Abstract
The vitro antifungal activity of retigeric acid B (RAB), a pentacyclic triterpenoid from the lichen species Lobaria kurokawae, was evaluated alone and in combination with fluconazole, ketoconazole, and itraconazole against Candida albicans using checkerboard microdilution and time-killing tests. The MICs for RAB against 10 different C. albicans isolates ranged from 8 to 16 μg/ml. A synergistic action of RAB and azole was observed in azole-resistant strains, whereas synergistic or indifferent effects were observed in azole-sensitive strains when interpreted by a separate approach of the fractional inhibitory concentration index and ΔE model (the difference between the predicted and measured fungal growth percentages). In time-killing tests, we used both colony counts and a colorimetric assay to evaluate the combinational antifungal effects of RAB and azoles, which further confirmed their synergistic interactions. These findings suggest that the natural product RAB may play a certain role in increasing the susceptibilities of azole-resistant C. albicans strains.
The incidence of candidiasis has increased during the last several decades due to the widespread use of antibacterials, corticosteroids, immunosuppressive agents, radiotherapy, and antitumoral chemotherapy (2, 4, 21, 23). The azole antifungal agents have excellent efficacy-toxicity profiles and play an important role in the treatment of Candida infections (12). However, concomitant with their widespread use, reports of clinical failure and correlations with elevated MICs to azole have begun to appear (3, 21). Moreover, some of these clinical isolates exhibit cross-resistance to a variety of different azole drugs (15). At present, although three echinocandins (caspofungin, micafungin, and anidulafungin) and voriconazole are available for the treatment of infection caused by azole-resistant isolates, the cost is too high for the patients. New antifungal agent research and development is still needed. Moreover, identification of small molecules that synergize with current antifungals against azole-resistant Candida strains may be a better way to overcome antifungal drug resistance.
Natural products with diverse bioactivities and structures are an important source of novel chemicals with pharmaceutical potentials (1, 26). Lichens, the symbiotic organisms of fungi and algae, are found commonly worldwide and can survive a variety of harsh environmental conditions. Lichens are inherently resistant to microbial infection due to the production of large numbers of unique secondary metabolites (6, 9). Therefore, we have focused our attention on lichens and their metabolites in an effort to find novel, naturally occurring antifungal potentiators. A thin-layer chromatography-bioautography screening guided phytochemical investigation for antifungal constituents from a lichen, Lobaria kurokawae Yoshim., led to the isolation of a pentacyclic triterpenoid, retigeric acid B (RAB) (Fig. 1), as the main active constituent (27). Interestingly, Lobaria kurokawae Yoshim. has been applied as a folk medicine for the treatment of hypopepsia, malnutritional stagnation, and abdominal distention in South China.
FIG. 1.
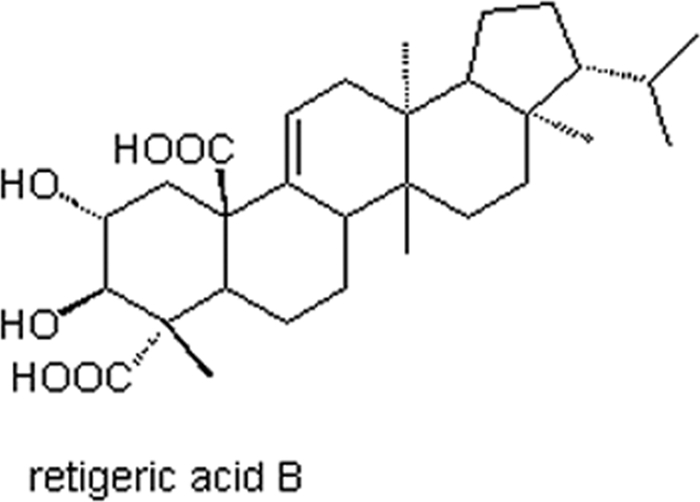
Structure of RAB.
Preliminary studies using the agar disk diffusion method have shown that RAB has excellent anti-Candida activity when combined with azoles (data not shown). In the present study, we investigated the antifungal activity of RAB alone and in combination with azoles, mainly fluconazole (FLC), ketoconazole (KCZ), and itraconazole (ITR), against clinical isolates of Candida albicans by the checkerboard broth microdilution method and time-killing tests.
MATERIALS AND METHODS
Strains.
The 10 clinical isolates of C. albicans used in this study were kindly provided by the Shandong Provincial Qianfoshan Hospital, Jinan, China. Their sensitivities to the tested azoles were evaluated according to the CLSI standard M27-A2 guidelines (16). Quality control was performed on each day of testing using CLSI-recommended reference strain C. albicans ATCC 10231. Frozen stocks of isolates were stored at −70°C in culture medium supplemented with 10% (vol/vol) glycerol and were subcultured twice at 35°C before each experiment.
Chemicals.
FLC was obtained from the Institute of Biopharmaceuticals of Shandong, KCZ was purchased from the National Institute for the Control of Pharmaceutical Biological Products, and ITR was obtained from Xian-Janssen Pharmaceutical Co., Ltd., China. RAB was isolated from the lichen L. kurokawae in our laboratory, and its purity is over 96% as analyzed by high-performance liquid chromatography. Stock solutions were prepared in dimethyl sulfoxide at 5,120 μg/ml for KCZ and ITR and 20,480 μg/ml for RAB. Stock solutions of FLC at 5,120 μg/ml were made in sterile distilled water.
Antifungal activities of RAB and azoles.
The antifungal activities of all tested drugs were tested by the broth microdilution method according to CLSI standard M27-A2 (16) with a final inoculum of 0.5 × 103 to 2.5 × 103 cells/ml. The test was carried out in RPMI 1640 medium (adjusted to pH 7.0 with 0.165 M morpholinepropanesulfonic acid [MOPS] buffer) in 96-well flat-bottomed microtitration plates (Costar). After incubation at 35°C for 48 h, MICs were determined by measuring the optical density at 490 nm with a spectrophotometer, and background optical densities were subtracted from that of each well. The MICs were defined as the concentrations of drug that reduced growth by 80% compared to that of organisms grown in the absence of drug. All experiments were performed in triplicate.
Interactions between RAB and azoles.
Drug interactions were assessed by broth microdilution checkerboard assays (24). Drugs dilutions were initially prepared at four times the desired final concentration. Aliquots of 50 μl of each concentration of azoles were added to columns 2 to 12, and then 50 μl of RAB was added to rows A to G. Row H and column 1 contained only the azole and RAB, respectively, and the well at the intersection of row H and column 1 (well H1) was the drug-free well that served as the growth control. An exploratory study was carried out to choose the appropriate range of concentrations for different drugs against strains with different susceptibilities. The final drug concentrations after the addition of 100 μl of inoculum ranged from 0.008 to 8 μg/ml for FLC, 0.001 to 1 μg/ml for KCZ and ITR, and 0.25 to 16 μg/ml for RAB, and the final inoculum size was 0.5 × 103 to 2.5 × 103 CFU/ml. The microtiter plates were incubated at 35°C for 48 h. The growth in each well was quantified by a spectrophotometer in a manner similar to that for the sensitivity assay. The growth inhibitory effects of the drugs alone and in combination were then calculated based on the results. All the experiments were performed in triplicate.
Drug interaction models.
To assess the in vitro interactions between the three azoles and RAB, the data obtained from the checkerboard tests were analyzed by nonparametric models based on the following two no-interaction theories: the Loewe additivity model (LA) and the Bliss independence (BI) theory. The LA theory is based on the idea that a drug cannot interact with itself, while the BI theory is based on the idea that two drugs act independently with the probabilistic sense of independence (13, 25).
LA-based model.
The nonparametric approach of fractional inhibitory concentration index (FICI) was used and expressed as follows: ∑ FIC = FICA+ FICB = MICAB/MICA + MICBA/MICB, where MICA and MICB are the MICs of drugs A and B when acting alone and MICAB and MICBA are the MICs of drugs A and B when acting in combination, respectively. Among all of the ∑ FIC values calculated for each data set, the FICI was determined as ∑ FICmin (the lowest ∑ FIC) when ∑ FICmax (the highest ∑ FIC) was less than 4; otherwise, the FICI was determined as ∑ FICmax. Synergy was defined as an FICI of ≤0.5, while antagonism was defined as an FICI value of >4. An FICI result between 0.5 and 4 (0.5 < FICI ≤ 4) was considered indifferent (17). In addition, isobolograms were plotted. The characteristic shape of the isobologram was used to visualize synergistic and antagonistic drug interactions (8).
BI-based model.
The nonparametric approach of BI is based on the Prichard model, defined as Ei = EA × EB, where Ei is the predicted percentage of growth of the theoretical noninteractive combination of the drugs A and B and EA and EB are the experimental percentages of growth of each drug acting alone. Interaction is defined by the difference (ΔE) between the predicted and measured percentages of growth with drugs at various concentrations (ΔE = Epredicted − Emeasured). In each of the three independent experiments, the observed percentage of growth obtained from the experimental data was subtracted from the predicted percentage, and then the average difference of three experiments was calculated. When the average difference as well as its 95% confidence interval among the three replicates was positive, statistically significant synergy was defined; when the difference as well as its 95% confidence interval was negative, significant antagonism was defined. In any other case, BI was concluded. The ΔE value obtained for each combination can be depicted on the z axis to construct a 3-D surface plot. Peaks above and below the zero plane indicate synergistic and antagonistic combinations, respectively, while the zero plane indicates the absence of statistically significant interaction.
To summarize the interaction surface, the sums of the percentages of all statistically significant synergistic (∑ SYN) and antagonistic (∑ ANT) interactions were calculated. Interactions with <100% statistically significant interactions were considered weak, interactions with 100% to 200% statistically significant interactions were considered moderate, while interactions with >200% were considered strong, as described previously (24). In addition, the numbers of statistically significant synergistic and antagonistic combinations among the 77 combinations tested were calculated for each strain.
Time-killing test.
In order to further evaluate the effect of RAB alone and in combination with azoles on the resistant strain, CA10 was used for the time-kill experiments. CA10 was grown in RPMI 1640 medium at the starting inoculum of 105 CFU/ml. The concentrations for RAB, FLC, and ITR were all 8 μg/ml and that for KCZ was 2 μg/ml, respectively. A drug-free sample served as a growth control. Dimethyl sulfoxide comprised <1% of the total testing volume. Samples (100 μl) were removed from the cultures just before treatment and at 6 h, 12 h, 24 h, and 48 h. The samples were then diluted, plated, and incubated at 35°C for 48 h for colony counts.
Meanwhile, 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) quantification of metabolic activity was performed with a different 100-μl sample of culture in order to estimate the cell viability after drug treatment according to methods described previously (10). Briefly, at each time point, a 100-μl aliquot was removed from every treatment mixture and transferred to a well of a 96-well microplate, and then a 100-μl aliquot of XTT-menadione solution was added. (XTT and menadione were purchased from Sigma Chemical Co. Prior to each assay, XTT was dissolved in a saturated solution at 0.5 mg/ml in Ringer's lactate. The solution was filtered through a 0.22-μm-pore-size filter. Menadione was prepared as a 10-mM stock solution in acetone and stored at −20°C.) The final concentrations of XTT and menadione were 0.25 mg/ml and 5 μM, respectively. The plate was then incubated in the dark for up to 2 h at 35°C. After incubation, the absorbance of the XTT reduction product, formazan, was read at 490 nm with a microtiter plate reader. All experiments were done in triplicate, and the results were presented as mean values. Thus, growth and metabolic inhibitory effects of each drug alone and in combination were measured based on colony counts and absorbance.
Synergism and antagonism were defined as a ≥2 log10 CFU/ml increase or a ≥2 log10 CFU/ml decrease of antifungal activity produced by the combination compared with that by the most active agent alone, respectively. If a <2 log10 CFU/ml change was observed, the interaction was considered indifferent (22). Each experiment was performed in triplicate, and the results were presented as mean values.
The correlation between the viable cell counts determined by colony counting and the optical density values by XTT-menadione colorimetric readings was evaluated before interpretation of results.
RESULTS
Susceptibility and interaction of drugs.
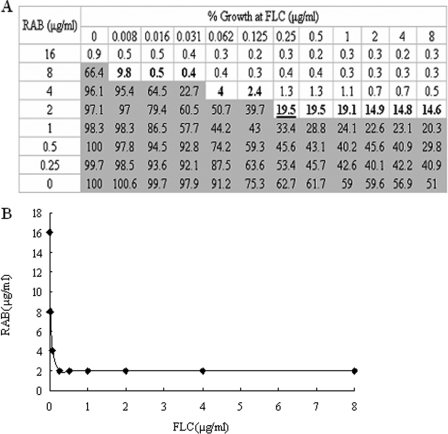
The MICs of RAB and azoles, alone and in combination against C. albicans, are shown in Table 1. The MIC of FLC against the quality control strain C. albicans ATCC 10231 was 0.5 to 2 μg/ml, within the reference range (11). According to the interpretive breakpoints for FLC (≤8 and ≥64 μg/ml, respectively), ITR (≤0.1 and ≥1 μg/ml, respectively) (24), and KCZ (≤0.125 and ≥1 μg/ml, respectively) (14), C. albicans isolates CA10, CA135, CA137, and CA138 are resistant to FLC, KCZ, and ITR; the others are sensitive. RAB has antifungal activity against sensitive and resistant strains alike, especially considering it is a natural product. The MIC range of RAB for all tested strains, based on an 80% reduction in growth, was 8 to 16 μg/ml as reported in Table 1. When a MIC-like assay was performed for the azoles in the presence of a fixed subinhibitory concentration of RAB, the median MICs of FLC, KCZ, and ITR decreased from two- to 16-fold for azole-sensitive strains while even greater reductions were observed against the azole-resistant strains. In fact, the MICs of azoles against the resistant strains in the presence of RAB were comparable to those of the sensitive strains. For example, in combination with RAB, the MICs of FLC, KCZ, and ITR against the azole-resistant C. albicans strain CA10 decreased more than 1,000-fold, 500-fold, and 1,000-fold, respectively, with RAB. The interaction between FLC and RAB against the azole-resistant strain CA10 is shown in Fig. 2A, and the corresponding isobologram is presented in Fig. 2B.
TABLE 1.
Susceptibilities of drugs alone and in combination against 10 clinical isolates of C. albicans by checkerboard microdilution assay
| Drug | Strain | Median MIC (range) of drug (μg/ml) used:
|
|||
|---|---|---|---|---|---|
| Alone
|
In combination
|
||||
| Azole | RAB | Azole | RAB | ||
| FLC | CA1 | 0.25 (0.25-0.5) | 8 | 0.062 (0.062-0.125) | 2 |
| CA2 | 0.5 (0.25-0.5) | 16 | 0.062 (0.062-0.125) | 2 | |
| CA3 | 0.25 (0.25-0.5) | 16 | 0.062 (0.062-0.125) | 4 | |
| CA4 | 1 (0.5-1) | 8 | 0.125 (0.125-0.5) | 1 | |
| CA127 | 1 (0.5-1) | 16 | 0.125 (0.125-0.25) | 4 | |
| CA132 | 0.5 (0.5-1) | 8 | 0.25 | 2 | |
| CA10 | 256 (256-512) | 16 | 0.25 | 2 | |
| CA135 | 128 (64-128) | 16 | 0.25 (0.125-0.25) | 2 | |
| CA137 | 64 (64-128) | 16 | 0.125 (0.125-0.25) | 4 | |
| CA138 | 128 (128-256) | 8 | 0.25 | 2 | |
| KCZ | CA1 | 0.031 (0.031-0.062) | 8 | 0.004 (0.004-0.008) | 2 |
| CA2 | 0.016 (0.016-0.031) | 16 | 0.002 (0.002-0.004) | 2 | |
| CA3 | 0.062 (0.062-0.125) | 16 | 0.004 (0.004-0.008) | 2 | |
| CA4 | 0.062 (0.062-0.125) | 8 | 0.031 (0.031-0.062) | 2 | |
| CA127 | 0.031 (0.031-0.062) | 16 | 0.004 (0.004-0.008) | 8 | |
| CA132 | 0.031 (0.031-0.062) | 8 | 0.004 (0.004-0.008) | 4 | |
| CA10 | 16 | 16 | 0.031 (0.031-0.062) | 4 | |
| CA135 | 16 (8-16) | 16 | 0.002 | 2 | |
| CA137 | 16 (8-16) | 16 | 0.002 | 4 | |
| CA138 | 16 | 8 | 0.062 (0.031-0.062) | 2 | |
| ITR | CA1 | 0.125 (0.125-0.25) | 8 | 0.031 (0.031-0.062) | 4 |
| CA2 | 0.125 (0.125-0.25) | 16 | 0.016 (0.016-0.031) | 4 | |
| CA3 | 0.031 (0.031-0.062) | 16 | 0.004 | 4 | |
| CA4 | 0.031 (0.031-0.062) | 8 | 0.004 | 4 | |
| CA127 | 0.016 (0.016-0.031) | 16 | 0.004 | 2 | |
| CA132 | 0.031 (0.031-0.062) | 8 | 0.016 (0.016-0.031) | 2 | |
| CA10 | 128 (64-128) | 16 | 0.125 (0.125-0.25) | 4 | |
| CA135 | 32 (16-32) | 16 | 0.125 (0.125-0.25) | 4 | |
| CA137 | 16 (16-32) | 16 | 0.125 (0.125-0.25) | 4 | |
| CA138 | 64 (64-128) | 8 | 0.25 | 2 | |
FIG. 2.
In vitro assessment of the interaction between FLC and RAB against the clinical azole-resistant strain CA10 based on the FICI. (A) Checkerboard showing the percentage of growth for each combination and the combination with more than 20% growth (light gray area). The isoeffective combinations, on the basis of which the FICIs were calculated, are shown in bold. Among all the FICIs calculated based on the isoeffective combinations, FICImax was <4, and so FICImin (0.126) was reported as the FICI. The underlined combination is that with the lowest FICI. (B) Corresponding isobologram of the MICs obtained with combinations of FLC and RAB. The isobole is concave, which indicates synergism.
The results of the checkerboard analysis interpreted by the FICI and ΔE method are summarized in Table 2. In the checkerboard microtiter plate format, synergism was consistently concluded in all four resistant isolates analyzed by the FICI and ΔE models for FLC, KCZ, and ITR. However, different interpretations were obtained for the azole-sensitive isolates when the conclusions from the FICI and ΔE models were compared for the different azole drug treatments. For the RAB-FLC combination, indifference was observed against two strains and synergism was observed against four strains analyzed by both models. For the RAB-KCZ combination, synergism was observed in three strains by FICI and in two strains by ΔE; others showed indifference. For the RAB-ITR combination, synergism was observed in three strains by FICI and in four strains by ΔE; others revealed indifference.
TABLE 2.
In vitro interactions between RAB and azoles as determined by nonparametric methods FICI and the ΔE modela
| Drug used in combination with RAB | Strain | FICI
|
ΔE modelb
|
|||
|---|---|---|---|---|---|---|
| Median (range) | INT | ∑SYN (n) | ∑ANT (n) | INT | ||
| FLC | CA1 | 0.5 | SYN | 751.2 (51) | −77.2 (13) | SYN |
| CA2 | 0.25 (0.25-0.375) | SYN | 712.6 (42) | −71.1 (20) | SYN | |
| CA3 | 0.5 | SYN | 150.7 (13) | −62.8 (12) | SYN | |
| CA4 | 0.625 (0.375-0.625) | IND | 41.0 (21) | −20.3 (15) | IND | |
| CA127 | 0.5 (0.375-0.5) | SYN | 211.7 (39) | −37.7 (14) | SYN | |
| CA132 | 0.75 (0.5-0.75) | IND | 88.0 (21) | −85.8 (24) | IND | |
| CA10 | 0.126 (0.125-0.126) | SYN | 1,365.6 (38) | 0 | SYN | |
| CA135 | 0.127 (0.126-0.127) | SYN | 877.7 (51) | −91.8 (13) | SYN | |
| CA137 | 0.252 | SYN | 1,464.7 (64) | 0 | SYN | |
| CA138 | 0.252 (0.251-0.252) | SYN | 981.3 (54) | −12.5 (14) | SYN | |
| KCZ | CA1 | 0.375 | SYN | 42.8 (35) | −16.0 (30) | IND |
| CA2 | 0.253 (0.137-0.253) | SYN | 25.0 (20) | −64.8 (53) | IND | |
| CA3 | 0.25 (0.187-0.25) | SYN | 106.8 (45) | −40.0 (30) | SYN | |
| CA4 | 0.75 | IND | 39.5 (16) | −21.3 (6) | IND | |
| CA127 | 0.625 (0.562-0.75) | IND | 116.4 (47) | −23.8 (27) | SYN | |
| CA132 | 0.625 (0.562-0.75) | IND | 21.2 (30) | −10.7 (25) | IND | |
| CA10 | 0.254 (0.252-0.254) | SYN | 910.7 (39) | −68.1 (31) | SYN | |
| CA135 | 0.125 | SYN | 728.3 (39) | −26.8 (18) | SYN | |
| CA137 | 0.25 (0.25-0.251) | SYN | 814.2 (51) | −28.8 (18) | SYN | |
| CA138 | 0.254 (0.252-0.254) | SYN | 413.5 (52) | −23 (16) | SYN | |
| ITR | CA1 | 0.75 | IND | 116.3 (36) | −38.8 (32) | SYN |
| CA2 | 0.375 (0.313-0.5) | SYN | 197.7 (36) | −84.4 (33) | SYN | |
| CA3 | 0.375 (0.312-0.375) | SYN | 105.7 (37) | −15.1 (9) | SYN | |
| CA4 | 0.625 (0.562-0.625) | IND | 69.6 (22) | −58.4 (14) | IND | |
| CA127 | 0.375 (0.25-0.375) | SYN | 321.1 (42) | −20.8 (35) | SYN | |
| CA132 | 0.75 | IND | 60.5 (22) | −68.7 (25) | IND | |
| CA10 | 0.252 (0.251-0.252) | SYN | 520.9 (48) | −15.9 (20) | SYN | |
| CA135 | 0.254 (0.254-0.258) | SYN | 905.6 (44) | −25.3 (8) | SYN | |
| CA137 | 0.258 | SYN | 541.3 (60) | −14.2 (17) | SYN | |
| CA138 | 0.254 (0.252-0.254) | SYN | 553.0 (41) | −20.7 (30) | SYN | |
INT, interpretation; IND, indifference. Synergy was defined as an FICI of ≤0.5, antagonism was defined as an FICI of >4.0, and indifference was defined as an FICI of >0.5 to 4 (i.e., no interaction).
n, number of drug combinations (among the 77 drug combinations for each strain) with statistically significant synergy or antagonism.
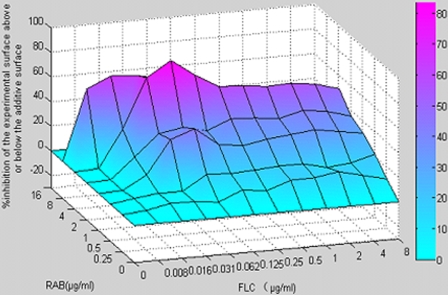
Additionally, the ΔE values obtained for each combination were illustrated in a 3-D plot made by MATLAB7 (Fig. 3). Peaks above and below the zero plane indicate synergistic and antagonistic combination, respectively, while the zero plane indicates the absence of statistically significant interaction.
FIG. 3.
The 3-D plot of the percent synergy calculated with the ΔE model. The mean ΔE values obtained for three separate experiments are shown on the z axis of the graph. Higher ΔE values suggested stronger synergistic interaction between FLC and RAB.
Time-killing test.
Time-kill curves showed the effect of drug combination on cell viability and supported the results of the broth checkerboard microdilution assays. First, we demonstrated that the XTT viability assay correlated to viable cell count with a correlation coefficient (R2) of 0.9664. The results of RAB combined with azoles from the time-kill study are shown in Table 3. Given the initial inoculums of 105 CFU/ml, RAB alone had a very weak antifungal effect at 8 μg/ml at 48 h, but the combination yielded a 2.03-log10 CFU/ml decrease compared with 8 μg/ml FLC alone, a 2.10-log10 CFU/ml decrease compared with 2 μg/ml KCZ alone, and a 2.11-log10 CFU/ml decrease compared with 8 μg/ml ITR alone at 48 h. There was no obvious difference in the features of drug action among the three combinations.
TABLE 3.
Decrease in log10 CFU/ml of strain CA10 using RAB combined with azoles at 48 ha
| Drug in combination with RAB | Mean (±SD) decrease in log10 CFU/ml as measured by:
|
|
|---|---|---|
| Colony count | XTT reduction assay | |
| FLC | 2.15 (0.09) | 2.03 (0.02) |
| KCZ | 2.12 (0.12) | 2.10 (0.10) |
| ITR | 2.15 (0.09) | 2.11 (0.05) |
All drug combinations were interpreted as synergistic.
DISCUSSION
Methods for studying antifungal combinations in vitro have differed considerably over time and among investigators (17, 19, 20). Different results might be obtained by choosing different approaches and models for the assessment of in vitro drug interaction (17, 20). To accurately evaluate the combinational effect of drugs, we used a spectrophotometric method in the broth checkerboard microdilution assay, which is a more objective measurement of yeast growth in the presence of inhibitory agents than subjective visual assessment. The data analysis was done with Microsoft Excel, which makes endpoint readings quantitative and more objective than visual endpoint determination. In this study, we chose the more stringent endpoint of 80% inhibition in growth rather than the standard 50%. For the antifungal agents that did not have sharp endpoints, an 80% endpoint was found to be useful for resolving problems of reproducibility (7). In addition, we found good reproducibility when susceptibility tests were repeated. Two nonparametric approaches, FICI and ΔE models, were used to assess the nature and intensity of the in vitro interactions between the three azoles and RAB. Between them, the FICI approach is popular among bacteriologists and mycologists to quantify drug interactions, although the interpretation of the FICI model in concluding synergy or antagonism can be problematic (8). For example, the results obtained with the FICI model are dependent on the MIC endpoints and the cutoff values by which synergism and antagonism are defined. To overcome these problems, a response surface approach based on the BI theory (ΔE model) was then used to conclude the interactions between RAB and the three azoles. The ΔE model has been used extensively to describe drug-drug interactions, especially in the area of antiviral drugs (5, 18) and has considerable advantages over conventional methods. The fitting of a model to the whole data surface not only allows the optimal use of information in the data but also allows the determination of error estimates of the interaction coefficient, thereby indicating whether the interaction is significant or not (25). In our study, the ΔE model and the FICI model showed good agreement in the interpretation of the results.
Besides the checkerboard method, we used the time-killing test to assess antimicrobial combination in vitro. We performed the time-killing test by both the XTT assay and colony counts. Repetitive counting of CFU is labor intensive and tedious, which seriously limits the number of antifungal concentrations and combinations that can be tested in any one experiment. The XTT assay largely avoided these disadvantages, showing an excellent correlation between colorimetric readings and cell numbers, and the data from the XTT assay correlate well with the colony-counting results.
In conclusion, the findings of the present study are very encouraging. RAB exhibited antifungal activity alone against both azole-sensitive and -resistant C. albicans isolates. Furthermore, when RAB was combined with azoles, strong synergy was observed against azole-resistant strains, with synergistic or indifferent effects observed against azole-sensitive strains, analyzed by both the FICI and ΔE models. RAB is an acid with antifungal activity that possibly has activity either in facilitating the uptake of azoles or in enhancing the membrane damage associated with the action of the azoles. Further studies will be performed to discern the mechanism of growth inhibition by RAB alone and the mechanism of synergy between RAB and azoles.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 30730109, no. 30672531, and no. 30801431) and the Special Foundation for State Major Basic Research Program of China (973 program, no. 2006CB708511).
Footnotes
Published ahead of print on 26 January 2009.
REFERENCES
- 1.Barrett, D. 2002. From natural products to clinically useful antifungals. Biochim. Biophys. Acta 1587:224-233. [DOI] [PubMed] [Google Scholar]
- 2.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 3.Denning, D. W., G. G. Baily, and S. V. Hood. 1997. Azole resistance in Candida. Eur. J. Clin. Microbiol. Infect. Dis. 16:261-280. [DOI] [PubMed] [Google Scholar]
- 4.Diz Dios, P., I. Otero Varela, I. Iglesias Martín, A. Ocampo Hermida, and C. Martínez Vázquez. 1999. Failure of indinavir to inhibit Candida albicans in vitro. Eur. J. Clin. Microbiol. Infect. Dis. 18:755-756. [DOI] [PubMed] [Google Scholar]
- 5.Drusano, G. L., D. Z. D'Argenio, W. Symonds, P. A. Bilello, J. McDowell, B. Sadler, A. Bye, and J. A. Bilello. 1998. Nucleoside analog 1592U89 and human immunodeficiency virus protease inhibitor 141W94 are synergistic in vitro. Antimicrob. Agents Chemother. 42:2153-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elix, J. A. 1996. Biochemistry and secondary metabolites, p. 154-180. In T. H. Nash (ed.), Lichen biology. Cambridge University Press, Cambridge, United Kingdom.
- 7.Galgiani, J. N. 1993. Susceptibility testing of fungi: current status of the standardization process. Antimicrob. Agents Chemother. 37:2517-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haynes, M. P., H. R. Buckley, M. L. Higgins, and R. A. Pieringer. 1994. Synergism between the antifungal agents amphotericin B and alkyl glycerol ethers. Antimicrob. Agents Chemother. 38:1523-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huneck, S. 1999. The significance of lichens and their metabolites. Naturwissenschaften 86:559-570. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn, D. M., M. Balkis, J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2003. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J. Clin. Microbiol. 41:506-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, Y., S. Sun, Q. Guo, L. Ma, C. Shi, L. Su, and H. Li. 2008. In vitro interaction between azoles and cyclosporin A against clinical isolates of Candida albicans determined by the chequerboard method and time-kill curves. J. Antimicrob. Chemother. 61:577-585. [DOI] [PubMed] [Google Scholar]
- 12.Loeffler, J., and D. A. Stevens. 2003. Antifungal drug resistance. Clin. Infect. Dis. 36(Suppl.):S31-S41. [DOI] [PubMed] [Google Scholar]
- 13.Meletiadis, J., J. W. Mouton, J. F. G. M. Meis, and P. E. Verweij. 2003. In vitro drug interaction modeling of combination of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother. 47:106-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milan, E. P., M. N. Burattini, E. G. Kallas, O. Fischmann, P. R. Costa, and A. L. Colombo. 1998. Azole resistance among oral Candida species isolates from AIDS patients under ketoconazole exposure. Diagn. Microbiol. Infect. Dis. 32:211-216. [DOI] [PubMed] [Google Scholar]
- 15.Müller, F. M. C., M. Weig, J. Peter, and T. J. Walsh. 2000. Azole cross-resistance to ketoconazole, fluconazole, itraconazole and voriconazole in clinical Candida albicans isolates from HIV-infected children with oropharyngeal candidosis. J. Antimicrob. Chemother. 46:338-341. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 17.Odds, F. C. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 18.Patick, A. K., T. J. Boritzki, and L. A. Bloom. 1997. Activities of the human immunodeficiency virus type 1 (HIV-1) protease inhibitor nelfinavir mesylate in combination with reverse transcriptase and protease inhibitors against acute HIV-1 infection in vitro. Antimicrob. Agents Chemother. 41:2159-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prichard, M. N., and J. Charles Shipman. 1990. A three-dimensional model to analyze drug-drug interactions. Antivir. Res. 14:181-206. [DOI] [PubMed] [Google Scholar]
- 20.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruhnke, M., A. Eigler, I. Tennagen, B. Geiseler, E. Engelmann, and M. Trautmann. 1994. Emergence of fluconazole-resistant strains of Candida albicans in patients with recurrent oropharyngeal candidosis and human immunodeficiency virus infection. J. Clin. Microbiol. 32:2092-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahuquillo Arce, J. M., E. Colombo Gainza, A. Gil Brusola, R. Ortiz Estévez, E. Cantón, and M. Gobernado. 2006. In vitro activity of linezolid in combination with doxycycline, fosfomycin, levofloxacin, rifampicin and vancomycin against methicillin-susceptible Staphylococcus aureus. Rev. Esp. Quimioter. 19:252-257. [PubMed] [Google Scholar]
- 23.Sobel, J. D., H. C. Wiesenfeld, M. Martens, P. Danna, T. M. Hooton, A. Rompalo, M. Sperling, C. Livengood III, B. Horowitz, J. Von Thron, L. Edwards, H. Panzer, and T. C. Chu. 2004. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N. Engl. J. Med. 351:876-883. [DOI] [PubMed] [Google Scholar]
- 24.Sun, S., Y. Li, Q. Guo, C. Shi, J. Yu, and L. Ma. 2008. In vitro interactions between tacrolimus and azoles against Candida albicans determined by different methods. Antimicrob. Agents Chemother. 52:409-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Te Dorsthorst, D. T. A., P. E. Verweij, J. Meletiadis, M. Bergervoet, N. C. Punt, J. F. G. M. Meis, and J. W. Mouton. 2002. In vitro interaction of flucytosine combined with amphotericin B or fluconazole against thirty-five yeast isolates determined by both the fractional inhibitory concentration index and the response surface approach. Antimicrob. Agents Chemother. 46:2982-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vicente, M. F., A. Basilio, A. Cabello, and F. Peláez. 2003. Microbial natural products as a source of antifungals. Clin. Microbiol. Infect. 9:15-32. [DOI] [PubMed] [Google Scholar]
- 27.Wang, L., T. Narui, H. Harada, C. F. Culberson, and W. L. Culberson. 2001. Ethnic uses of lichens in Yunnan, China. Bryologist 104:345-349. [Google Scholar]