Abstract
The pharmacokinetics of amoxicillin were studied in umbilical cord and neonatal sera relative to maternal concentrations in prevention of neonatal group B streptococcus infection. The subjects were 44 pregnant women receiving amoxicillin as 1 or 2 g as an intravenous infusion. To measure the concentrations, blood samples were obtained from the mother, the arterial and venous umbilical cord, and the neonate. The pharmacokinetics were characterized by a five-compartment model by using nonlinear mixed-effects (population) modeling. The population estimates for the clearance, central volume of distribution, and the two peripheral maternal volumes of distribution were 19.7 ± 0.99 liters/h, 6.40 ± 0.61 liters, and 5.88 ± 0.83 liters (mean ± standard error), respectively. The volume of distribution of the venous umbilical cord and the neonatal volume of distribution were 3.40 liters and 11.9 liters, respectively. The pharmacokinetic parameter estimates were used to simulate the concentration-time profiles in maternal, venous umbilical cord, and neonatal sera. The peak concentration in the venous umbilical cord serum was 18% of the maternal peak concentration. It was reached 3.3 min after the maternal peak concentration. The concentration-time profile in neonatal serum was determined by the profile in venous umbilical cord serum, which in turn depended on the profile in maternal serum. Furthermore, the simulated concentrations in maternal, venous umbilical cord, and neonatal sera exceeded the MIC for group B streptococcus for more than 90% of the 4-h dosing interval. In a first approximation, the 2-g infusion to the mother appears to be adequate for the prevention of group B streptococcal disease. However, to investigate the efficacy of the prophylaxis, further studies of the interindividual variability in pharmacokinetics are indicated.
Amoxicillin (amoxicilline), a penicillin derivative, is an antibiotic used for the prevention of neonatal group B streptococcal (GBS) disease. Neonates from mothers colonized with GBS are at risk for vertical transmission, because they might be exposed to GBS in utero or in the vagina during delivery. While protection of the fetus is the actual objective of the prophylaxis, the procedure used for the prophylaxis of GBS is administration of antibiotics to the pregnant woman. Since antibiotics reach the fetus only after transport over the placenta via the umbilical cord, adequate concentrations in maternal serum are a prerequisite, but no guarantee, for adequate venous and arterial umbilical cord and fetal serum concentrations. While data on ampicillin concentrations in umbilical cord blood have been reported (2, 4), data for amoxicillin are not available. The pharmacokinetics (PKs) of amoxicillin in pregnant women before labor have been described previously and have been shown to exceed the MIC for an adequate percentage of the dosing interval for treatment of the infection in the mother (7). To assess whether the administration of amoxicillin also protects the fetus from GBS infection, data on the PKs in umbilical cord serum or fetal serum are necessary.
The study of the PKs in fetal or umbilical cord serum faces ethical and practical difficulties. Because sampling of the blood of the fetus during delivery is not possible, blood samples from the arterial or the venous umbilical cord taken after birth are in most instances the only samples directly available for the collection of information on the concentrations in the fetal blood. Unfortunately, these samples can be obtained only at a single point in time for each individual. Alternatively, the concentration in blood samples from the neonate taken by heel puncture shortly after birth can be considered a good approximation of the concentration in fetal serum. However, such samples are difficult to obtain because of the poor blood supply to the extremities directly after birth. When neonatal blood samples are taken hours later, these concentrations might not truly reflect the concentrations in fetal blood, because of the differences in the rates and routes of elimination before and after birth.
By necessity, the time intervals between administration of the last antibiotic dose and birth are different for each individual. Therefore, the presentation of either individual or average concentrations in the umbilical cord blood samples at birth is of little value. To obtain useful information, the PKs (i.e., the concentration-versus-time profile) in the maternal serum, the arterial or venous umbilical cord serum, and the neonatal serum should be known. The use of a population PK approach can be useful for the analysis of such data (6, 9, 3, 11). The objective of this study was to describe the concentration-time profile of amoxicillin in umbilical cord and neonatal serum in relation to the concentration-time profile in maternal serum.
MATERIALS AND METHODS
Patients.
During the period between 7 February 2005 and 28 February 2007, all women who needed treatment with amoxicillin or amoxicillin-clavulanic acid (co-amoxiclav; Augmentin) shortly before or during labor were eligible for this study. To take full advantage of all data available to us for the development of a population PK model of amoxicillin in pregnant women, the umbilical cord, and neonates, the present study includes part of a data set from a recently published study (7). In the previous study, 416 blood samples from pregnant women with preterm premature rupture of membranes (PPROM) were used. None of the blood samples from the umbilical cord or the neonate were used in the previous study. According to local guidelines, women were treated with amoxicillin for the prevention of GBS disease when no signs of infection were present but the woman had a proven or unknown Streptococcus agalactiae carriage status and had generally recognized risk factors for neonatal GBS disease (10). In the case of suspected intra-amniotic infection, the women were treated with co-amoxiclav. Delivery was induced when signs of infection were present. The study was approved by the Medical Ethics Committee. Written informed consent was obtained from all patients. Women were excluded from the study if (i) they had been treated with oral or intramuscular antibiotics within 2 days before the start of therapy, (ii) were unwilling to comply with the requirements of the study, (iii) were known to be allergic to amoxicillin or other penicillins, or (iv) were receiving another medication that exhibits an interaction with amoxicillin. All patients were at least 18 years of age.
All patients received a standard workup, which included a medical history and biochemical and hematological examinations. Furthermore, the blood pressure, pulse, oral temperature, and body weight were recorded. The amount of edema was scored semiquantitatively from 0 (no edema) to 3 (above the knee). The weight of the placenta with the umbilical cord was measured. For the neonate, the birth weight and the Apgar scores after 1, 5, and 10 min were recorded.
Drug administration and blood sampling.
Before the administration of amoxicillin or co-amoxiclav, an intravenous catheter was placed in each arm. Antibiotics were administered by following local guidelines. Treatment with amoxicillin started with an intravenous infusion of 2 g amoxicillin (50 mg/ml) administered over 30 min, followed by a second infusion after 4 h of 1 g amoxicillin (50 mg/ml) over 15 min. Treatment with co-amoxiclav (consisting of 1 g amoxicillin [50 mg/ml] with 200 mg clavulanic acid) consisted of an infusion for 15 min every 8 h. Blood samples of 2 ml were collected from the second catheter in the contralateral arm at timed intervals beginning at 1 min after the start of the infusion and at 7 and 15 min (for the 1-g infusion) or 15 and 30 min (for the 2-g infusion) during the first two amoxicillin administrations. After the infusion, sampling was scheduled at 3, 6, 10, 16, and 36 min and every 30 min after that until the next antibiotic dosage. The exact sampling times were recorded.
After the umbilical cord was clamped, it was cleaned with normal saline and a sponge to prevent contamination of the umbilical cord blood samples with maternal blood. Both arterial and venous cord blood samples of 5 to 10 ml were taken. A blood sample of approximately 0.5 ml was obtained from the neonate by heel puncture, after signed informed consent was obtained from both parents. These blood samples were taken at least 10 min after birth, depending on the physical condition of the neonate. In all cases in which samples from the umbilical cord and neonate were taken, samples were also obtained from the mother before, during, and after delivery.
All blood samples were immediately placed on ice, allowed to clot, and processed within 1 h after collection. The samples were centrifuged at 1,200 × g for approximately 10 min. The supernatants were transferred into plastic storage tubes and frozen at −70°C until analysis.
High-performance liquid chromatography.
Amoxicillin concentrations were determined by an isocratic high-pressure liquid chromatography (Shimadzu, Den Bosch, The Netherlands) method with an octyldecyl silane Gemini column (Bester, Amstelveen, The Netherlands) and with 0.066 M KH2PO4 solution containing 10% methanol as the mobile phase. A perchloric acid solution of 0.1 ml was added to the sample in an equal volume, and after the mixture was vortexed, it was added to 0.56 ml 0.028 M citric acid containing cefadroxil (Sigma, Zwijndrecht, The Netherlands) as an internal standard. The assay was linear over the concentration range measured. Controls were included in every run. The lower limit of detection and the lower limit of quantification were 0.2 mg/liter, and the between-run coefficient of variation was <4%.
PK analysis.
The PK parameters were estimated by nonlinear mixed-effect (population) modeling (NONMEM). The model was implemented in the NONMEM ADVAN5 subroutine, and the analysis was performed by the FOCE (first-order conditional estimation) method with the additional option INTERACTION. All fitting procedures were performed with the Compaq Computer Corporation (Euston, TX) Visual FORTRAN standard edition (version 6.6) and the NONMEM software package (version VI, release 1.2; ICON Development Solutions, Ellicott City, MD).
To determine the basic structural PK parameters, various three-, four-, and five-compartment models were tested. The data for the arterial and venous umbilical cord and the neonate were analyzed simultaneously with the data for the mother. The point of departure for the present analysis was the previously described three-compartment PK population model for pregnant women with PPROM, which was used to describe the time course of amoxicillin in maternal blood (7). However, because it is unknown whether one of the peripheral maternal compartments might be explained by the presence of the fetus, we also explored the possibility of the presence of one or two maternal compartments. Furthermore, we investigated whether the data for the venous umbilical cord and maternal sera could be considered one compartment and whether arterial and venous cord sera could be considered one compartment. The data for the arterial umbilical cord serum were also combined with the data for neonatal serum. Because only venous cord blood enters the umbilical cord after passage through the placenta, the antibiotic exchange between the compartments might not be equal. Therefore, we used k values in our model to describe the umbilical cord and neonatal data.
Model selection and the identification of variability were based on the likelihood ratio test, PK parameter point estimates and their respective confidence intervals, and goodness-of-fit plots. For the likelihood ratio test on differences between two models, the objective function value (OFV) with a prespecified level of significance of a P value of <0.001 was used. NONMEM minimizes an objective function in performing nonlinear regression analysis. To detect systematic deviations in the model fits, the goodness-of-fit plots were visually inspected. The data for individual observations versus individual or population predictions should be randomly distributed around the line of identity. The weighted residuals versus time or population predictions should be randomly distributed around zero. Population values were estimated for the parameters clearance (CL), volume of distribution (V), and intercompartmental clearance (Q).
Individual estimates for PK parameters were assumed to follow a log-normal distribution. Therefore, an exponential distribution model was used to account for interindividual variability. The correlations between the various random parameters for interindividual variability were tested by using the forward addition and backward deletion method in the NONMEM Omega block option. Intersubject variabilities were tested for all maternal, umbilical cord, and neonatal parameters.
Selection of an appropriate residual error model was based on the likelihood ratio test and inspection of the goodness-of-fit plots. The residual variability between the observed concentrations and the concentrations predicted by the model was described by using a proportional error model. The residual error term contains all the error terms which cannot be explained and refers to, for example, measurement and experimental errors and structural model misspecification.
To refine the model, covariate analysis was also performed. The estimated PK parameters on which interindividual variability was found were plotted independently against the covariates maternal body weight and body mass index, gestational age, blood pressure, pulse, oral temperature, the amount of edema, birth weight, singleton versus twin pregnancy, and mode of delivery to determine whether these covariates influenced the PKs. Covariate analysis was performed by the forward addition of each candidate covariate into the model structure until no further improvement of the goodness of fit was observed. A further criterion for acceptance of the covariate effects was for the estimated confidence interval of the covariate effect to not overlap zero. The contribution of each covariate to the final model was confirmed by the backward elimination of each covariate from the model to account for possible interactions between covariates. The residual intra- and interindividual variabilities were visually evaluated.
The accuracy of the final population model for the entire population was established by using the bootstrap option in NONMEM, which consists of repeated random sampling with replacement from the original data. This resampling was repeated 100 times. The estimated parameters from the bootstrap analysis were compared to the estimates from the original data.
Simulations.
Simulations were performed to determine the relation in time between the maternal, venous umbilical cord, and neonatal serum concentration-time profiles. The simulations were performed by using the Berkeley Madonna, Inc., program (version 8.3.5; University of California). The mean population parameter values of the PK model derived with NONMEM in the first part of the analysis were used in the simulations.
RESULTS
A total of 53 umbilical cord blood samples consisting of 25 arterial and 28 venous cord blood samples were obtained from 44 pregnant patients. Both arterial and venous cord blood samples were obtained from 23 women. Four umbilical cord samples were collected from one twin pregnancy. A total of 904 maternal serum samples were collected. Due to the unpredictable and variable duration of labor and the various emotional conditions of the patients, the number of maternal blood samples that could be obtained from each patient varied (range, 3 to 41 samples per patient). The time interval between administration of the last antibiotic dose and birth varied from 24.4 min to 316.8 min. The patients gave birth at gestational ages that ranged from 30.0 to 42.4 weeks. The birth weights of the neonates ranged from 1,340 g to 4,470 g. Fourteen blood samples could be obtained from 13 neonates between 14.2 min and 199.8 min after birth. The characteristics of the study patients and their neonates are presented in Table 1.
TABLE 1.
Characteristics of study patients and their neonates
| Characteristic | Unit | Mean | SD | No. of patients |
|---|---|---|---|---|
| Maternal age | yr | 30.0 | 6.85 | 44 |
| Maternal wt | kg | 79.4 | 13.9 | 43 |
| Body mass index | kg/m2 | 29.4 | 5.3 | 43 |
| Edema (none/around the ankle/up to knee) | 29/12/3 | |||
| Leukocytes | 109/liter | 12.8 | 5.14 | 44 |
| Creatinine | μmol/liter | 47.4 | 11.5 | 44 |
| Amenorrhea | wk | 36 6/7 | 2.7 | 44 |
| Nulliparity | 22 | |||
| Twin pregnancy | 3 | |||
| Mode of delivery (vaginal/vacuum extraction/emergency cesarean delivery) | 23/1/3a | |||
| Placental wt | g | 546.5 | 165.0 | 40 |
| Birth wt of neonate | g | 2,887.4 | 627.9 | 46 |
| Apgar score at: | ||||
| 1 min | 8.7 | 1.05 | 41 | |
| 5 min | 9.6 | 0.84 | 41 | |
| 10 min | 9.8 | 0.59 | 40 |
Only patients from whom umbilical cord serum was obtained.
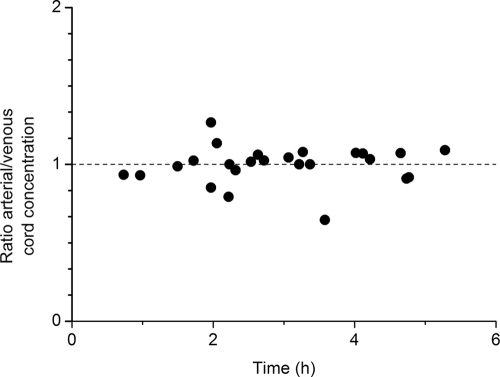
The concentrations in cord serum ranged from 1.0 to 16.8 mg/liter in arterial umbilical cord serum and from 1.1 to 18.0 mg/liter in venous umbilical cord serum. The ratios of the arterial umbilical cord serum concentrations to the venous umbilical cord serum concentrations are shown in Fig. 1. None of the deliveries occurred within the period of 30 min in which the antibiotic was infused. Therefore, none of the ratios was obtained before the maximum concentration in maternal serum was reached. Apparently, equilibrium between the arterial and the venous umbilical cord serum concentrations was reached soon after the maximum concentration in maternal serum had been reached. The ratio was not significantly different from 1. The differences between the arterial and the venous umbilical cord serum concentrations were too small for analysis of these concentrations by the use of two separate compartments.
FIG. 1.
Ratio of arterial umbilical cord serum concentrations to venous umbilical cord serum concentrations as a function of time. The ratio of the arterial umbilical cord serum concentrations to the venous umbilical cord serum concentrations was plotted versus the interval of time between the time of the last antibiotic administration to the mother and the time of birth of the child.
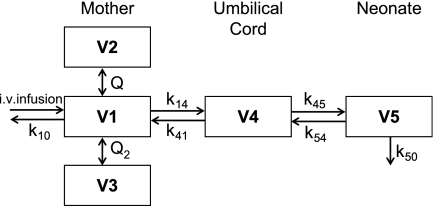
In the population modeling, data on the concentrations in maternal, arterial and venous umbilical cord, and neonatal sera were analyzed simultaneously. Of all models tested, a multicompartment PK model with three compartments for the mother, one compartment for the venous umbilical cord, and one compartment for the neonate best described the data (Fig. 2). Peripheral compartments 2, 3, and 4 are connected to the central compartment. Compartment 5 is attached to compartment 4, and the antibiotics in compartment 5 are transferred back to compartment 4 and eliminated from the system. During the analysis, the values of V for compartments 2 and 3 (V2 and V3, respectively) were comparable (difference in the final model with separate estimates for V2 and V3, <0.5 liter). The coefficients of variation of these estimates exceeded 51%, and therefore, for the final analysis, V3 was assumed to be equal to V2. The interindividual variability was mainly due to differences in CL, V1, and the residual error. A correlation between the random parameters for interindividual variability was found and was accounted for in the model. The residual error was found to be proportional to the blood concentrations.
FIG. 2.
Five-compartment model. The structure of the final five-compartment model consists of the central volume of distribution of the mother (V1), the peripheral volumes of distribution of the mother (V2 and V3), the volume of distribution for the venous umbilical cord (V4), and the volume of distribution for the neonate (V5). The k values represent the intercompartmental exchange rate constants.
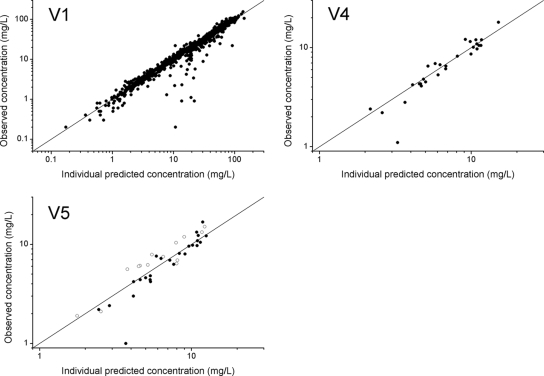
The demographic and clinical characteristics of the patients were examined as potential covariates on the parameters CL, V1, the constant for the transfer of the antibiotics in compartment 4 to compartment 5 (k45), and the residual error. According to the specified criterion of the change in OFV (ΔOFV), both gestational age (ΔOFV = −10.5) and body mass index (ΔOFV = −8.5) influenced V. Although a correlation between gestational age and body mass index was noted, the incorporation of these two covariates on V did not result in a significant decrease in OFV (ΔOFV = −3.3). Because the decrease in OFV was larger after the incorporation of gestational age than after the incorporation of body mass index, only gestational age was incorporated into the final model. The effects of other potential covariates were also assessed. This resulted in maximum decreases in OFV of −0.5 for body weight, −0.3 for blood pressure, and −0.2 for pulse. All models in which the temperature was incorporated resulted in running errors. The following equation represents the covariate model in the final model, including the gestational age as a covariate for V: V1 = θ2·[1 + θ12·(GA − 36.8)]·exp(η2), where V1 is the volume of distribution of the central compartment, θ2 is the estimate for V1, θ12 is the estimate for the effect of gestational age, and GA is the gestational age centered by its median value in the study population (36.8 weeks). η2 estimates the interindividual variability on V1. An increase in V1 of 4.2% per week was found and was incorporated into the model. The scatter plots of the observed concentrations versus the model-predicted concentrations were randomly distributed, illustrating the unbiased model fit for maternal, venous umbilical cord, and neonatal serum concentrations (Fig. 3). Table 2 shows the estimated values for the PK parameters of the final model. The values of V for the venous umbilical cord and the neonate were calculated and were found to be 3.4 liters for the venous umbilical cord and 11.9 liters for the neonate.
FIG. 3.
Individual predicted versus observed concentrations of amoxicillin for the central compartment of the mother (V1), the venous umbilical cord compartment (V4), and the neonatal compartment (V5). Scatter plots of the individual predicted concentrations versus the observed concentrations of amoxicillin for 44 patients (V1), 28 measures of the venous umbilical cord (V4), and 25 measures of the arterial umbilical cord with 14 measures for the neonates (V5). The open symbols in V5 represent the neonatal serum concentrations, and the filled symbols represent the arterial umbilical cord concentrations. The three panels show the individual data points and the line of identity (x = y).
TABLE 2.
Estimated values for the PK parameters of the final model
| Parameter | Structural model parameters
|
Variance model parameters
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CL (liters/h) | V1 (liters) | V2 (liters) | V3 (liters) | Q1 (liters/h) | Q2 (liters/h) | k14 | k41 | k45 | k54 | k50 | Interindividual variability in CL | Interindividual variability in V1 | Residual variability | |
| Mean | 19.7 | 6.4 | 5.88 | 5.88 | 56.6 | 10.7 | 0.76 | 1.4 | 5.1 | 1.4 | 0.16 | 0.076 | 0.038 | 0.03 |
| SE | 0.99 | 0.61 | 0.83 | 0.83 | 9.5 | 2.2 | 0.28 | 0.31 | 2 | 0.31 | 0.033 | 0.026 | 0.013 | 0.004 |
| 95% confidence interval | 17.8-21.6 | 5.2-7.6 | 4.2-7.5 | 4.2-7.5 | 38.0-75.2 | 6.3-15.1 | 0.21-1.3 | 0.83-2.1 | 1.1-9.1 | 0.83-2.1 | 0.098-0.23 | 0.026-0.13 | 0.014-0.063 | 0.022-0.037 |
The bootstrap validation of the model of the entire population was performed with 100 runs. The bootstrap validation was successful for 91 runs. The mean parameter estimates of the runs obtained from the bootstrap analysis deviated 1 to 36% from the predicted values from the NONMEM PK analysis, indicating that the accuracy of the final model is good.
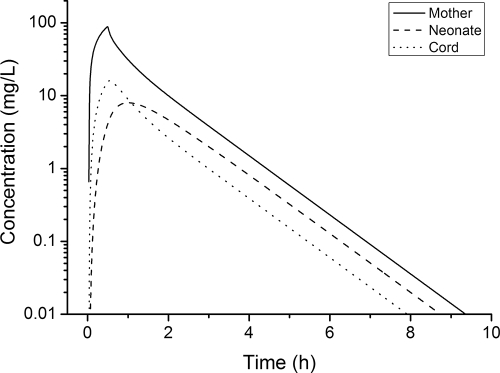
The parameter estimates obtained by the population modeling analysis were used to simulate the concentration-time profiles of amoxicillin in maternal, venous umbilical cord, and neonatal sera after the administration of a single dose of 2 g amoxicillin. Figure 4 shows the concentration-time profiles obtained for the initial dose of 2 g amoxicillin infused into the mother over 30 min. The maternal peak concentration (88.7 mg/liter) was reached at the end of the antibiotic infusion at 30 min. The peak concentration in venous umbilical cord serum was lower and was delayed compared to the time that the maternal peak concentration was reached. The peak concentration in venous umbilical cord serum was 16.0 mg/liter (18% of the maternal peak concentration) and was reached 3.3 min after the maternal peak concentration.
FIG. 4.
Simulated concentration-time profiles for the mother, venous umbilical cord, and neonate. The concentration-time profile of amoxicillin in maternal, venous umbilical cord, and neonatal sera was simulated after the administration of a single dose of 2 g amoxicillin infused over 30 min. The simulations were performed with PK parameter estimates based on the final five-compartment model and were carried out for 12 h after the administration of a single dose of antibiotic.
The few neonatal concentration data were analyzed simultaneously with the maternal and arterial and venous umbilical cord serum concentrations. The restricted blood supply to the extremities directly after birth and the need for informed consent from both parents were the main reasons for the limited number of samples from the neonates. The peak concentration in neonatal serum achieved after the infusion of 2 g into the mother was 8.0 mg/liter (whereas it was 16.0 mg/liter in venous umbilical cord serum). Similar concentrations were reached 1.1 h after the start of the infusion, and afterwards, the neonatal serum concentrations exceeded the venous umbilical cord serum concentrations.
An intrapartum dose of 2 g amoxicillin is commonly used to prevent neonatal GBS disease by attempting to achieve bactericidal concentrations in the fetus for a sufficient amount of time. According to the clinical breakpoints determined by EUCAST, for GBS, concentrations of at least 0.25 mg/liter should be reached (5). The simulations showed that the concentration of amoxicillin after the administration of a single 2-g dose in the maternal, venous umbilical cord, and neonatal sera exceeded the MIC for more than 90% of the dosing interval.
DISCUSSION
To our knowledge, the five-compartment model described here is the first in which data for the mother, umbilical cord, and neonate have been analyzed simultaneously to describe the overall concentration-versus-time profile in maternal plasma, umbilical cord, and neonatal plasma. Similar to our previous study, the PKs in the mother were best described by the use of three compartments, indicating that none of the peripheral maternal compartments represents the presence of the fetus. The total CL in the current five-compartment model was slightly lower (19.7 liters/h) than that in the model with three compartments in women with PPROM (22.8 liters/h). This can be explained by the additional route of elimination toward the neonate in the current model. The neonatal compartment was best described by the inclusion of both arterial umbilical cord serum and neonatal serum samples. The peak concentrations in venous umbilical cord and neonatal sera were lower and delayed compared to the peak concentration in maternal serum. At approximately 1 h after the start of the antibiotic administration, the concentration in neonatal serum reached its highest level and thereafter exceeded the concentration in venous umbilical cord serum. The results indicate that amoxicillin concentrations in maternal, venous umbilical cord, and neonatal sera exceeded the MIC for >90% of the dosing interval after the administration of a 2-g dose.
Due to the stressful conditions that exist during delivery and the consequent ethical implications, data for the umbilical cord and neonate are limited by necessity, whereas data for the mother are more plentiful, as samples can more easily be obtained from the mother. Population PK modeling is particularly helpful here, because by this approach all data for the entire study population (i.e., all mothers and fetuses) are taken into account. An important feature of population PK modeling is that it enables the analysis of data from studies with unbalanced designs (e.g., unequal groups) and of incomplete data sets (6, 9). Specifically, nonlinear mixed-effects (population) modeling can be used to connect the sparse data for umbilical cord and neonatal blood concentrations to the more detailed information on the PKs in the mother (3, 11).
In the final analysis, we adopted a simplified approach in which the arterial umbilical cord and neonatal serum concentrations were placed in the same compartment. Since arterial cord blood originates directly from the fetal circulation, the arterial cord blood concentrations can be considered fetal blood concentrations. Venous cord blood concentrations are achieved after passage of the antibiotic through the placenta. Therefore, those concentrations were analyzed separately. Strictly speaking, the arterial umbilical cord and neonatal serum concentrations should be analyzed separately as well, because several differences exist between the two. Before clamping of the umbilical cord, antibiotics are eliminated from the fetus by transplacental transport to the mother and by fetal renal excretion. After clamping of the cord, the elimination of amoxicillin from the neonate takes place only by neonatal renal excretion. Because elimination from the neonate to the mother was included in the model, the model-predicted neonatal concentrations were slightly lower than the observed concentrations, as seen in Fig. 3. Furthermore, many physiological changes occur immediately after birth in the neonate. These changes might influence the PKs in the neonate and are more diverse than the differences in maternal PKs. The limited data available for umbilical cord and neonatal sera forced us to combine the arterial cord serum concentrations and the neonatal serum concentrations, and combined with the fact that there is a larger variation in physiology in newborns than there is in adults, this resulted in larger confidence intervals for the parameter estimates for the umbilical cord and the neonate than for the mother.
Studies of the PKs in umbilical cord serum are scarce and, in general, do not distinguish between arterial and venous umbilical cord sera. We hypothesized that the ratio between the arterial cord serum concentration and the venous umbilical cord serum concentration would be <1 when the umbilical cord was clamped shortly after the administration of the last antibiotic dose. As in our study we had no deliveries in which umbilical cord samples could be obtained before the maximum concentration in maternal serum had been reached, we were not able to confirm or reject this hypothesis. Our data do indicate, however, that an equilibrium between these concentrations (i.e., the ratio is not significantly different from 1) is reached fast, and therefore, it is possible to compare our data with those from previous studies that did not distinguish between arterial and venous umbilical cord serum.
For ampicillin, an antibiotic closely related to amoxicillin, two studies have been performed with women during elective delivery by cesarean section (2, 4). In comparison with our study, there are some important differences that must be considered. Because physical changes during labor might influence the transplacental blood flow, the transfer of amoxicillin across the placental barrier during vaginal delivery might be different from that during cesarean delivery. Furthermore, the data from the two previous studies were not analyzed by use of a population PK approach. Nevertheless, the results of both studies are in line with our results. The ampicillin concentrations in cord serum exceeded the MIC for GBS during the study periods of 7 to 71 min (2) and 32 to 343 min (4) after the start of the antibiotic infusion. Colombo et al. (4) focused on the ratio of the concentration in cord serum to the concentration in maternal serum. They reported that the initial concentrations in cord serum were lower than the concentrations in maternal serum, but after approximately 80 min, the concentrations in venous umbilical cord serum exceeded the concentrations in maternal serum. In our study, the venous cord serum concentrations did not exceed the maternal serum concentrations.
The concentrations in venous umbilical cord serum are sometimes used as a representative for the concentrations in fetal serum. In the simulated concentration-time profiles, the concentration in neonatal serum was slightly higher than the concentration in venous umbilical cord serum. This might be explained by a change in the route of elimination after birth. Before the umbilical cord is clamped, amoxicillin is eliminated by both the mother and the fetus. After birth, amoxicillin is eliminated only by the neonate. Together with the decreased glomerular filtration rate in neonates, this results in a lower elimination rate and higher serum concentrations. This indicates that venous umbilical cord amoxicillin concentrations may be slightly different from those in fetal serum but that the use of venous umbilical cord concentrations will at least not overestimate the concentrations in fetal serum.
Finally, a remark should be made about the fact that both patients receiving amoxicillin and patients receiving co-amoxiclav were included in the study. In the literature, it has been described that the addition of clavulanic acid does not influence the PKs of amoxicillin after intravenous administration (1, 8, 12). Our data confirmed this finding (unpublished observations). Therefore, we consider it justified to assume that the PKs of amoxicillin in the two sets of patients are similar and that all patients could be analyzed simultaneously.
In our study, the simulated concentration-time profiles in maternal, venous umbilical cord, and neonatal sera have been used to describe the PKs of amoxicillin in relation to each other. In a first approximation, an amoxicillin infusion of 2 g seems to be adequate for the prevention of neonatal GBS disease in the average patient. However, to evaluate the efficacy of the dosing regimen as recommended by the CDC (10) for all pregnant women and to investigate possible improvements to the dosing regimen, further studies are needed, such as Monte Carlo simulations of the concentrations in the various compartments. Such studies should take into account the interindividual variability, the correlation between the PK parameter estimates, knowledge of the concentration-effect relationship, and finally, the desired action of the antibiotic in the fetus.
Acknowledgments
This work was supported by grant SNO-T-06-31 from the Stichting Nuts Ohra.
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Adam, D., I. de Visser, and P. Koeppe. 1982. Pharmacokinetics of amoxicillin and clavulanic acid administered alone and in combination. Antimicrob. Agents Chemother. 22:353-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom, S. L., S. M. Cox, R. E. Bawdon, and L. C. Gilstrap. 1996. Ampicillin for neonatal group B streptococcal prophylaxis: how rapidly can bactericidal concentrations be achieved? Am. J. Obstet. Gynecol. 175:974-976. [DOI] [PubMed] [Google Scholar]
- 3.Bonate, P. L. 2005. Recommended reading in population pharmacokinetic pharmacodynamics. AAPS J. 7:E363-E373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo, D. F., J. L. Lew, C. A. Pedersen, J. R. Johnson, and P. Fan-Havard. 2006. Optimal timing of ampicillin administration to pregnant women for establishing bactericidal levels in the prophylaxis of group B streptococcus. Am. J. Obstet. Gynecol. 194:466-470. [DOI] [PubMed] [Google Scholar]
- 5.EUCAST. Antimicrobial wild type distributions of microorganisms. EUCAST, Basel, Switzerland. http://217.70.33.99/Eucast2/.
- 6.Liefaard, L. C., B. A. Ploeger, C. F. Molthoff, R. Boellaard, A. A. Lammertsma, M. Danhof, and R. A. Voskuyl. 2005. Population pharmacokinetic analysis for simultaneous determination of B(max) and K(D) in vivo by positron emission tomography. Mol. Imaging Biol. 7:411-421. [DOI] [PubMed] [Google Scholar]
- 7.Muller, A. E., J. DeJongh, P. M. Oostvogel, R. A. Voskuyl, P. J. Dorr, M. Danhof, and J. W. Mouton. 2008. Amoxicillin pharmacokinetics in pregnant women with preterm premature rupture of the membranes. Am. J. Obstet. Gynecol. 198:108.e1-108.e6. [DOI] [PubMed] [Google Scholar]
- 8.Reed, M. D. 1996. Clinical pharmacokinetics of amoxicillin and clavulanate. Pediatr. Infect. Dis. J. 15:949-954. [DOI] [PubMed] [Google Scholar]
- 9.Schoemaker, R. C., and A. F. Cohen. 1996. Estimating impossible curves using NONMEM. Br. J. Clin. Pharmacol. 42:283-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrag, S., R. Gorwitz, K. Fultz-Butts, and A. Schuchat. 2002. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm. Rep. 51(RR-11):1-22. [PubMed] [Google Scholar]
- 11.Sheiner, B. L., and S. L. Beal. 1981. Evaluation of methods for estimating population pharmacokinetic parameters. II. Biexponential model and experimental pharmacokinetic data. J. Pharmacokinet. Biopharm. 9:635-651. [DOI] [PubMed] [Google Scholar]
- 12.Weber, D. J., N. E. Tolkoff-Rubin, and R. H. Rubin. 1984. Amoxicillin and potassium clavulanate: an antibiotic combination. Mechanism of action, pharmacokinetics, antimicrobial spectrum, clinical efficacy and adverse effects. Pharmacotherapy 4:122-136. [DOI] [PubMed] [Google Scholar]