Abstract
Small-colony variant (SCV) strains of Staphylococcus aureus show reduced antibiotic susceptibility and intracellular persistence, potentially explaining therapeutic failures. The activities of oxacillin, fusidic acid, clindamycin, gentamicin, rifampin, vancomycin, linezolid, quinupristin-dalfopristin, daptomycin, tigecycline, moxifloxacin, telavancin, and oritavancin have been examined in THP-1 macrophages infected by a stable thymidine-dependent SCV strain in comparison with normal-phenotype and revertant isogenic strains isolated from the same cystic fibrosis patient. The SCV strain grew slowly extracellularly and intracellularly (1- and 0.2-log CFU increase in 24 h, respectively). In confocal and electron microscopy, SCV and the normal-phenotype bacteria remain confined in acid vacuoles. All antibiotics tested, except tigecycline, caused a net reduction in bacterial counts that was both time and concentration dependent. At an extracellular concentration corresponding to the maximum concentration in human serum (total drug), oritavancin caused a 2-log CFU reduction at 24 h; rifampin, moxifloxacin, and quinupristin-dalfopristin caused a similar reduction at 72 h; and all other antibiotics had only a static effect at 24 h and a 1-log CFU reduction at 72 h. In concentration dependence experiments, response to oritavancin was bimodal (two successive plateaus of −0.4 and −3.1 log CFU); tigecycline, moxifloxacin, and rifampin showed maximal effects of −1.1 to −1.7 log CFU; and the other antibiotics produced results of −0.6 log CFU or less. Addition of thymidine restored intracellular growth of the SCV strain but did not modify the activity of antibiotics (except quinupristin-dalfopristin). All drugs (except tigecycline and oritavancin) showed higher intracellular activity against normal or revertant phenotypes than against SCV strains. The data may help rationalizing the design of further studies with intracellular SCV strains.
Small-colony variant (SCV) strains are increasingly recognized as a major cause of the persistence and recurrence of Staphylococcus aureus infections, and specific therapeutic options remain so far ill explored (58). Microbiologically, SCV strains constitute a naturally occurring subpopulation of bacteria with a series of metabolic alterations that confer a particular phenotype characterized by slow growth, reduced hemolytic activity, and auxotrophy for hemin, menadione, or thymidine. SCVs easily revert to the normal phenotype and therefore often escape detection. Moreover, even if stable, they remain difficult to recognize in routine testing unless specifically looked for (42). Genetically, the expression of virulence regulators (agr and sarA) or the alternative stress regulator sigB and its dependent virulence genes (hla and spa) is downregulated in SCV strains compared with isogenic normal strains (26, 38). Clinically, SCV strains are preferentially isolated in chronic infections of skin, soft tissues, joint, and bone (41, 59, 62), as well as in the respiratory tract of cystic fibrosis (CF) patients (12, 25, 44, 57). They can be found even in antibiotic-treated patients (45, 60) and appear to be selected during treatment (25).
The eradication of SCV strains is difficult for two main reasons. First, SCVs show reduced susceptibility to many antibiotics, a phenomenon best understood for aminoglycosides and cell-wall-acting drugs against hemin-dependent strains and for cotrimoxazole against thymidine-dependent strains (43). Second, SCV strains have a propensity to invade cells and persist intracellularly longer than a normal-phenotype strain (58), as shown in models of infected keratinocytes (59), endothelial cells (61), bronchial epithelial cells (63), or monocytes (5). This is probably the consequence of increased production of fibronectin-binding proteins (37, 55) combined with decreased production of alpha-toxin (6).
This makes the choice of antibiotics quite difficult since it must be based on not only in vitro susceptibility but also the capacity of the drug to penetrate and act against intracellular forms. However, as recently reviewed (50), there is no simple correlation between the extent of cellular accumulation and the activity demonstrated by a given antibiotic against intracellular bacteria. Accordingly, models have been developed to directly assess the intracellular activity of a large number of antistaphylococcal antibiotics against the normal phenotype of S. aureus strains of various resistance phenotypes (7, 9, 30). Appropriate in vitro models are, however, critically lacking to test for the susceptibility of intracellular SCVs (56, 58). Available data suggest that clarithromycin, clindamycin, and ciprofloxacin are inefficient despite their known accumulation in eucaryotic cells (40). Daptomycin, gentamicin, and rifampin are poorly active when used alone at low concentrations, but improvement can be obtained when they are combined with rifampin (5).
In the present study, we have established a model of infected THP-1 macrophages that allow quantitative assessment of the activity of antibiotics against both extracellular and intracellular forms of SCVs by using a pharmacodynamic approach based on a method developed to study normal-phenotype S. aureus isolates (9). The approach has been applied to a stable, thymidine-dependent SCV strain isolated from a CF patient (57) for comparison with its revertant and the isogenic normal-phenotype strain isolated from the same patient.
MATERIALS AND METHODS
Bacterial strains, identification, and growth conditions.
A pair of S. aureus strains isolated from a chronically infected CF patient during a national survey and presenting a normal or an SCV phenotype (57) was used in this study. The isogenic relationship between these two isolates was confirmed by pulsed-field gel electrophoresis (21), and identification of S. aureus was confirmed by PCR amplification of the nuc gene (35); the isolates belong to the agr group 2, as determined by multiplex PCR (23), which corresponds to clonal complex 5 by multilocus sequence typing and to the pulsed-field gel electrophoresis group C (22). The SCV strain was thymidine dependent as normal growth on Mueller-Hinton (MH) agar was restored around a disk impregnated with 1.5 μg of thymidine (Sigma-Aldrich, St. Louis, MO). Compared to its normal-phenotype counterpart, the SCV strain grew as very small, nonpigmented, nonhemolytic colonies on Columbia blood agar (Becton Dickinson, Le Pont de Claix, France) (28) (Fig. 1). The stability of the SCV phenotype was assessed by checking colony phenotype over six successive subcultures. With more prolonged subculturing (at least 10 passages), a few revertants exhibiting all characteristics of a normal S. aureus phenotype, such as growth as large, golden and hemolytic colonies (Fig. 1), were isolated and also included in our study. For each experiment, a new aliquot stored at −80°C was used, subcultured only once on Columbia blood agar, and checked for SCV phenotype by visual inspection of colonies. Unless stated otherwise, bacteria were grown in an aerobic atmosphere in cation-adjusted MH II broth (CA-MH; Becton Dickinson, Le Pont de Claix, France).
FIG. 1.

Morphological appearance of S. aureus colonies on Columbia blood agar after incubation for 24 h at 37°C. Shown are normal-phenotype and SCV and spontaneous revertant strains. Differences in colony size, shape, and hemolytic activity are evident.
Susceptibility testing and killing curves in broth.
MICs were determined by the broth microdilution method in CA-MH broth according to the CLSI recommendations (20). MICs of oritavancin were measured in the presence of 0.002% polysorbate-80 using non-tissue culture-treated 96-well polystyrene microtiter plates (Costar, Corning, NY) in order to prevent binding of the drug to plastic surfaces (3), as recommended by the manufacturer (Targanta Therapeutics Corp., Cambridge, MA) and in accordance with CLSI M100-S18 guidelines (20). Readings were made after 24 h for the normal phenotype and revertant strains and after 48 h for SCVs (19). Killing curves were performed in CA-MH broth as described earlier (9) except that 0.002% polysorbate-80 was added when oritavancin was tested and that samples plated on brain heart infusion agar (Fluka-Biochemica, Buchs, Switzerland) were incubated for 48 h before SCV colonies were counted. Bactericidal activity was defined as a reduction of 99.9% (≥3 log10) of the total count of CFU/ml in the original inoculum (39).
Intracellular infection and assessment of intracellular activity.
THP-1 cells (ATCC TIB-202), a human cell line displaying macrophage-like activity (4), were used for all experiments. Infection with SCV, normal-phenotype, and revertant strains was performed as described earlier for S. aureus ATCC 25923 (9), except that CFU counting was performed after a 48-h incubation of cell lysate on brain heart infusion agar for SCVs. For all experiments, antibiotics were added after phagocytosis and elimination of the extracellular bacteria (see details and validation in reference 9), and cells were incubated for 24 to 72 h in the presence of the drug. In some experiments with SCVs, 100 mg/liter thymidine was added to the culture medium together with the antibiotic under study. All concentrations refer to total drug. The same criterion for bactericidal activity was used as for extracellular bacteria. Since not all antibiotics could be tested simultaneously, each set of experiments included at least one antibiotic that had been used in other experiments to check for consistency of the response between experiments.
Confocal and transmission electron microscopy.
For confocal microscopy, infection was done as described above with bacteria labeled with 0.25 g/liter fluorescein isothiocyanate (Molecular Probes, Eugene, OR). To stain the acidic compartments of THP-1 macrophages, 100 μM LysoTracker Red DND-99 (Molecular Probes, Leiden, The Netherlands) was added to the medium 1 h prior to harvesting of the cells. At 3 h postinfection, cells were washed three times with phosphate-buffered saline and fixed with 3.7% paraformaldehyde in phosphate-buffered saline at room temperature for 20 min. After cells were washed, specimens were dried and mounted in 2.5% 1,4-diacylbicyclo(2,2,2)octane (Dabco; Sigma-Aldrich) in Mowiol (Calbiochem-NovaBiochem GmbH, Bad Soden, Germany). Observations were made under oil immersion conditions with a 63× objective mounted on an MRC1024 confocal microscope (Bio-Rad Laboratories, Richmond, CA). Images were digitally recorded with a Focus Graphics image recorder and processed with the Confocal Assistant software (version 4.02). For transmission electron microscopy, infection of macrophages was performed as described above but using a bacterium/macrophage ratio of approximately 20 to allow visualization of a sufficiently large number of bacteria; all other conditions were otherwise unchanged. At 24 h postinfection, cells were harvested, washed, fixed in 1% glutaraldehyde, rinsed in 0.1 mM cacodylate buffer, postfixed in 2% osmic acid, dehydrated in graded ethanol solutions, and embedded in Epone resin-propylene oxide. Ultrathin sections (50 nm) were colored with uranyl acetate and lead citrate and observed in an EM 208 Philips microscope.
Antibiotics.
The following antibiotics were obtained as microbiological standards from their corresponding manufacturers: moxifloxacin from Bayer AG, Wuppertal, Germany; linezolid from Pfizer Corp., Kalamazoo, MI; quinupristin-dalfopristin (Synercid) from Norden Pharma, Paris, France; daptomycin from Novartis Pharma AG, Basel, Switzerland; tigecycline from Wyeth, Pearl River, NY; telavancin from Theravance Inc., San Francisco, CA; and oritavancin from Targanta Therapeutics Corp., Cambridge, MA. Oxacillin, clindamycin, and fusidic acid were purchased from Sigma-Aldrich. The other antibiotics were procured as commercial products registered in Belgium for parenteral use from their respective marketing authorization holders or sellers (gentamicin as Geomycin and vancomycin as Vancocin from Glaxo-SmithKline and rifampin as Rifadine from Aventis).
Curve-fitting and statistical analyses.
Curve-fitting and statistical analyses were performed with GraphPad Prism (version 4.02) and GraphPad Instat (version 3.06) for Windows, respectively (GraphPad Prism Software, San Diego, CA).
RESULTS
Susceptibility testing.
Table 1 shows the MICs of a series of antibiotics representing current first-line or alternative therapeutic options for an S. aureus SCV strain or agents currently in development, in the absence or in the presence of 100 mg/liter thymidine (26), compared with MICs for the revertant and the isogenic normal-phenotype strains. No major differences in MICs were noted between strains, with the most marked changes consisting of a two-dilution increase in the MICs of oxacillin, fusidic acid, gentamicin, and rifampin for the normal-phenotype strain. Likewise, the addition of thymidine did not modify MICs. Among the panel of antibiotics tested, rifampin, oritavancin, and fusidic acid showed the lowest MIC, and linezolid showed the highest MIC against the SCV strain. MICs were also determined in broth adjusted at pH 5.5 (see Table SP1 in the supplemental material). Gentamicin and moxifloxacin MICs were higher, and oxacillin MICs were lower, but this effect was similar for both the SCV and normal-phenotype strains. The MICs of the other antibiotics were not significantly affected by pH change.
TABLE 1.
MICs of antibiotics for SCV, normal-phenotype, and revertant strains compared to the human serum Cmax
| Antibiotica | MIC (mg/liter) for the indicated strain type
|
Human Cmax (mg/liter)b | % Free drug | Reference | |||
|---|---|---|---|---|---|---|---|
| SCV
|
Revertant | Normal phenotype | |||||
| Without thymidine | With 100 mg/liter thymidine | ||||||
| OXA | 0.125 | 0.25 | 0.25 | 0.5 | 64 | 7 | 16 |
| FA | 0.03 | 0.03 | 0.06 | 0.125 | 30 | 5 | 2 |
| CLI | 0.125 | 0.125 | 0.125 | 0.125 | 4 | 10 | 2 |
| GEN | 0.125 | 0.125 | 0.5 | 0.5 | 18 | 100 | 2 |
| RIF | 0.0005 | 0.002 | 0.0005 | 0.002 | 18 | 15 | 2 |
| VAN | 0.5 | 0.5 | 0.5 | 0.5 | 50 | 50 | 52 |
| LZD | 2 | 2 | 2 | 4 | 16 | 31 | 48 |
| Q-D | 0.5 | 0.5 | 0.5 | 0.5 | 11 | 40/80 | 33 |
| DAP | 0.125 | 0.25 | 0.25 | 0.125 | 57 | 10 | 29 |
| TGC | 0.125 | 0.125 | 0.125 | 0.125 | 1 | 20 | 47 |
| MXF | 0.125 | 0.125 | 0.125 | 0.25 | 4 | 50 | 2 |
| TLV | 0.125 | 0.125 | 0.125 | 0.125 | 90 | 7 | 52 |
| ORI | 0.015 | 0.015 | 0.015 | 0.03 | 25 | 12 | 52 |
OXA, oxacillin; FA, fusidic acid; CLI, clindamycin; GEN, gentamicin; RIF, rifampin; VAN, vancomycin; LZD, linezolid; Q-D, quinupristin-dalfopristin; DAP, daptomycin; TGC, tigecycline; MXF, moxifloxacin; TLV, televancin; ORI, oritavancin.
Total drug.
Characterization of the model.
The growth rate of the SCV was first compared to that of its normal-phenotype counterpart in both extracellular and intracellular milieus (Fig. 2). Extracellularly, SCV grew slowly, achieving only a 1-log increase in the number of CFU over 24 h compared to 3 logs for the normal-phenotype strain. Likewise, in infected macrophages, only a small change in intracellular inoculum was detected at 24 h for the SCV (0.1- to 0.2-log increase) while the normal-phenotype strain gained 1 log, as previously found for different S. aureus strains in the same model (7, 9). The intracellular localization of both strains was then examined by confocal and electron microscopy. Figure 3 shows a clear-cut colocalization with LysoTracker dye in both cases, suggesting that bacteria are confined in acidic compartments, irrespective of their phenotypes. Electron microscopy (Fig. 4) showed that both strains were present in membrane-bound vacuoles, a few of which contained several bacteria, suggesting that some degree of intracellular multiplication could have occurred.
FIG. 2.
Comparison of the growth curves for S. aureus normal-phenotype and SCV strains in CA-MH broth (extracellular) or in THP-1 macrophages exposed to 1× MIC of gentamicin to control the extracellular growth of bacteria. The graphs show the change in the number of CFU over time (Δlog CFU from the initial inoculum) per ml of culture medium (extracellular) or per mg of cell protein (intracellular). Data are means ± standard deviations (n = 3); most error bars are smaller than the symbols.
FIG. 3.
Confocal microscopy of THP-1 macrophages observed 3 h after phagocytosis of S. aureus normal-phenotype and SCV strains. Bacteria were labeled with fluorescein isothiocyanate (green signal), and the acidic compartments of the cells were labeled with LysoTracker Red DND-99 (red signal). For both strains, merged images show a clear-cut colocalization of the two fluorescent tracers in round vesicles.
FIG. 4.
Electron microscopy of THP-1 macrophages fixed 24 h after phagocytosis of S. aureus. Both normal and SCV phenotypes were seen in membrane-bound structures with, in some cases, the appearance of several bacteria in a single vacuole, suggestive of intracellular multiplication. Arrows indicate membranes. Bar, 0.3 μm.
Activity of antibiotics at a fixed concentration against intracellular and extracellular SCVs.
The activity of antibiotics against intracellular SCVs after 5, 24, or 72 h of infection was first examined at total drug concentrations mimicking their maximal concentration (Cmax) values in human serum or plasma (Fig. 5, left panel). At 5 h, there was no marked change in the number of CFU compared to the postphagocytosis inoculum, except with the use of rifampin, moxifloxacin, and oritavancin, which caused a 1-log decrease. No or little change occurred at 24 h except with the use of oritavancin, which caused a 2.5-log decrease in that time frame. At 72 h, however, all antibiotics had achieved at least a 1-log decrease, with quinupristin-dalfopristin, moxifloxacin, oritavancin, and rifampin causing a decrease of 2 to 3.5 logs (drugs are ordered from smaller to greater effects). To determine whether the poor or slow activity could be due to the SCV character itself or to the intracellular localization of the bacteria, we also measured the effect of the antibiotics toward bacteria in broth after 5 or 24 h of incubation using the same concentrations as used for the intracellular assay (Fig. 5, right panel). All antibiotics known as bactericidal (oxacillin, gentamicin, rifampin, vancomycin, quinupristin-dalfopristin, daptomycin, moxifloxacin, telavancin, and oritavancin) achieved a 3-log reduction within 24 h, with four of them (gentamicin, daptomycin, quinupristin-dalfopristin, and oritavancin) achieving this effect after only 5 h. Fusidic acid, linezolid, and tigecycline caused a ∼1-log reduction, and clindamycin produced a ∼2-log reduction from the initial inoculum.
FIG. 5.
Comparative intracellular and extracellular activities of different antibiotics against SCVs exposed for the indicated times at fixed extracellular concentrations (total drug, corresponding to the human Cmax [Table 1]). Antibiotics are ordered from classical first-line drugs to more recent alternatives and drugs under development. No data were obtained for control cells at 72 h (massive infection and cell death). The graphs show the change in the number of CFU over time (Δlog CFU from the initial inoculum) per mg of cell protein (intracellular) or per ml of culture medium (extracellular). Data are means ± standard deviations (n = 3); most error bars are smaller than the symbols. The dotted lines highlight changes of −1, −2, and −3 logs to facilitate comparisons between drugs. The limit of detection is set at −4.5 logs. Drug abbreviations are given in footnote a to Table 1. Contr, control.
Concentration-effect relationships against SCVs.
The activity of all antibiotics was then examined against SCVs at 24 h using a wide range of drug concentrations (total drug) to obtain full pharmacological dose-response curves while at the same time covering the full range of clinically meaningful concentrations. Data were used for fitting a previously described dose-response model based on Hill's equation (see details in reference 9). Simple sigmoidal functions could be fitted satisfactorily (R2 > 0.91) to all data, except for the intracellular activity of oritavancin (see below). The pertinent regression parameters and pharmacological descriptors (Emax, maximum effect in terms of the log CFU decrease of inoculum for an infinitely large antibiotic concentration; EC50, the extracellular antibiotic concentration in multiples of the MIC causing a reduction of the inoculum to halfway between the number of CFU extrapolated for an infinitely low antibiotic concentration [E0] and the Emax) are presented in Table 2, together with statistical analysis of their differences. Data for rifampin, moxifloxacin, and oritavancin (the three most active antibiotics intracellularly) are shown graphically in Fig. 6 for illustrative purposes (see Fig. SP1 in the supplemental material for graphical data of all other antibiotics). Intracellularly, the Emax ranged from a 0.3- to 0.6-log CFU decrease relative to the starting inoculum for most drugs but reached −1 to −2 log CFU for tigecycline, moxifloxacin, and rifampin (drugs are ordered from smaller to greater effects). For oritavancin, inspection of the data suggested a bimodal effect, allowing the fitting of two successive sigmoidal functions, with a maximal effect (Emax(2)) of about −3 log CFU. A static effect against SCVs was obtained for all antibiotics at extracellular concentrations (total drug) between 1× and 6× MIC except for oritavancin, which required concentrations about 10-fold higher (65 × MIC). Extracellularly, all drugs were bacteriostatic against SCVs at concentrations close to their MICs and showed Emax values of −3 log CFU or more, with the exception of fusidic acid, clindamycin, and tigecycline (−1.1 to −2.6 log CFU).
TABLE 2.
Pertinent regression parameters of dose-response curves for intracellular and extracellular activity against the SCV straina
| Antibiotic | Intracellular activityb
|
Extracellular activityc
|
||||||
|---|---|---|---|---|---|---|---|---|
| Emax (log10 CFU)d | EC50e | Csf | R2 | Emax (log10 CFU)d | EC50e | Csf | R2 | |
| OXA | −0.39 (−0.52 to −0.27) Aa | 0.83 (0.58 to 1.17) Aa | 2.03 | 0.966 | −3.91 (−4.07 to −3.76) Ab | 5.50 (4.71 to 6.43) Ab | 1.66 | 0.993 |
| FA | −0.36 (−0.52 to −0.20) Aa | 10.70 (7.77 to 14.72) Ba | 6.29 | 0.970 | −1.43 (−1.52 to −1.34) Bb | 4.78 (4.15 to 5.51) Bb | 2.45 | 0.994 |
| CLI | −0.45 (−0.51 to −0.39) Ca | 0.47 (0.39 to 0.57) Aa | 1.47 | 0.990 | −2.59 (−2.85 to −2.23) Cb | 2.52 (1.79 to 3.55) Cb | 1.47 | 0.967 |
| GEN | −0.58 (−0.76 to −0.40) Aa | 0.63 (0.41 to 0.97) Aa | 1.62 | 0.947 | <−4.5 Db | 2.33 (1.75 to 3.11) Cb | 1.04 | 0.986 |
| RIF | −1.72 (−2.04 to −1.40) Ba | 42.60 (29.42 to 61.66) Ca | 5.69 | 0.960 | −3.02 (−3;19 to −2.85) Eb | 43.35 (37.66 to 49.90) Db | 4.57 | 0.994 |
| VAN | −0.36 (−0.62 to −0.10) Aa | 6.61 (3.77 to 11.61) Da | 5.18 | 0.915 | −4.25 (−4.42 to −4.00) Fb | 4.99 (4.27 to 5;84) Eb | 1.58 | 0.992 |
| LZD | −0.54 (−0.84 to −0.23) Aa | 0.61 (0.38 to 0.97) Aa | 1.87 | 0.948 | −3.24 (−3.49 to −3.00) Eb | 2.72 (2.21 to 3.35) Cb | 1.43 | 0.989 |
| Q-D | −0.60 (−0.76 to −0.44) Aa | 0.25 (0.15 to 0.40) Aa | 1.02 | 0.934 | −3.50 (−3.70 to −3.30) Gb | 0.75 (0.58 to 0.97) Gb | 0.80 | 0.981 |
| DAP | −0.50 (−0.64 to −0.35) Aa | 4.17 (2.91 to 5.96) Ea | 3.45 | 0.964 | −3.78 (−4.03 to −3.53) Ab | 4.54 (3.58 to 5.76) Hb | 1.68 | 0.983 |
| TGC | −1.11 (−1.26 to 0.95) Ca | 0.80 (0.50 to 1.17) Aa | 1.23 | 0.960 | −1.75 (−1.85 to −1.64) Hb | 1.31 (1;08 to 1.59) Ib | 1.27 | 0.989 |
| MXF | −1.32 (−1.45 to −1.19) Ca | 2.49 (1.92 to 3.23) Fa | 1.85 | 0.980 | <−4.5 Db | 3.01 (1.45 to 6.26) Jb | 1.12 | 0.923 |
| TLV | −0.35 (−0.53 to −0.17) Aa | 0.52 (0.31 to 0.85) Aa | 3.89 | 0.931 | <−4.5 Db | 0.88 (0.64 to 1.21) Gb | 0.52 | 0.971 |
| ORIg | −0.43 (−0.56 to 0.30) NA | 41.61 (35.79 to 48.38) NA | 65 | 0.990 | <−4.5 Dn | 3.15 (3.05 to 3.24) NA | 1.50 | 0.976 |
| −3.13 (−3.22 to −3.00) NA | 1272 (1212 to 1334) NA | NA | 0.994 | |||||
Data are based on a 24-h postphagocytosis exposure. Regression parameters were calculated using all data from antibiotic concentrations of 10−2 to 103 to 105 times the MIC. The parameters described in the table were derived from analysis of the data shown in Fig. 6 for rifampin, moxifloxacin, and oritavancin and in Fig. SP1 in the supplemental material for the other antibiotics. Statistical analyses were performed as follows. For analysis per column, one-way analysis of variance with Tukey's posttest for multiple comparisons between each parameter for all drugs; figures with different uppercase letters are significantly different from each other (P < 0.05). For analysis per row, an unpaired, two-tailed t test between corresponding parameters of intracellular and extracellular activities was used; figures with different lowercase letters are significantly different from each other (P < 0.05). Confidence intervals (CI) are given in parentheses. See Table 1, footnote a, for a key to the drug abbreviations. NA, not applicable.
Determined in THP-1 macrophages with an initial phagocytosed inoculum of about 106 CFU/mg of protein.
Determined in MH broth with an initial inoculum of about 106 CFU/ml.
Log CFU decrease at 24 h from the corresponding original inoculum, as extrapolated for an infinitely large antibiotic concentration. Samples yielding less than −4.5 log CFU were considered below the detection level.
Concentrations (total drug; in multiples of the MIC) causing a reduction of the inoculum halfway between the initial (E0) and the maximal (Emax) values, as obtained from the Hill equation.
Static concentration, i.e., the concentration (total drug; in multiples of the MIC) resulting in no apparent bacterial growth (number of CFU identical to the initial inoculum), as determined by graphical intrapolation.
Two successive sigmoidal regressions were use to fit to actual intracellular data.
FIG. 6.
Dose-response curves of the three most active antibiotics against extracellular and intracellular SCVs. The graphs show the change in the number of CFU (Δlog CFU from the initial inoculum) per ml of broth (extracellular [extra]) or per mg of cell protein (intracellular [intra]) in THP-1 macrophages after a 24-h incubation at the extracellular concentrations (total drug) shown in the abscissa. Curves were constructed by sigmoidal regression using the Hill equation (Table 2 gives the regression parameters); for oritavancin, for which a bimodal curve is clearly seen intracellularly, two successive sigmoidal regressions with variable slopes were used to fit the data. The zone highlighted in gray corresponds to the clinically relevant concentration range (total drug). Data are means ± standard deviations (n = 3); most error bars are smaller than the symbols.
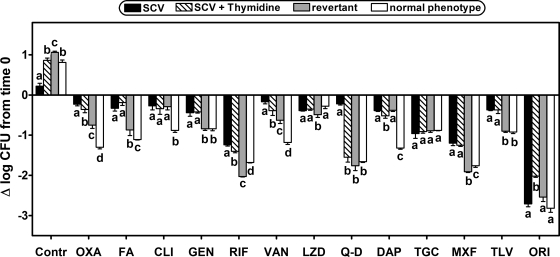
Effect of addition of thymidine and comparison with a revertant and the normal-phenotype isogenic strain.
Since the SCV strain used in this study was thymidine dependent, we also examined whether thymidine addition (100 mg/liter) (26) modified the intracellular cell growth and the bacterial response to antibiotics. Figure 7 shows that at 24 h, the addition of thymidine restored the intracellular growth of the SCV strain, making it comparable to that of the normal-phenotype strain, but did not markedly increase the intracellular susceptibility to antibiotics except for quinupristin-dalfopristin, for which intracellular activity became similar to that of the normal-phenotype strain (confirmed in full dose-response studies) (see Fig. SP2 in the supplemental material). In contrast, the revertant showed not only intracellular growth similar to that of the normal phenotype strain but also greater susceptibility to several antibiotics, except for oxacillin, fusidic acid, and vancomycin, for which activity was intermediate between the activities measured for the SCV and the normal-phenotype isogenic strains. Tigecycline and oritavancin both exhibit distinct behavior in showing similar activities against all strains but with a markedly greater reduction of the postphagocytosis inoculum for oritavancin (−2 logs).
FIG. 7.
Comparative intracellular activity toward isogenic S. aureus strains with different phenotypes: SCV in the absence or in the presence of 100 mg/liter thymidine in the culture medium and revertant and normal phenotypes. Infected THP-1 macrophages were exposed for 24 h at a fixed extracellular concentration (total drug) of each antibiotic (corresponding to the human Cmax [Table 1]). Thymidine was added at the same time as antibiotics. Antibiotics are ordered, left to right, from classical first-line drugs to more recent alternatives and drugs under development. The graph shows the change in the number of CFU per mg of cell protein over time (Δlog CFU from the initial inoculum). Data are means ± standard deviations (n = 3); most error bars are smaller than the symbols. Analysis of variance and multiple comparison by Tukey's test were used to determine significance; strains with different letters are significantly different from one another for each antibiotic (P < 0.05). Drug abbreviations are given in footnote a to Table 1. Contr, control.
DISCUSSION
The present study represents a first attempt to document, on a pharmacological basis, the response of SCVs to antibiotics, using a model developed specifically for the study of intracellular infections that has already been applied to normal-phenotype S. aureus strains with different resistance mechanisms (7, 9, 30). Compared to its normal-phenotype isogenic counterpart, the thymidine-dependent SCV isolate used in the present study allowed us to make three main observations that warrant critical analysis since they reveal specific properties with potential biological and clinical significance.
We first show that a thymidine-dependent SCV strain is characterized by very slow growth not only in broth, as already described (13, 26), but also in THP-1 macrophages. This is unlikely to be the consequence of the activity of host defenses as THP-1 cells are known to be poorly active in this respect (references 15 and 53 and references therein). In the present study, THP-1 macrophages allowed the normal-phenotype isogenic strain to grow intracellularly to a level similar to what has been observed for most of the other S. aureus strains studied to date (for details, see references 7, 9, 30, and 31). Similarly, the slow intracellular growth of the SCV strain cannot be ascribed to differences in phagocytosis and subcellular localization since quantitative measurements of the phagocytosis index and microscopy studies failed to differentiate it from its isogenic normal-phenotype counterpart or from other S. aureus strains studied in the same model (7, 9, 30, 46). The data rather suggest that the metabolic defects responsible for thymidine auxotrophy (17, 18) and the resulting low amount of this nucleoside inside bacteria (63) play the most critical role. We show, indeed, that intracellular growth is restored upon addition of thymidine in the culture medium of cells, as already described for extracellular growth in thymidine-supplemented broth (26, 27). Of note, thymidine dependence seems to universally depend upon random mutations in the thymidylate synthase-encoding thyA gene (leading to an intracellular lack of dTMP). This was not specifically tested here, but these mutants are known to be frequent not only in SCVs isolated from cystic fibrosis patients (as was the strain used here) but also many other infection sites of patients with chronic infections (14).
A second property of intracellular SCV that is documented by our data is the considerable decrease in maximal efficacy of most antibiotics, especially in the first 24-h period, not only in comparison with extracellular forms (as was already noted and largely documented for all S. aureus strains studied in our THP-1 macrophage model so far) (8, 9, 30, 46) but also, and most importantly, compared to the isogenic normal-phenotype strain (except for tigecycline and linezolid, probably due to their bacteriostatic character). This decrease cannot be due to differences in intrinsic susceptibilities since MICs were largely similar for both strains (as already reported for a thymidine-dependent SCV [5] and in contrast to hemin-dependent strains that tend to become less susceptible to some antibiotics [10, 19, 40]). This decrease also cannot be ascribed to the acidic pH, as the MICs of only 4 out of 13 antibiotics used were affected by the pH (and in opposite directions) with no difference between the SCV and normal-phenotype strains. Likewise, it cannot be explained by the slow-growth character of our strain since restoration of normal intracellular growth by the addition of thymidine failed to restore susceptibility to antibiotics, except for quinupristin-dalfopristin (but we were unable to explain this observation based on available data and/or what is known about the mode of action of this antibiotic) (54). Because this effect appears quite general (disregarding quinupristin-dalfopristin), it is also unlikely to result from specific changes in bacterial metabolism. The data, therefore, suggest the intervention of more general parameters such as the presence of protective mechanisms or global metabolic changes in the bacteria that will need to be systematically studied.
A third critical observation is that killing of intracellular bacteria can eventually be observed if infected cells are maintained in the presence of antibiotics for 72 h. This length of incubation is rarely used in in vitro experiments, which makes our model quite different from many others, with the downside that no antibiotic-free control can be run because of overwhelming extracellular bacterial growth at this late time point. Yet this allowed us to observe that four antibiotics were significantly more active, namely, quinupristin-dalfopristin, moxifloxacin, oritavancin, and rifampin (drugs are ordered from smaller to greater effects; for oritavancin, maximal activity is already reached after 24 h). Notably, these drugs are among those which proved most efficacious in models of intracellular infections by normal-phenotype strains (9, 30). The high level of activity seen with rifampin and moxifloxacin probably results from their substantial intrinsic activity, as witnessed by their low MICs. For moxifloxacin, this could be aided by its ability to quickly penetrate cells and thereby provide a high degree of bioavailability (for a review, see reference 50). For oritavancin, the explanation probably lies in the combined effects of its high level of intracellular accumulation (with a preferential accumulation in lysosomes) (51) and its dual mode of action that involves both the impairment of cell wall synthesis at low concentrations (1) and membrane activity, namely, dissipation of transmembrane potential (36), and also some bacterial lysis at higher concentrations (11) (ongoing electron microscopic studies at concentrations around 25 mg/liter show that oritavancin also lyses SCVs). This dual mode of action may explain the bimodal shape of the dose-response curve of intracellular SCVs (which could not be explored for extracellular bacteria in this setup because the speed of bactericidal activity of oritavancin was too fast). Although also known to have membrane-destabilizing effects toward S. aureus (24), telavancin failed to demonstrate bimodal dose responses toward SCVs, as it does toward various normal-phenotype S. aureus strains (7), and was therefore less bactericidal than oritavancin. Based on our observations of a greater accumulation of oritavancin than telavancin in macrophages and other cell types (8), we suggest that telavancin does not reach the concentration threshold in phagolysosomes that would allow it to demonstrate its membrane-destabilizing effects toward SCVs. It remains, however, uncertain whether the membrane-destabilizing effects of either drug will be expressed in vivo since these effects may require concentrations that may not be reached upon therapy with conventional dosages (see reference 7 for a discussion about bimodal concentration-effect response of telavancin with a normal-phenotype S. aureus strain). The behavior of quinupristin-dalfopristin with respect to its activity seen at 72 h (and the influence of thymidine that is unique to this antibiotic) is more difficult to explain. A critical aspect in quinupristin-dalfopristin pharmacology is that its two components must be maintained in a specific molar ratio range for optimal expression of bactericidal activity. No information is available on the ratio achieved in the infected intracellular compartments. We also do not know whether optimal activity is obtained for the same ratio in the intracellular milieu.
More broadly speaking, the present study also extends to SCVs our general observation that there is no simple correlation between the levels of cellular accumulation of antibiotics (as established from studies with noninfected cells [9; for a review, see reference 50]) and activity even if some trend is apparent (for example, the behavior of oritavancin; but note that rifampin was almost as active as oritavancin even though it was reported to accumulate at a considerably lower level than oritavancin in uninfected THP-1 macrophages [9]).
A first limitation of the present study is that we used a single strain with a metabolic defect in thymidine synthesis obtained from one CF patient. This is because we concentrated our efforts on a detailed pharmacological analysis aimed at characterizing a model and a general type of bacterial response to several drugs, which could not be done with several strains simultaneously. Yet we controlled the specificity of our observations regarding the SCV strain by using a revertant and an isogenic normal-phenotype strain. Thus, even though our conclusions may not extend directly to strains with other metabolic defects (such as those affecting hemin synthesis) or to those obtained from other types of patients, they nevertheless allow us to draw provisional conclusions of general interest concerning SCVs. A second limitation is that we could not measure the intracellular accumulation and subcellular disposition of antibiotics in infected cells because the extracellular concentrations at which intracellular dose effects can be studied create cellular drug levels that are too low for reliable, quantitative assay without using radiolabeled drugs (and these were not available for most antibiotics studied here). Some information can be obtained, nevertheless, from what is known about cellular uptake and subcellular disposition of antibiotics in uninfected cells exposed to higher concentrations (for a review, see reference 50) since there is often a fair degree of linearity in the concentration-accumulation responses and similarity in drug disposition between these two different experimental setups (50). A third limitation is that concentration-response studies could not be made at longer times than 24 h because of the marked growth of extracellular bacteria in samples subjected to low concentrations of antibiotics.
The clinical significance of our findings requires careful analysis. First, the slow-growing character of SCVs observed extracellularly also applies to intracellular forms, explaining why these forms easily escape attention. This calls for improved diagnostic approaches since SCVs are considered to be important determinants in the relapsing and recurrent character of many staphylococcal infections. Second, our data, with the limitations inherent to in vitro experiments, clearly illustrate that conventional susceptibility testing methods may poorly predict outcomes of an intracellular infection. This could explain failures in clinical situations despite therapeutic choices that, based on in vitro susceptibility testing results, should provide optimal outcomes. Both negative and positive results obtained with specific antibiotics in our model need to be interpreted with caution, however. For the most active drugs and for oritavancin in particular, maximal activity is obtained only upon prolonged exposure to large extracellular concentrations, which might not be attainable in patients. As discussed earlier (7, 9, 32), our model also does not take into account important parameters such as serum protein binding or variations in drug concentrations over time, making extrapolations to the in vivo situation and to difficult or indirect clinical applications. Protein binding under our culture conditions is lower than what occurs in vivo since the serum concentration is indeed limited to 10%. While this may lead to overestimation of the activity of highly protein-bound antibiotics, such as oritavancin or telavancin, it is possible to use the data generated by the concentration-dependent experiments to extrapolate what could be the activity in vivo at the desired free concentration (using the human protein binding data shown in Table 1); it must be remembered, however, that the negative effect of protein binding on the activity of antibiotics remains a controversial issue, especially for the new lipoglycopeptides used here (34, 49). Our studies should nevertheless facilitate the development of improved susceptibility testing methods that could help guide further studies (involving more strains and additional in vitro and in vivo models) in order to rationalize antibiotic selection for persistent infections involving SCVs.
Supplementary Material
Acknowledgments
This work was supported by the Institut d'encouragement de la Recherche Scientifique et de l'Innovation de Bruxelles (IRSIB)/Instituut ter Bevordering van het Wetenschappelijk Onderzoek en de Innovatie van Brussel (IWOIB) of the Brussels Capital Region, within the framework of the Research in Brussels program, by the Belgian Fonds National de la Recherche Scientifique Médicale (grant no. 3.4.597.06), and by grants-in-aid from Targanta Therapeutics Corp. and Theravance, Inc. H.A.N. was a postdoctoral fellow of the IRSIB/IWOIB, and F.V.B. is Maître de Recherches of the Belgian Fonds de la Recherche Scientifique-Fonds National de la Recherche Scientifique.
We warmly thank M. C. Cambier, S. Harag, C. Misson, and M. Vergauwen for their dedicated technical assistance.
Footnotes
Published ahead of print on 2 February 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Allen, N. E., and T. I. Nicas. 2003. Mechanism of action of oritavancin and related glycopeptide antibiotics. FEMS Microbiol. Rev. 26:511-532. [DOI] [PubMed] [Google Scholar]
- 2.Amsden, G. W. 2000. Tables of antimicrobial agents pharmacology, p. 635-700. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Churchill Livingstone, New York, NY.
- 3.Arhin, F. F., I. Sarmiento, A. Belley, G. A. McKay, D. C. Draghi, P. Grover, D. F. Sahm, T. R. Parr, Jr., and G. Moeck. 2008. Effect of Polysorbate-80 on oritavancin binding to plastic surfaces: implications for susceptibility testing. Antimicrob. Agents Chemother. 52:1597-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auwerx, J. 1991. The human leukemia cell line, THP-1: a multifaceted model for the study of monocyte-macrophage differentiation. Experientia 47:22-31. [DOI] [PubMed] [Google Scholar]
- 5.Baltch, A. L., W. J. Ritz, L. H. Bopp, P. Michelsen, and R. P. Smith. 2008. Activities of daptomycin and comparative antimicrobials, singly and in combination, against extracellular and intracellular Staphylococcus aureus and its stable small colony variant in human monocyte-derived macrophages and in broth. Antimicrob. Agents Chemother. 52:1829-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balwit, J. M., P. van Langevelde, J. M. Vann, and R. A. Proctor. 1994. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J. Infect. Dis. 170:1033-1037. [DOI] [PubMed] [Google Scholar]
- 7.Barcia-Macay, M., S. Lemaire, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2006. Evaluation of the extracellular and intracellular activities (human THP-1 macrophages) of telavancin versus vancomycin against methicillin-susceptible, methicillin-resistant, vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:1177-1184. [DOI] [PubMed] [Google Scholar]
- 8.Barcia-Macay, M., F. Mouaden, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2008. Cellular pharmacokinetics of telavancin, a novel lipoglycopeptide antibiotic, and analysis of lysosomal changes in cultured eukaryotic cells (J774 mouse macrophages and rat embryonic fibroblasts). J. Antimicrob. Chemother. 61:1288-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barcia-Macay, M., C. Seral, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob. Agents Chemother. 50:841-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumert, N., C. von Eiff, F. Schaaff, G. Peters, R. A. Proctor, and H. G. Sahl. 2002. Physiology and antibiotic susceptibility of Staphylococcus aureus small colony variants. Microb. Drug Resist. 8:253-260. [DOI] [PubMed] [Google Scholar]
- 11.Belley, A., B. Harris, T. Beveridge, T. R. Parr, Jr., and G. Moeck. 2008. Cell wall and membrane effects of oritavancin on Staphylococcus aureus and Enterococcus faecalis, abstr. P537. Abstr. 18th Eur. Cong. Clin. Microbiol. Infect. Dis., Barcelona, Spain, 19 to 22 April 2008.
- 12.Besier, S., C. Smaczny, C. von Mallinckrodt, A. Krahl, H. Ackermann, V. Brade, and T. A. Wichelhaus. 2007. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J. Clin. Microbiol. 45:168-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besier, S., J. Zander, B. C. Kahl, P. Kraiczy, V. Brade, and T. A. Wichelhaus. 2008. The thymidine-dependent small colony variant phenotype is associated with hypermutability and antibiotic resistance in clinical Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 52:2183-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besier, S., J. Zander, E. Siegel, S. H. Saum, K. P. Hunfeld, A. Ehrhart, V. Brade, and T. A. Wichelhaus. 2008. Thymidine-dependent Staphylococcus aureus small-colony variants: human pathogens that are relevant not only in cases of cystic fibrosis lung disease. J. Clin. Microbiol. 46:3829-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carryn, S., V. d. Van, F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2004. Impairment of growth of Listeria monocytogenes in THP-1 macrophages by granulocyte macrophage colony-stimulating factor: release of tumor necrosis factor-alpha and nitric oxide. J. Infect. Dis. 189:2101-2109. [DOI] [PubMed] [Google Scholar]
- 16.Chambers, H. F. 2000. Penicillins, p. 281-293. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Churchill Livingstone, New York, NY.
- 17.Chatterjee, I., M. Herrmann, R. A. Proctor, G. Peters, and B. C. Kahl. 2007. Enhanced post-stationary-phase survival of a clinical thymidine-dependent small-colony variant of Staphylococcus aureus results from lack of a functional tricarboxylic acid cycle. J. Bacteriol. 189:2936-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee, I., A. Kriegeskorte, A. Fischer, S. Deiwick, N. Theimann, R. A. Proctor, G. Peters, M. Herrmann, and B. C. Kahl. 2008. In vivo mutations of thymidylate synthase (encoded by thyA) are responsible for thymidine dependency in clinical small-colony variants of Staphylococcus aureus. J. Bacteriol. 190:834-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuard, C., P. E. Vaudaux, R. A. Proctor, and D. P. Lew. 1997. Decreased susceptibility to antibiotic killing of a stable small colony variant of Staphylococcus aureus in fluid phase and on fibronectin-coated surfaces. J. Antimicrob. Chemother. 39:603-608. [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing, 18th informational supplement. Approved standard M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 21.Denis, O., A. Deplano, R. De Ryck, C. Nonhoff, and M. J. Struelens. 2003. Emergence and spread of gentamicin-susceptible strains of methicillin-resistant Staphylococcus aureus in Belgian hospitals. Microb. Drug Resist. 9:61-71. [DOI] [PubMed] [Google Scholar]
- 22.Denis, O., A. Deplano, C. Nonhoff, M. Hallin, R. De Ryck, R. Vanhoof, R. De Mendonca, and M. J. Struelens. 2006. In vitro activities of ceftobiprole, tigecycline, daptomycin, and 19 other antimicrobials against methicillin-resistant Staphylococcus aureus strains from a national survey of Belgian hospitals. Antimicrob. Agents Chemother. 50:2680-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallin, M., O. Denis, A. Deplano, R. De Mendonca, R. De Ryck, S. Rottiers, and M. J. Struelens. 2007. Genetic relatedness between methicillin-susceptible and methicillin-resistant Staphylococcus aureus: results of a national survey. J. Antimicrob. Chemother. 59:465-472. [DOI] [PubMed] [Google Scholar]
- 24.Higgins, D. L., R. Chang, D. V. Debabov, J. Leung, T. Wu, K. M. Krause, E. Sandvik, J. M. Hubbard, K. Kaniga, D. E. Schmidt, Jr., Q. Gao, R. T. Cass, D. E. Karr, B. M. Benton, and P. P. Humphrey. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahl, B., M. Herrmann, A. S. Everding, H. G. Koch, K. Becker, E. Harms, R. A. Proctor, and G. Peters. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177:1023-1029. [DOI] [PubMed] [Google Scholar]
- 26.Kahl, B. C., G. Belling, P. Becker, I. Chatterjee, K. Wardecki, K. Hilgert, A. L. Cheung, G. Peters, and M. Herrmann. 2005. Thymidine-dependent Staphylococcus aureus small-colony variants are associated with extensive alterations in regulator and virulence gene expression profiles. Infect. Immun. 73:4119-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahl, B. C., G. Belling, R. Reichelt, M. Herrmann, R. A. Proctor, and G. Peters. 2003. Thymidine-dependent small-colony variants of Staphylococcus aureus exhibit gross morphological and ultrastructural changes consistent with impaired cell separation. J. Clin. Microbiol. 41:410-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kipp, F., B. C. Kahl, K. Becker, E. J. Baron, R. A. Proctor, G. Peters, and C. von Eiff. 2005. Evaluation of two chromogenic agar media for recovery and identification of Staphylococcus aureus small-colony variants. J. Clin. Microbiol. 43:1956-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, S. Y., H. W. Fan, J. L. Kuti, and D. P. Nicolau. 2006. Update on daptomycin: the first approved lipopeptide antibiotic. Expert. Opin. Pharmacother. 7:1381-1397. [DOI] [PubMed] [Google Scholar]
- 30.Lemaire, S., K. Kosowska-Shick, K. Julian, P. M. Tulkens, F. Van Bambeke, and P. C. Appelbaum. 2008. Activities of antistaphylococcal antibiotics towards the extracellular and intraphagocytic forms of S. aureus isolates from a patient with persistent bacteraemia and endocarditis. Clin. Microbiol. Infect. 14:766-777. [DOI] [PubMed] [Google Scholar]
- 31.Lemaire, S., A. Olivier, F. Van Bambeke, P. M. Tulkens, P. C. Appelbaum, and Y. Glupczynski. 2008. Restoration of susceptibility of intracellular methicillin-resistant Staphylococcus aureus to β-lactams: comparison between strains, cells, and antibiotics. Antimicrob. Agents Chemother. 52:2797-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemaire, S., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2005. Activity of three β-lactams (ertapenem, meropenem and ampicillin) against intraphagocytic Listeria monocytogenes and Staphylococcus aureus. J. Antimicrob. Chemother. 55:897-904. [DOI] [PubMed] [Google Scholar]
- 33.Leuthner, K. D., J. R. Aeschlimann, and M. J. Rybak. 2005. Quinupristin/dalfopristin, p. 367-382. In V. L. Yu, G. Edwards, P. S. McKinnon, C. Peloquin, and G. D. Morse (ed.), Antimicrobial therapy and vaccines. Esun Technologies, LLC, Pittsburgh, PA.
- 34.Leuthner, K. D., C. M. Cheung, and M. J. Rybak. 2006. Comparative activity of the new lipoglycopeptide telavancin in the presence and absence of serum against 50 glycopeptide non-susceptible staphylococci and three vancomycin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:338-343. [DOI] [PubMed] [Google Scholar]
- 35.Maes, N., J. Magdalena, S. Rottiers, Y. De Gheldre, and M. J. Struelens. 2002. Evaluation of a triplex PCR assay to discriminate Staphylococcus aureus from coagulase-negative staphylococci and determine methicillin resistance from blood cultures. J. Clin. Microbiol. 40:1514-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKay, G. A., I. Fadhil, S. Beaulieu, S. Ciblat, A. R. Far, G. Moeck, and Parr, T. R., Jr. 2006. Oritavancin disrupts transmembrane potential and membrane integrity concomitantly with cell killing in Staphylococcus aureus and vancomycin-resistant enterococci. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-682.
- 37.Mitchell, G., C. A. Lamontagne, E. Brouillette, G. Grondin, B. G. Talbot, M. Grandbois, and F. Malouin. 2008. Staphylococcus aureus SigB activity promotes a strong fibronectin-bacterium interaction which may sustain host tissue colonization by small-colony variants isolated from cystic fibrosis patients. Mol. Microbiol. 70:1540-1555. [DOI] [PubMed] [Google Scholar]
- 38.Moisan, H., E. Brouillette, C. L. Jacob, P. Langlois-Begin, S. Michaud, and F. Malouin. 2006. Transcription of virulence factors in Staphylococcus aureus small-colony variants isolated from cystic fibrosis patients is influenced by SigB. J. Bacteriol. 188:64-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Committee for Clinical Laboratory Standards. 1998. Methods for determining bactericidal activity of antimicrobial agents. Approved guideline M26A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 40.Proctor, R. A., and G. Peters. 1998. Small colony variants in staphylococcal infections: diagnostic and therapeutic implications. Clin. Infect. Dis. 27:419-422. [DOI] [PubMed] [Google Scholar]
- 41.Proctor, R. A., P. van Langevelde, M. Kristjansson, J. N. Maslow, and R. D. Arbeit. 1995. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin. Infect. Dis. 20:95-102. [DOI] [PubMed] [Google Scholar]
- 42.Proctor, R. A., C. von Eiff, B. C. Kahl, K. Becker, P. McNamara, M. Herrmann, and G. Peters. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4:295-305. [DOI] [PubMed] [Google Scholar]
- 43.Proctor, R. A., and A. von Humboldt. 1998. Bacterial energetics and antimicrobial resistance. Drug Resist. Updat. 1:227-235. [DOI] [PubMed] [Google Scholar]
- 44.Sadowska, B., A. Bonar, C. von Eiff, R. A. Proctor, M. Chmiela, W. Rudnicka, and B. Rozalska. 2002. Characteristics of Staphylococcus aureus, isolated from airways of cystic fibrosis patients, and their small colony variants. FEMS Immunol. Med. Microbiol. 32:191-197. [DOI] [PubMed] [Google Scholar]
- 45.Sendi, P., M. Rohrbach, P. Graber, R. Frei, P. E. Ochsner, and W. Zimmerli. 2006. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin. Infect. Dis. 43:961-967. [DOI] [PubMed] [Google Scholar]
- 46.Seral, C., F. Van Bambeke, and P. M. Tulkens. 2003. Quantitative analysis of gentamicin, azithromycin, telithromycin, ciprofloxacin, moxifloxacin, and oritavancin (LY333328) activities against intracellular Staphylococcus aureus in mouse J774 macrophages. Antimicrob. Agents Chemother. 47:2283-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein, G. E., and W. A. Craig. 2006. Tigecycline: a critical analysis. Clin. Infect. Dis. 43:518-524. [DOI] [PubMed] [Google Scholar]
- 48.Tsuji, B. T., G. W. Kaatz, and M. J. Rybak. 2005. Linezolid and other oxazolidinones, p. 223-241. In V. L. Yu, G. Edwards, P. S. McKinnon, C. Peloquin, and G. D. Morse (ed.), Antimicrobial therapy and vaccines. Esun Technologies, LLC, Pittsburgh, PA.
- 49.Tsuji, B. T., S. N. Leonard, P. R. Rhomberg, R. N. Jones, and M. J. Rybak. 2008. Evaluation of daptomycin, telavancin, teicoplanin, and vancomycin activity in the presence of albumin or serum. Diagn. Microbiol. Infect. Dis. 60:441-444. [DOI] [PubMed] [Google Scholar]
- 50.Van Bambeke, F., M. Barcia-Macay, S. Lemaire, and P. M. Tulkens. 2006. Cellular pharmacodynamics and pharmacokinetics of antibiotics: current views and perspectives. Curr. Opin. Drug Discov. Devel. 9:218-230. [PubMed] [Google Scholar]
- 51.Van Bambeke, F., S. Carryn, C. Seral, H. Chanteux, D. Tyteca, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2004. Cellular pharmacokinetics and pharmacodynamics of the glycopeptide antibiotic oritavancin (LY333328) in a model of J774 mouse macrophages. Antimicrob. Agents Chemother. 48:2853-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Bambeke, F., Y. Van Laethem, P. Courvalin, and P. M. Tulkens. 2004. Glycopeptide antibiotics: from conventional molecules to new derivatives. Drugs 64:913-936. [DOI] [PubMed] [Google Scholar]
- 53.Van de Velde, S., H. A. Nguyen, F. Van Bambeke, P. M. Tulkens, J. Grellet, V. Dubois, C. Quentin, and M. C. Saux. 2008. Contrasting effects of human THP-1 cell differentiation on levofloxacin and moxifloxacin intracellular accumulation and activity against Staphylococcus aureus and Listeria monocytogenes. J. Antimicrob. Chemother. 62:518-521. [DOI] [PubMed] [Google Scholar]
- 54.Vannuffel, P., and C. Cocito. 1996. Mechanism of action of streptogramins and macrolides. Drugs 51(Suppl. 1):20-30. [DOI] [PubMed] [Google Scholar]
- 55.Vaudaux, P., P. Francois, C. Bisognano, W. L. Kelley, D. P. Lew, J. Schrenzel, R. A. Proctor, P. J. McNamara, G. Peters, and C. von Eiff. 2002. Increased expression of clumping factor and fibronectin-binding proteins by hemB mutants of Staphylococcus aureus expressing small colony variant phenotypes. Infect. Immun. 70:5428-5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaudaux, P., W. L. Kelley, and D. P. Lew. 2006. Staphylococcus aureus small colony variants: difficult to diagnose and difficult to treat. Clin. Infect. Dis. 43:968-970. [DOI] [PubMed] [Google Scholar]
- 57.Vergison, A., O. Denis, A. Deplano, G. Casimir, G. Claeys, F. DeBaets, K. DeBoeck, N. Douat, H. Franckx, J. Gigi, M. Ieven, C. Knoop, P. Lebeque, F. Lebrun, A. Malfroot, F. Paucquay, D. Pierard, J. Van Eldere, and M. J. Struelens. 2007. National survey of molecular epidemiology of Staphylococcus aureus colonization in Belgian cystic fibrosis patients. J. Antimicrob. Chemother. 59:893-899. [DOI] [PubMed] [Google Scholar]
- 58.von Eiff, C. 2008. Staphylococcus aureus small colony variants: a challenge to microbiologists and clinicians. Int. J. Antimicrob. Agents 31:507-510. [DOI] [PubMed] [Google Scholar]
- 59.von Eiff, C., K. Becker, D. Metze, G. Lubritz, J. Hockmann, T. Schwarz, and G. Peters. 2001. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier's disease. Clin. Infect. Dis. 32:1643-1647. [DOI] [PubMed] [Google Scholar]
- 60.von Eiff, C., D. Bettin, R. A. Proctor, B. Rolauffs, N. Lindner, W. Winkelmann, and G. Peters. 1997. Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin. Infect. Dis. 25:1250-1251. [DOI] [PubMed] [Google Scholar]
- 61.von Eiff, C., C. Heilmann, R. A. Proctor, C. Woltz, G. Peters, and F. Gotz. 1997. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J. Bacteriol. 179:4706-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Eiff, C., G. Peters, and K. Becker. 2006. The small colony variant (SCV) concept: the role of staphylococcal SCVs in persistent infections. Injury 37(Suppl. 2):S26-S33. [DOI] [PubMed] [Google Scholar]
- 63.Zander, J., S. Besier, S. H. Saum, F. Dehghani, S. Loitsch, V. Brade, and T. A. Wichelhaus. 2008. Influence of dTMP on the phenotypic appearance and intracellular persistence of Staphylococcus aureus. Infect. Immun. 76:1333-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.