja, J. Burgos, A. Leon-Rossell, J. Concepcion, A. Schijman, M. Levin, E. Oldfield, and J. Urbina. 2006. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J. Med. Chem. 49:892-899. [DOI] [PubMed] [Google Scholar]
- 8.Berman, J. D. 1989. Biochemistry of pentostan resistant Leishmania. Am. J. Trop. Med. Hyg. 40:159-164. [DOI] [PubMed] [Google Scholar]
- 9.Courchesne, W. E. 2002. Characterization of a novel, broad-based fungicidal activity for the antiarrythmic drug amiodarone. J. Pharmacol. Exp. Ther. 300:195-199. [DOI] [PubMed] [Google Scholar]
- 10.Courchesne, W. E., and S. Ozturk. 2003. Amiodarone induces a caffeine inhibited, MID1-dependent rise in free cytoplasmic calcium in Saccharomyces cerevisiae. Mol. Microbiol. 47:223-234. [DOI] [PubMed] [Google Scholar]
- 11.Croft, S. L., K. Seifert, and M. Duchene. 2003. Antiprotozoal activity of phospholipid analogues. Mol. Biochem. Parasitol. 126:165-172. [DOI] [PubMed] [Google Scholar]
- 12.Croft, S. L., and G. H. Coombs. 2003. Leishmaniasis: current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 11:502-508. [DOI] [PubMed] [Google Scholar]
- 13.Davies, C., P. Kaye, S. Croft, and S. Sundar. 2005. Leishmaniasis: new approaches to disease control. Br. Med. J. 326:377-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis, A., and L. Kedzierski. 2005. Recent advances in antileishmanial drug development. Curr. Opin. Investig. Drugs 6:163-169. [PubMed] [Google Scholar]
- 15.Docampo, R., and A. Vercesi. 1989. Ca2+ transport by coupled Trypanosoma cruzi mitochondria in situ. J. Biol. Chem. 264:108-111. [PubMed] [Google Scholar]
- 16.Docampo, R., D. Scott, A. Vercesi, and S. N. J. Moreno. 1995. Intracellular Ca2+ storage in acidocalcisomes of Trypanosoma cruzi. Biochem. J. 310:1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Docampo, R., and S. N. J. Moreno. 2001. The acidocalcisome. Mol. Biochem. Parasitol. 33:151-159. [DOI] [PubMed] [Google Scholar]
- 18.Grynkiewicz, G., M. Poenie, and R. Tsien. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440-3450. [PubMed] [Google Scholar]
- 19.Gupta, S. S., V.-K. Ton, V. Beaudry, S. Rulli, K. Cunningham, and R. Rao. 2003. Antifungal activity of amiodarone is mediated by disruption of calcium homeostasis. J. Biol. Chem. 278:28831-28839. [DOI] [PubMed] [Google Scholar]
- 20.Handman, E. 2001. Leishmaniasis: current status of vaccine development. Clin. Microbiol. Rev. 14:229-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herwaldt, B. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 22.Mazmuder, S., T. Mukherjee, J. Gosh, M. Ray, and A. Bhaduri. 1992. Allosteric modulation of Leishmania donovani plasma membrane Ca2+-ATPase by endogenus calmodulin. J. Biol. Chem. 267:18440-18446. [PubMed] [Google Scholar]
- 23.Mendoza, M., G. Uzcanga, R. Pacheco, H. Rojas, L. M. Carrasquel, Y. Garcia-Marchan, X. Serrano-Martin, G. Benaim, J. Bubis, and A. Mijares. 2008. Anti-VSG antibodies induce an increase in Trypanosoma evansi intracellular Ca2+ concentration. Parasitology 135:1303-1315. [DOI] [PubMed] [Google Scholar]
- 24.Moreno, S. N. J., and R. Docampo. 2003. Calcium regulation in protozoan parasites. Curr. Opin. Microbiol. 6:359-364. [DOI] [PubMed] [Google Scholar]
- 25.Pandey, S., S. Suryawanshi, S. Gupta, and L. Srivastava. 2005. Chemotherapy of leishmaniasis. Part II. Synthesis and bioevaluation of substituted arylketene dithioacetals as antileishmanial agents. Eur. J. Med. Chem. 40:751-756. [DOI] [PubMed] [Google Scholar]
- 26.Paniz-Mondolfi, A., A. Perez-Alvarez, O. Reyes-Jimenez, G. Socorro, O. Zerpa, D. Slova, and J. L. Concepción. 2008. Concurrent Chagas' disease and borderline disseminated cutaneous leishmaniasis: the role of amiodarone as an antitrypanosomatidae drug. Ther. Clin. Risk Manag. 4:659-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigues, C. F., M. Attias, C. Rodriguez, J. A. Urbina, and W. de Souza. 2002. Ultrastructural and biochemical alterations induced by 22,26-azasterol, a 24(25)-sterol methyltransferase inhibitor, on promastigote and amastigote forms of Leishmania amazonensis. Antimicrob. Agents Chemother. 46:487-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohloff, P., A. Montalvetti, and R. Docampo. 2004. Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi. J. Biochem. Mol. Biol. 279:52270-52281. [DOI] [PubMed] [Google Scholar]
- 29.Serrano-Martín, X., G. Payares, and A. Mendoza-León. 2006. Glibenclamide, a blocker of K+ATP channels, shows antileishmanial activity in experimental cutaneous leishmaniasis. Antimicrob. Agents Chemother. 50:4214-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsien, R. Y. 1988. Fluorescence measurement and photochemical manipulation of cytosolic free calcium. Trends Neurosci. 11:419-424. [DOI] [PubMed] [Google Scholar]
- 31.Urbina, J., and R. Docampo. 2003. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 19:495-501. [DOI] [PubMed] [Google Scholar]
- 32.Vieira, M. C., and S. N. J. Moreno. 2000. Mobilization of intracellular calcium upon attachment of Toxoplasma gondii tachyzoites to human fibroblasts is required for invasion. Mol. Biochem. Parasitol. 106:157-162. [DOI] [PubMed] [Google Scholar]
- 33.Visbal, G., A. Alvarez, B. Moreno, and G. San-Blas. 2003. S-Adenosyl-l-methionine inhibitors: Δ24-sterol methyl transferase and Δ24(28)-sterol methyl reductase as possible agents against Paracoccidioides brasiliensis. Antimicrob. Agents Chemother. 47:2966-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]







