Abstract
A topical microbicide that women can use to prevent sexually transmitted diseases (STDs) is essential, and many microbicide candidates are being tested for activity against human immunodeficiency virus and other STDs, including Chlamydia trachomatis. Screening assays for assessing the activity of microbicides against C. trachomatis are typically done with laboratory-adapted strains, but it is possible that recent clinical isolates may have different susceptibilities to microbicides, as has been seen with Neisseria gonorrhoeae and Lactobacillus spp. (B. J. Moncla and S. L. Hillier, Sex. Transm. Dis. 32:491-494, 2005). We utilized three types of microbicides to help define this aspect of our assay to test microbicides against C. trachomatis in vitro. To simulate conditions of transmission, we used an assay that we previously developed in which we exposed chlamydial elementary bodies to microbicides prior to contact with epithelial cells. We first determined the toxicity of microbicides to the cells used to culture Chlamydia trachomatis in the assay and, if necessary, modified the assay to eliminate toxicity at the concentrations tested. We compared the sensitivities of recent clinical isolates of Chlamydia trachomatis versus laboratory strains of the same serovar and found major differences in sensitivity to nonoxynol-9 (non-9), but only minor differences were seen with the other microbicides. We thus conclude that when assessing activity of potential topical microbicides versus the obligate intracellular bacteria C. trachomatis, the use of recent clinical isolates may not be necessary to draw a conclusion about a microbicide's effectiveness. However, it is important to keep in mind that differences (like those seen with non-9) are possible and that clinical isolates could be included in later stages of testing.
Topical microbicides are a promising method for the prevention of sexually transmitted diseases (STDs) in women who are not able to persuade their partners to use condoms. Interest in the antimicrobial capabilities of spermicides began with products containing nonoxynol-9 (non-9), a nonionic detergent that is the active ingredient in many existing spermicides. Non-9 has been shown to inhibit human immunodeficiency virus (12), Chlamydia trachomatis (2, 5, 14, 20), Neisseria gonorrhoeae (23), herpes simplex virus type 2 (3, 24), and Trichomonas vaginalis (22) both in vitro and in vivo. Its potential value as a microbicide is controversial, however, because results from testing have not been consistent and because it is toxic to cells used for in vitro testing (1, 7, 10, 13, 15). It can increase susceptibility to human papillomavirus in mice (21), and with frequent use, non-9 appears to actually increase a person's chance of contracting human immunodeficiency virus because of the epithelial lesions it causes (8, 26). For these reasons, attention has recently turned from surfactants (like non-9) to other compounds, such as lipids and peptides, as possible topical microbicide candidates (11, 16, 18, 19, 27, 28).
The ideal topical microbicide would prevent multiple STDs, but the experiments reported here have focused on C. trachomatis specifically because it is the most commonly reported STD in the United States. In 2006, more than one million new chlamydia infections were reported to the CDC, which is almost three times the number of new cases of Neisseria gonorrhoeae reported in the same year (6). Many chlamydia infections are asymptomatic and not reported but, if left untreated, can cause infertility, ectopic pregnancy, and pelvic inflammatory disease (6). Further, C. trachomatis is an obligate intracellular parasite with a unique biphasic developmental cycle. The infectious form, the elementary body (EB), is found in genital secretions and would be exposed to microbicides during transmission. Topical microbicides are intended to prevent infection, and thus, we previously developed the minimum -cidal concentration (MCC) assay, which focused on microbicide action against the extracellular EBs (16). This assay mimics what would happen in the human vagina during exposure to C. trachomatis, because EBs would come in contact with the microbicide before reaching the target cells. With whichever microbicide is being tested, careful consideration must be given to the design of the in vitro assay used in order to produce reliable results that are relevant to infection in humans.
It is difficult and time-consuming to grow high titers of C. trachomatis in the laboratory. Clinical isolates are particularly difficult to propagate because they have not yet adapted to growth in vitro, and most research facilities do not have easy access to patient samples. For this reason, most antichlamydial testing is done with strains that have been passaged for decades in the laboratory and have adapted to in vitro growth. Although convenient, we hypothesized that such laboratory-passaged strains may not be the most relevant models of clinical infection. Thus, in these experiments we tested whether recent clinical isolates of C. trachomatis had different sensitivities to microbicides than laboratory-adapted strains of the same serovars.
MATERIALS AND METHODS
Cell culture.
McCoy mouse fibroblasts (ATCC CRL 1696) were maintained in antibiotic-free Eagle's minimal essential medium supplemented with 10% fetal calf serum, 0.017 M glucose, 0.02 M HEPES, 0.084% sodium bicarbonate, and 2 mM l-glutamine (CMGH). The McCoy cells were tested once per month for mycoplasma contamination by PCR.
Inoculum.
Laboratory prototype strains of C. trachomatis serovars D (UW-3/Cx), E (UW-5/Cx), F (UW-6/Cx), J (UW-36/Cx), and L2 (434/Bu) and recent clinical isolates of the same serovars were purified from McCoy cells as previously described (4). Clinical strains used were provided by the Chlamydia clinical laboratory at the University of Washington, and they were passaged as few times as possible in order to obtain the high titers required for testing. We were able to achieve the appropriate titers for all clinical isolates in less than or equal to 17 passages in our laboratory. The laboratory strains have all been passaged hundreds or thousands of times over decades in the laboratory. Table 1 describes the number of passages for each isolate and against which compound(s) they were tested. Immediately before use, purified organisms were thawed and diluted to the appropriate concentrations in sucrose-phosphate-glutamate buffer (SPG). All purified chlamydial isolates were tested for mycoplasma contamination by PCR. The serotype of each isolate was confirmed with a plate typing method (25).
TABLE 1.
Designation, number of passages in the laboratory, and compounds tested against each C. trachomatis serovar used in the MCC assays
| Prototype or clinical strain of indicated serovar | Designation | No. of passagesa | Compounds tested |
|---|---|---|---|
| Prototype strain | |||
| D | UW-3/Cx | N/A | All compoundsb |
| E | UW-5/Cx | N/A | Non-9, peptides |
| F | UW-6/Cx | N/A | Non-9 |
| J | UW-36/Cx | N/A | Non-9, 1-OG, surgilube |
| L2 | 434/Bu | N/A | Non-9, peptides |
| Clinical strain | |||
| D | 9939 | 4 | Non-9, peptides, surgilube |
| 9427 | 13, 14 | Non-9, 1-OG | |
| E | 9424 | 5 | Non-9 |
| 89 | 17 | Non-9, peptides | |
| F | 9397 | 10 | Non-9 |
| J | 9379 | 14 | Non-9, 1-OG |
| 1178 | 17 | Non-9, surgilube | |
| L2 | 663 | 13, 16 | Non-9, peptides |
N/A, not applicable.
Includes non-9, peptides, surgilube, and 1-OG.
Microbicides.
Pure non-9 (no. N1217, lot no. PSO145; Spectrum) was donated to us by Lisa Rohan (Magee-Womens Research Institute, Pittsburgh, PA) and stored at room temperature. Novispirin G10 (G10) and SMAP-29 peptides were synthesized and provided to us by Robert Lehrer (UCLA David Geffen School of Medicine, Los Angeles, CA). All peptides were rehydrated in sterile water, aliquoted, and stored at −20°C until use. A 1-O-octyl-sn-glycerol (1-OG) lipid provided to us by Charles Isaacs was synthesized (NYS Institute for Basic Research, Staten Island, NY), reconstituted in 100% ethanol, and stored at 4°C until use. Surgilube, a sterile surgical lubricant gel containing 0.25% chlorhexidine, was purchased from E. Fougera & Co. (a division of Altana Inc., Melville, NY) and stored at room temperature until use.
Controls.
Penicillin G (61K1045; Sigma) and polymyxin B (P4932; Sigma) were used as negative and positive inhibition controls, respectively, in the MCC assay. An inoculum control, in which no drug was added, was included for each C. trachomatis strain at each time point to monitor normal inclusion formation. Percent inhibition of inclusion formation was calculated using this inoculum control. A cell control (no drug and no inoculum) was included in order to monitor McCoy cell morphology and possible cross-contamination. SPG and 10% (vol/vol) Triton X-100 (T8787; Sigma) were used as negative and positive toxicity controls, respectively, in the Alamar blue cytotoxicity assays (see below).
MCC assay.
We used our previously published preinoculation assay (16) to test the antichlamydial activity of the microbicides. On the day prior to performing the assay, 96-well tissue culture plates were seeded with 5 × 104 mycoplasma-free McCoy cells in 0.2 ml CMGH per well. Plates were incubated at 37°C in 5% CO2 overnight. On the day of the assay, between 5 and 10 twofold dilutions of each test compound (starting at a concentration of 50% [vol/vol] for surgilube, 200 μM for the G10 and SMAP-29 peptides, 50 mM for the 1-OG lipid, 1.44 mM for polymyxin B, and 5.37 mM for penicillin G) were made in SPG. The remainder of the assay was performed as previously published. At the conclusion of the assay, McCoy cell monolayers were fixed with methanol and stained with a primary antibody to C. trachomatis lipopolysaccharide, E6-H1 (provided by Harlan Caldwell). Secondary staining was done with an anti-mouse immunoglobulin G fluorescein isothiocyanate-conjugated antibody (F-9006; Sigma) diluted 1:250 in Evans blue counterstain (0.5% Evans blue, 5% sodium azide, 94.5% phosphate-buffered saline). Each concentration was plated in triplicate, three fields per well were counted, and the inclusion-forming unit (IFU) counts between triplicates were averaged. The percent inhibition of inclusion formation was calculated with the following formula: [(average IFU in the inoculum control − average IFU in the test)/(average IFU in the inoculum control)] × 100 = percent inhibition of C. trachomatis IFU formation. Assays were performed twice, on different days. The results of the two independent assays were averaged, and the standard deviations (SD) between the two assays at each concentration were calculated. Results were reported as the percent inhibition of C. trachomatis inclusion formation compared to that of a no-drug control. The MCC was defined as the lowest concentration of a test compound that completely inhibited C. trachomatis inclusion formation.
MCC assay adapted for non-9.
We slightly modified our previously published preinoculation assay (16) to adjust for the cytotoxicity of non-9 as follows. Plates (96-well) seeded with McCoy cells were set up as described in the previous section. On the day of the assay, 10 fourfold dilutions of non-9 (starting at a concentration of 2% [vol/vol]), polymyxin B (starting at a concentration of 1.44 mM), and penicillin G (starting at a concentration of 5.37 mM) were made in SPG. C. trachomatis inoculum was added to the samples in the same manner as before except at a higher concentration (1 × 107 IFU). After incubation at room temperature for 120 min, the reaction mixture was diluted 1:1,600 in SPG to effectively eliminate the non-9. The remainder of the assay was performed as described previously, except that 0.2 ml of the inoculum instead of 0.1 ml was added to each McCoy cell monolayer.
Alamar blue cytotoxicity assay.
Prior to the MCC assay being performed, we measured the cytotoxicity of the dilutions of all test microbicides to McCoy cells in the MCC assay. The MCC assay procedure, as described above, was followed except that no inoculum was added to the test compounds. The compounds were instead diluted by half with SPG to imitate the dilution when an inoculum was added. After cells were incubated at 37°C for 48 h, the CMGH in each well was replaced with fresh CMGH containing 10% Alamar blue reagent (BioSource, Inc.). Plates were incubated for an additional 4 hours, and cytotoxicity was determined spectrophotometrically according to the manufacturer's instructions.
RESULTS
Cytotoxicity of microbicides to McCoy cells as determined by the Alamar blue assay.
With our standard MCC assay, all compounds were exposed to EBs, and then compound-organism mixtures were diluted 1:40 in SPG before addition to McCoy cells, resulting in exposure of the cells to maximum concentrations of 0.625%, 2.5 μM, and 0.625 mM for surgilube, the peptides, and 1-OG, respectively. With our toxicity-adapted MCC assay, EBs were exposed to maximum non-9 concentrations of 1%, and non-9-organism mixtures were diluted 1:1,600 before addition to McCoy cells, resulting in exposure of the cells to a maximum non-9 concentration of 0.000625%. We tested whether any residual microbicide in the dilution was toxic to the McCoy cells used to culture viable organisms in this assay system. None of the concentrations of the microbicides were toxic to McCoy cells under our assay conditions. Any reduction of C. trachomatis inclusion formation was therefore due to the action of the tested microbicides on the chlamydial EBs, not the host cells.
MCC of microbicides against clinical isolates and laboratory prototype strains of C. trachomatis serovars D, E, F, J, and L2.
To determine whether laboratory strains of C. trachomatis had different sensitivities to treatment with surfactants (surgilube, non-9), peptides (G10, SMAP-29), and lipids (1-OG) than recently isolated clinical strains, we tested two or more of a variety of laboratory prototype serovars, D, E, F, J, and L2, and at least one clinical isolate from each serovar in parallel. The serovars chosen are representative of the three C. trachomatis serological groups (9). For surgilube and 1-OG, we tested clinical and laboratory strains of serovars J and D. For G10 and SMAP-29, we compared the sensitivities of clinical and laboratory strains of serovars D and E. We tested non-9 against clinical and laboratory strains of serovars D, E, F, J, and L2. Two different clinical isolates were included in the non-9 assays against serovars D, E, and J. In each assay, we included polymyxin B as an active control and penicillin G as a minimally active control. Both of these control compounds behaved as expected in all assays.
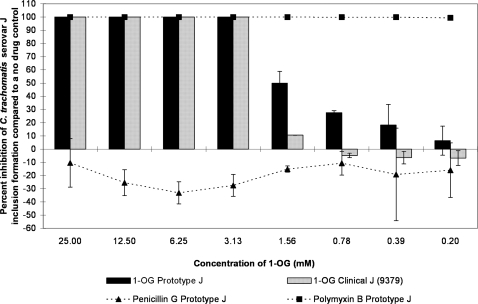
1-OG was 100% active for concentrations greater than or equal to 3.13 mM, and then activity rapidly dropped below 50% inhibition (Fig. 1). The MCC for 1-OG was statistically the same for clinical and prototype strains of serovars J and D (3.13 mM). However, at concentrations below 3.13 mM, there was a statistically significant difference between clinical and prototype activities for serovar J, with the prototype strain being more sensitive to the lipid than the clinical strain (Fig. 1). Prototype strain D was also significantly more sensitive than clinical isolate D to 1-OG at 1.56 mM (data not shown).
FIG. 1.
The MCC preinoculation assay was used to compare the activity of 1-OG with those of the laboratory prototype strain and clinical isolate 9379 of C. trachomatis serovar J after 120 min of exposure. Two dilutions of the negative and positive controls (penicillin G and polymyxin B, respectively) were also run against the prototype strain of serovar J. The highest test concentrations of penicillin G and polymyxin B were 2.69 mM and 0.72 mM, respectively. The percent inhibition of inclusion formation was calculated based on the number of inclusions in the no-drug control using the following formula: [(mean IFU no-drug control − mean IFU test)/mean IFU no-drug control] × 100. Each test was performed twice, on different days, and the reported results are the averages of those two tests. The SD of the results are indicated with error bars.
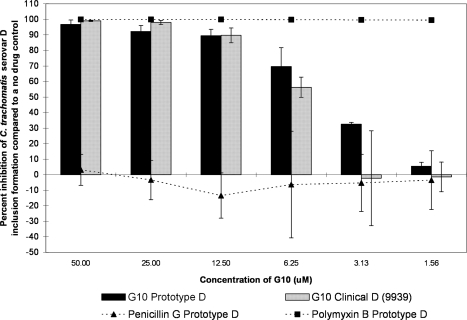
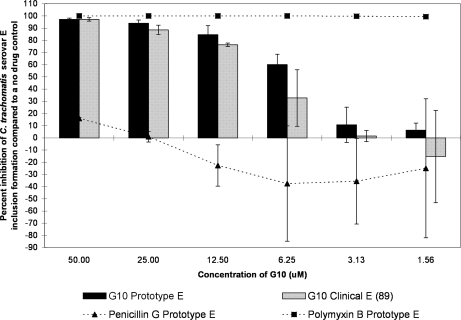
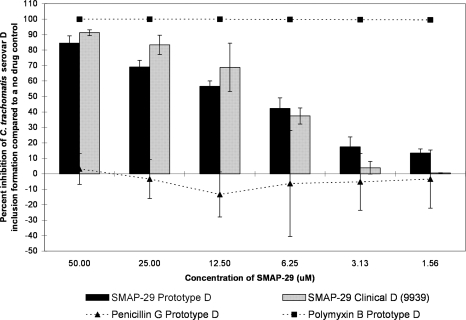
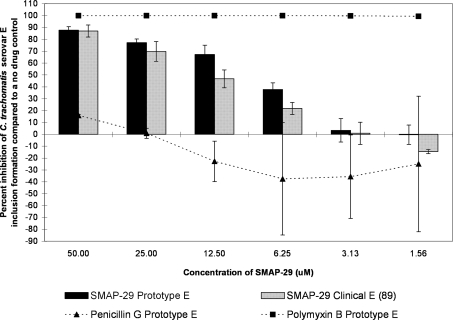
The G10 and SMAP-29 peptides each showed more of a classical dose response curve; activity was high for the higher test concentrations and then gradually fell off as the dilutions decreased in concentration (Fig. 2, 3, 4, and 5). G10 and SMAP-29 activity levels were similar against serovars D and E, but G10 was slightly more active. There were very few differences when comparing the sensitivities of clinical and prototype strains to both peptides.
FIG. 2.
The MCC preinoculation assay was used to compare the activity of the G10 peptide with those of the laboratory prototype strain and clinical isolate 9939 of C. trachomatis serovar D after 120 min of exposure. All assay details described in the legend to Fig. 1 were used.
FIG. 3.
The MCC preinoculation assay was used to compare the activity of the G10 peptide with those of the laboratory prototype strain and clinical isolate 89 of C. trachomatis serovar E after 120 min of exposure. All assay details described in the legend to Fig. 1 were used.
FIG. 4.
The MCC preinoculation assay was used to compare the activity of the SMAP-29 peptide with those of the laboratory prototype strain and clinical isolate 9939 of C. trachomatis serovar D after 120 min of exposure. All assay details described in the legend to Fig. 1 were used.
FIG. 5.
The MCC preinoculation assay was used to compare the activity of the SMAP-29 peptide with those of the laboratory prototype strain and clinical isolate 89 of C. trachomatis serovar E after 120 min of exposure. All assay details described in the legend to Fig. 1 were used.
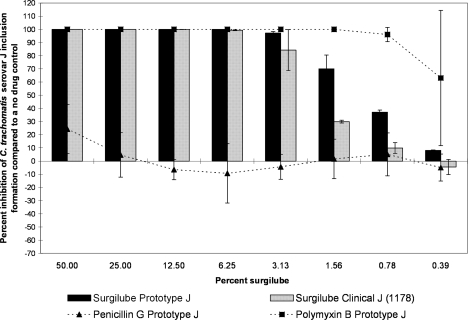
Surgilube was very active (>75% inhibition) against serovars D and J at concentrations of ≥3.13% after a 120-min exposure. The only differences between clinical and prototype isolates of the same serovar were seen with serovar J after exposure to 1.56%, 0.78%, and 0.39% surgilube, where the prototype isolate was statistically significantly more sensitive to treatment than the clinical isolate (Fig. 6).
FIG. 6.
The MCC preinoculation assay was used to compare the activity of surgilube with those of the laboratory prototype strain and clinical isolate 1178 of C. trachomatis serovar J after 120 min of exposure. All assay details described in the legend to Fig. 1 were used.
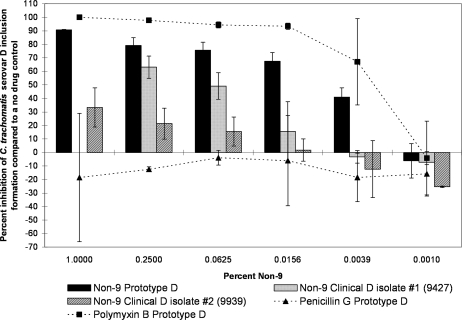
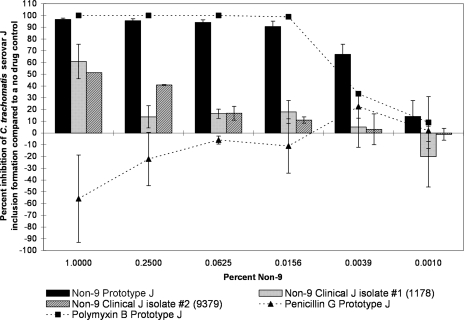
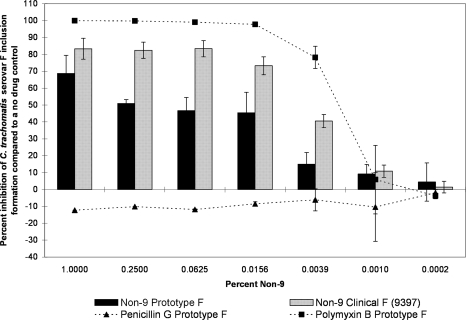
However, we observed marked differences between the clinical and prototype isolates of the same serovar in some but not all serovars when tested against another surfactant, non-9. For serovars D and J, the differences between clinical and prototype strains were large, highly significant, and present over a large range of concentrations, with the clinical strain showing much more resistance to the non-9 treatment (Fig. 7 and 8). For example, when exposed to 0.25% non-9, the serovar D laboratory prototype strain infectivity was reduced by 79.1% (SD, 5.8) compared to that of the no-drug control, and one recent clinical isolate of serovar D (designation 9427) was inhibited by 63.1% (SD, 8.2) (Fig. 7). However, a second clinical isolate (designation 9939) was only inhibited by 21.4% (SD, 11.4) when exposed to the same concentration of non-9. When exposed to 0.0625% non-9, C. trachomatis serovar J prototype strain infectivity was reduced by 94.2% (SD, 2.2), whereas a recently isolated clinical strain of the same serovar (designation 1178) was only reduced by 16.4% (SD, 3.9) (Fig. 8). A second clinical isolate of serovar J (designation 9379) was inhibited by 16.8% (SD, 5.9) when exposed to the same concentration of non-9. The clinical isolates were not always less sensitive to non-9 than the laboratory strains. Prototype F was found to be statistically significantly more resistant to the non-9 treatment than a clinical F strain at some concentrations, though the difference was not as large as those seen in serovars J and D (Fig. 9). The clinical and laboratory prototype strains of serovar L2 had no significant difference in sensitivity to non-9 (data not shown). The clinical and laboratory prototype strains of serovar E had minor differences in susceptibility at some of the lower concentrations, but in general, the activity was the same (data not shown).
FIG. 7.
The MCC preinoculation assay was used to compare the activity of non-9 with those of the laboratory prototype strain and clinical isolates 9427 and 9939 of C. trachomatis serovar D after 120 min of exposure. All assay details described in the legend to Fig. 1 were used. No data are available for clinical isolate 9427 against 1% non-9 due to partial cytotoxicity.
FIG. 8.
The MCC preinoculation assay was used to compare the activity of non-9 with those of the laboratory prototype strain and clinical isolates 1178 and 9379 of C. trachomatis serovar J after 120 min of exposure. All assay details described in the legend to Fig. 1 were used. There are no SD data available for isolate 9379 against 1% non-9 due to partial cytotoxicity in one of the experiments.
FIG. 9.
The MCC preinoculation assay was used to compare the activity of non-9 with those of the laboratory prototype strain and clinical isolate 9397 of C. trachomatis serovar F after 120 min of exposure. All assay details described in the legend to Fig. 1 were used.
DISCUSSION
It is challenging to test microbicidal activity against C. trachomatis in the laboratory because many compounds are toxic to the McCoy cells used to grow the test organisms. Since C. trachomatis is an obligate intracellular parasite, measuring the health of host cells is crucial in determining the number of surviving EBs, and test concentrations must thus be limited to those which will produce reliable data. With our MCC preinoculation assay, the compound is not required to enter the host cells, which makes it the ideal assay for testing compounds whose ultimate purpose will be to act on chlamydial EBs before they infect host cells. This assay is versatile and can be employed to test numerous chlamydial strains against multiple concentrations of a compound at any reasonable length of exposure.
Using our MCC assay, we found major differences in susceptibility to non-9 between C. trachomatis laboratory prototype strains and recent clinical isolates of the same serovar. We also found minor differences between prototype and clinical strains after exposure to some concentrations of another surfactant (surgilube), to two peptides, and to a lipid, although these differences were not as dramatic as those with non-9. The mode of action of a surfactant (like non-9) is on the lipid component of the outer membrane. Our results with non-9 suggest that the outer surface of prototype strains may be different from that of clinical isolates. The membrane components of prototype strains could have been altered during passages in the laboratory, resulting in a change in sensitivity to non-9. An alternative explanation is that the clinical isolates may have altered membrane components in order to survive repeated exposure to non-9 in the environment. The latter explanation may be more plausible because surgilube, another surfactant, did not affect clinical and prototype isolates in the same way. Surgilube has been used in the medical environment for a long time, but it is used as a sterile surgical lubricant, not as a method of birth control. This hypothesis is strengthened by the results of Moncla and Hillier (17), showing that N. gonorrhoeae and Lactobacillus laboratory and clinical strains have various susceptibilities to non-9, indicating the development of resistance (17).
For our experiments, when exposed to various concentrations of microbicidal compounds, recent clinical isolates of C. trachomatis did not have markedly different sensitivities than those of laboratory-adapted strains of the same serovar. The exception was non-9 (0.000004% to 1% [vol/vol]); when EBs were exposed for 120 min, recent clinical isolates of C. trachomatis often behaved significantly differently than laboratory strains of the same serovar. Though more-extensive testing is needed in order to draw a definitive conclusion, these results suggest that extensive testing of C. trachomatis clinical isolates may not be necessary when screening microbicides for antichlamydial activity, especially when testing those compounds which have not been present in the environment in another form (such as in a spermicide). However, microbicides that have shown promising activity could be tested against clinical isolates to fully characterize the potency of the microbicide.
Testing of clinical isolates could potentially be used as an additional step between in vitro screening with prototype strains and human or animal trials. Additional studies should be undertaken to determine whether clinical strains behave similarly when exposed to other microbicides. In summary, while it is true that some major differences were seen between the susceptibilities of some clinical and prototype isolates of C. trachomatis to non-9, major differences in susceptibility to other compounds have not yet been demonstrated. Testing topical microbicide candidates against clinical isolates as well as laboratory-adapted strains could lead to a better understanding of the range of activity of the compounds in vitro, but it may not always result in drawing a different conclusion about the overall usefulness of a compound.
Acknowledgments
We thank Robert Suchland for generously providing the recent clinical isolates used in this study. We also thank Robert Lehrer for providing us with the G10 and SMAP-29 peptides, Charles Isaacs for providing the 1-OG lipid, Lisa Rohan for providing the non-9, and Harlan Caldwell for providing the E6-H1 antichlamydial lipopolysaccharide antibody.
This work was supported in part by Public Health Service grant PO 1 AI 39061 from the National Institutes of Health.
Footnotes
Published ahead of print on 2 February 2009.
REFERENCES
- 1.Achiles, S. L., P. B. Shete, K. J. Whaley, T. R. Moench, and R. A. Cone. 2002. Microbicide efficacy and toxicity tests in a mouse model for vaginal transmission of Chlamydia trachomatis. Sex. Transm. Dis. 29:655-664. [DOI] [PubMed] [Google Scholar]
- 2.Amortegui, A. J., and M. P. Meyer. 1987. The in vitro effect of chemical intravaginal contraceptives on Chlamydia trachomatis. Contraception 36:481-487. [DOI] [PubMed] [Google Scholar]
- 3.Asculai, S. S., M. T. Weis, M. W. Rancourt, and A. B. Kupferberg. 1978. Inactivation of herpes simplex viruses by nonionic surfactants. Antimicrob. Agents Chemother. 13:686-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballweber, L. M., J. E. Jaynes, W. E. Stamm, and M. F. Lampe. 2002. In vitro microbicidal activities of cecropin peptides D2A21 and D4E1 and gel formulations containing 0.1 to 2% D2A21 against Chlamydia trachomatis. Antimicrob. Agents Chemother. 46:34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benes, S., and W. M. McCormack. 1985. Inhibition of growth of Chlamydia trachomatis by nonoxynol-9 in vitro. Antimicrob. Agents Chemother. 27:724-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. 2007. Sexually transmitted disease surveillance, 2006. U.S. Department of Health and Human Services, Atlanta, GA.
- 7.Cone, R. A., T. Hoen, X. Wong, R. Abusuwwa, D. J. Anderson, and T. R. Moench. 2006. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect. Dis. 6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fichorova, R. N., L. D. Tucker, and D. J. Anderson. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 184:418-428. [DOI] [PubMed] [Google Scholar]
- 9.Grayston, J. T., and S. Wang. 1975. New knowledge of chlamydiae and the diseases they cause. J. Infect. Dis. 132:87-105. [DOI] [PubMed] [Google Scholar]
- 10.Herold, B. C., A. Siston, J. Bremer, R. Kirkpatrick, G. Wilbanks, P. Fugedi, C. Peto, and M. D. Cooper. 1997. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob. Agents Chemother. 41:2776-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaacs, C. E., L. Rohan, W. Xu, J. H. Jia, T. Mietzner, and S. L. Hillier. 2006. Inactivation of herpes simplex virus clinical isolates by using a combination microbicide. Antimicrob. Agents Chemother. 50:1063-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennings, R., and A. Clegg. 1993. The inhibitory effect of spermicidal agents on replication of HSV-2 and HIV-1 in-vitro. J. Antimicrob. Chemother. 32:71-82. [DOI] [PubMed] [Google Scholar]
- 13.Kappus, E. W., and T. C. Quinn. 1986. The spermicide nonoxynol-9 does not inhibit Chlamydia trachomatis in vitro. Sex. Transm. Dis. 13:134-137. [DOI] [PubMed] [Google Scholar]
- 14.Kelly, J. P., R. B. Reynolds, S. Stagno, W. C. Louv, and W. J. Alexander. 1985. In vitro activity of the spermicide nonoxynol-9 against Chlamydia trachomatis. Antimicrob. Agents Chemother. 27:760-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight, S. T., S. H. Lee, C. H. Davis, D. R. Moorman, R. L. Hodinka, and P. B. Wyrick. 1987. In vitro activity of nonoxynol-9 on McCoy cells infected with Chlamydia trachomatis. Sex. Transm. Dis. 14:165-173. [DOI] [PubMed] [Google Scholar]
- 16.Lampe, M. F., L. M. Ballweber, C. E. Isaacs, D. L. Patton, and W. E. Stamm. 1998. Killing of Chlamydia trachomatis by novel antimicrobial lipids adapted from compounds in human breast milk. Antimicrob. Agents Chemother. 42:1239-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moncla, B. J., and S. L. Hillier. 2005. Why nonoxynol-9 may have failed to prevent acquisition of Neisseria gonorrhoeae in clinical trials. Sex. Transm. Dis. 32:491-494. [DOI] [PubMed] [Google Scholar]
- 18.Moncla, B. J., K. Pryke, and C. E. Isaacs. 2008. Killing of Neisseria gonorrhoeae, Streptococcus agalactiae (group B streptococcus), Haemophilus decreyi, and vaginal Lactobacillus by 3-O-octyl-sn-glycerol. Antimicrob. Agents Chemother. 52:1577-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori, T., B. R. O'Keefe, R. C. Sowder, S. Bringans, R. Gardella, S. Berg, P. Cochran, J. A. Turpin, R. W. J. Buckheit, J. B. McMahon, and M. R. Boyd. 2005. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 280:9345-9353. [DOI] [PubMed] [Google Scholar]
- 20.Patton, D. L., S.-K. Wang, and C.-C. Kuo. 1992. In vitro activity of nonoxynol 9 on HeLa 229 cells and primary monkey cervical epithelial cells infected with Chlamydia trachomatis. Antimicrob. Agents Chemother. 36:1478-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts, J. N., C. B. Buck, C. D. Thompson, R. Kines, M. Bernardo, P. L. Choyke, D. R. Lowy, and J. T. Schiller. 2007. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 13:857-861. [DOI] [PubMed] [Google Scholar]
- 22.Singh, B., J. C. Cutler, and H. M. Utidjian. 1972. Studies on development of a vaginal preparation providing both prophylaxis against venereal disease, other genital infections and contraception. III. In vitro effect of vaginal contraceptive and selected vaginal preparations of Candida albicans and Trichomonas vaginalis. Contraception 5:401-411. [DOI] [PubMed] [Google Scholar]
- 23.Singh, B., J. C. Cutler, and H. M. Utidjian. 1972. Studies on the development of a vaginal preparation providing both prophylaxis against venereal disease and other genital infections and contraception. II. Effect in vitro of vaginal contraceptive and non-contraceptive preparations on Treponema pallidum and Neisseria gonorrhoeae. Br. J. Vener. Dis. 48:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh, B., B. Posti, and J. C. Cutler. 1976. Virucidal effect of certain chemical contraceptives on type 2 herpesvirus. Am. J. Obstet. Gynecol. 126:422-425. [DOI] [PubMed] [Google Scholar]
- 25.Suchland, R. J., and W. E. Stamm. 1991. Simplified microtiter cell culture method for rapid immunotyping of Chlamydia trachomatis. J. Clin. Microbiol. 29:1333-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, H. Rees, P. Sirivongrangson, L. Mukenge-Tshibaka, V. Ettiegne-Traore, C. Uaheowitchai, S. S. Karim, B. Masse, J. Perriens, and M. Laga. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomized controlled trial. Lancet 360:971-977. [DOI] [PubMed] [Google Scholar]
- 27.Yasin, B., M. Pang, and E. A. Wagar. 2004. A cumulative experience examining the effect of natural and synthetic antimicrobial peptides vs. Chlamydia trachomatis. J. Pept. Res. 64:65-71. [DOI] [PubMed] [Google Scholar]
- 28.Yasin, B., M. Pang, E. A. Wagar, and R. I. Lehrer. 2002. Examination of Chlamydia trachomatis infection in environments mimicking normal and abnormal vaginal pH. Sex. Transm. Dis. 29:514-519. [DOI] [PubMed] [Google Scholar]