Abstract
Topical microbicides are being developed as a preventative approach to reduce the sexual transmission of human immunodeficiency virus type 1 (HIV-1) and other infections. For them to be efficacious, it is believed that they should avoid inducing inflammation while allowing the vaginal epithelium to initiate protective Toll-like receptor (TLR)-mediated innate responses against pathogens. In this study, human cervical and vaginal epithelial cells were exposed to polyanionic HIV entry inhibitors and the following synthetic TLR ligands: (i) the bacterial lipoprotein Pam3CSK4, binding cell surface TLR1/TLR2; (ii) macrophage activating lipopeptide 2 (MALP-2), binding cell surface TLR2/TLR6; and (iii) the viral double-stranded RNA analog poly(I:C), recognized by intracellular TLR3. Cell activation was assessed by nuclear factor κB (NF-κB) reporter gene transactivation and cytokine production. In spite of enhancing TLR-triggered NF-κB activation, the polyanionic microbicide compounds dextran sulfate and polystyrene sulfonate significantly inhibited TLR-mediated cytokine production. They decreased cytokine mRNA and protein levels of proinflammatory (interleukin-8 [IL-8] and IL-1β) and antiviral (beta interferon) cytokines following epithelial cell stimulation with Pam3CSK4, MALP-2, or poly(I:C). These activities were associated with the sulfate/sulfonate moieties of the polyanionic compounds, since the unsulfated dextran control did not show any effects. Our data demonstrate that these microbicide compounds are capable of selectively interfering with TLR-mediated epithelial responses at different points in their signaling pathways and underscore the importance of expanding the assessment of microbicide compatibility with vaginal innate immune function. Further studies are warranted to determine the impact of this interference on HIV-1 transmission risk.
Topical microbicides are a promising strategy for reducing the sexual transmission of human immunodeficiency virus type 1 (HIV-1) and other genital tract infections (16, 42). In order to be effective, these products must support the natural barrier function of the genital tract mucosa. Topical agents with toxic and proinflammatory properties have been associated with an increased risk of HIV-1 transmission due to epithelial erosion, release of inflammatory cytokines and chemokines, and activation of HIV-1 host immune cells (2, 27, 52). A number of recent studies have focused on the importance of selecting microbicidal agents that lack toxic and proinflammatory potential (20, 22, 36). However, topical agents should also be assessed for interference with the protective innate immunoinflammatory responses against other sexually transmitted infections (STIs). It is important that such compounds do not impair the ability of the genital mucosa to mount protective responses against bacterial and viral infections, since women at high risk for HIV-1 infection are also exposed to other STIs. Furthermore, susceptibility to HIV-1 transmission is increased in the presence of other STIs (53, 69), and bacterial determinants, e.g., lipopolysaccharide and lipoproteins, may drive HIV-1 expression via interaction with Toll-like receptors (TLRs) on host cells (5, 18). It is therefore essential to investigate the potential impact of vaginal microbicides and spermicides on these immunomodulatory events.
Epithelial cells sense microbial pathogens through different TLRs that recognize common bacterial, viral, fungal, and protozoan determinants and trigger innate host defense responses that influence adaptive immunity (63). As a result of TLR stimulation, followed by activation of multiple intracellular signaling pathways, primarily the nuclear factor κB (NF-κB) pathway, the production of proinflammatory cytokines and chemokines is induced and a protective inflammatory reaction is initiated (29, 56). Among the 10 TLR members identified in human cells so far, most are expressed by human cervical and vaginal epithelial cells (3, 19, 23). Under sterile in vitro conditions, TLR4 and the coreceptors MD2 and CD14 are undetectable in cervical and vaginal epithelial cells, consistent with their unresponsiveness to lipopolysaccharide (23), although TLR4 expression has been reported for female reproductive tract epithelial tissues (19, 35, 51), suggesting that it may be inducible in vivo. In the vaginal epithelium, TLR1, TLR2, and TLR6 are critical for the recognition of gram-positive and -negative bacterial or mycoplasmal determinants (23, 29). TLR2 forms heterodimers with TLR1 or TLR6, initiating responses to bacterial lipoproteins and peptidoglycans and leading to NF-κB activation and subsequent production of a myriad of cytokines (62, 65). Antiviral responses are mediated by TLR3, TLR7, and TLR9 (1, 8, 34). TLR3 recognizes viral double-stranded RNA and is essential for the production of type I interferons (IFNs), which are the first line of defense against viral pathogens (1). Therefore, impairment of TLR1/2-, TLR2/6-, or TLR3-mediated responses may lead to increased susceptibility to bacterial or viral infection.
This study assessed the effects of the anti-HIV microbicides dextran sulfate (DxS) and polystyrene sulfonate (PSS) on TLR signaling, using unsulfated dextran (Dx) as an inactive control. These two microbicides belong to the class of polyanionic HIV entry inhibitors (17, 49). The clinical effectiveness evaluation of cellulose sulfate (CS), the leading candidate in this chemical class of microbicides, was recently stopped due to a trend toward increased susceptibility to infection (68). Since CS and other members of the same chemical class were previously found to be safe in animal models and numerous phase I trials (7), a plausible explanation for the failure of the phase III trial would be a previously unidentified alteration of the mucosal immune barrier (15). To test this hypothesis, we challenged cervical and vaginal epithelial cells with TLR1/2, TLR2/6, and TLR3 ligands in the presence or absence of two prototype anionic polymers previously in development as microbicides and evaluated antibacterial and antiviral innate immune responses by assessing NF-κB activation and cytokine production and secretion.
MATERIALS AND METHODS
Test agents.
DxS and Dx were purchased from Sigma, St. Louis, MO, and PSS was purchased from Aldrich, Milwaukee, WI. The synthetic bacterial lipoprotein Pam3CSK4 (InvivoGen, San Diego, CA) was used as a TLR1/TLR2 ligand (46, 50). The synthetic analog of Mycoplasma fermentans lipopeptide macrophage activating lipopeptide 2 (MALP-2) (Alexis Biologicals, San Diego, CA) was used as a TLR2/TLR6 agonist (46, 50). Poly(I:C) (InvivoGen, San Diego, CA) is a synthetic analog of viral double-stranded RNA, which is produced during the replication cycle of many viral pathogens, and was used as a TLR3 agonist (1, 30). Endotoxin contamination of each reagent and compound was ruled out using the Endosafe system (Charles River Laboratories, Charleston, SC), based on a Limulus amebocyte lysate test (39) with a sensitivity of 0.05 endotoxin units/ml.
Cell culture and treatment.
Previously established immortalized endocervical (End1/E6E7), ectocervical (Ect1/E6E7), and vaginal (Vk2/E6E7) epithelial cell lines were grown in keratinocyte serum-free medium (Invitrogen, Carlsbad, CA) as described previously (24). The immortalized cell lines resemble their healthy tissues of origin (21, 24) and have the same TLR expression profiles and responses as primary epithelial cells in culture (23, 29). In the last 10 years, these cell lines have been used as a model of vaginal epithelial immune function that has been validated by comparisons with primary cell cultures, tissues, animal models, and clinical findings (12, 22, 26-28, 38, 60, 61). Cells were seeded at 104 cells/well in 96-well tissue culture plates (Fisher Scientific, Hampton, NH). Confluent epithelial cell monolayers were treated for 24 h with a broad twofold dose range of all test compounds and TLR ligands. Nontoxic, biologically active doses of TLR ligands, e.g., 5 μg/ml Pam3CSK4, 100 nM MALP-2, and 25 μg/ml poly(I:C), were selected for stimulation in the presence of microbicidal compounds. In some experiments, cells were pretreated with TLR ligands or compounds for 30 min, washed twice with phosphate-buffered saline (PBS) (Invitrogen), and then treated for an additional 6 h with compounds or TLR ligands, respectively. At the end of each incubation period, the culture supernatants were collected for measurement of secreted cytokines and cell lysates were prepared for assessment of cell-bound cytokines. Key experiments were repeated with VEC-100 tissue derived from primary ectocervical epithelial cells (Mattek Corporation, Ashland, MA) as described previously (67).
Cell viability test.
The CellTiter 96 3-(4,5-dimethylthiazol-2,5-diphenyltetrazolium bromide (MTT) assay (Promega, Madison, WI) was used following the manufacturer 's recommendations. In this assay, viable cells convert the MTT into a blue formazan product. The colorimetric reaction is measured by determining the absorbance at 570 nm, with a reference wavelength of 630 nm. Absorbance was read using a Victor2 1420 multilabel microplate counter with Wallac 2.01 software (Perkin-Elmer Life Sciences, Boston, MA). Tissue viability is presented as a percentage of the average optical density measured for untreated (medium alone) control cells.
Cytokine assays.
Concentrations of interleukin-8 (IL-8), IL-1β, and IFN-β in cell culture supernatants and cell lysates were measured with a Sector Imager 2400 instrument from Meso Scale Discovery (MSD), Gaithersburg, MD, in electrochemiluminescence (ECL) cytokine assays. For measurement of intracellular cytokines, epithelial cells were washed with ice-cold PBS (Invitrogen) and lysed in 0.5% Triton X-100 (Sigma, St. Louis, MO) in PBS. The lysates were cleared of cellular debris by centrifugation at 10,000 × g at 4°C for 10 min and then kept at −80°C. Compound interference with cytokine detection in the ECL assays was ruled out by spiking known amounts of recombinant cytokine standards (MSD) into compound solutions prepared in cell culture medium and by measuring the percent cytokine recovery from compound-supplemented medium versus that from a plain medium control as described previously (20, 25).
Gene expression analysis.
To determine if the polyanionic compounds affected stimulated cytokine production at the mRNA transcription level, we used a multiplex quantitative nuclease protection assay (qNPA) by High Throughput Genomics (HTG), Tucson, AZ. The qNPA is a chemiluminescence assay simultaneously measuring the expression levels of multiple target genes, including housekeeping genes, in the same total cellular RNA mixture, thus allowing normalization and quantitation similar to those by routine real-time reverse transcription-PCR. The qNPA has previously been validated by comparison to real-time PCR (41, 54, 55). In contrast to real-time PCR, the qNPA does not require RNA isolation, reverse transcription to cDNA, and amplification. It is based on hybridization of a target mRNA sequence to small single-stranded antisense probes so that only mRNA specific for the gene of interest is protected from subsequent nuclease digestion. The reaction is highly specific, and even a single nucleotide mismatch between the target RNA and the antisense probe would cause nuclease digestion (66). In our study, epithelial cells were grown to confluence in 12-well plates, stimulated for 2 h, and lysed directly in the culture dish, using lysis buffer provided by HTG. The qNPA was further performed as described previously. In brief, after incubation of cell lysates with gene-specific antisense probes at 60°C, excess probes and unhybridized RNA were degraded using S1 nuclease solution (Promega, Madison, WI). A plant gene served as a control for the S1 nuclease. Following neutralization, the solutions were hybridized with HTG ArrayPlates and exposed consecutively to detection linker, enzyme conjugate, and chemiluminescent peroxidase substrate (Atto-PSTM; Lumigen, Southfield, MI). The signal was read by an Omix imager (HTG), and the readings were analyzed using VueScript software (HTG) to determine the expression level of each gene. Gene intensities were normalized to those of two housekeeping genes (glyceraldehyde-3-phosphate dehydrogenase and β-actin) by use of the following equation: normalized gene intensity = [(gene value)/(sum of two housekeeping gene intensities)] × 2,000.
NF-κB luciferase assay.
Endocervical epithelial cells seeded at 1 × 104 cells/well in 96-well plates were transfected with pHTS-NF-κB firefly luciferase reporter vector, which contains binding sites for NF-κB (Biomyx Technology, San Diego, CA), using a gene juice transfection protocol as described previously (26). Cells were treated for 6 h or 24 h as described above, washed with PBS to remove residual medium and compound, and lysed in Glo Lysis buffer per the manufacturer's instructions (Promega, Madison, WI). Luciferase activity was measured in the cell lysates by using the Bright-Glo luciferase assay system (Promega). Luminescence was measured in relative units using a Victor2 1420 multilabel microplate counter with Wallac 2.01 software (Perkin-Elmer Life Sciences, Boston, MA).
Statistical analysis.
One-way analysis of variance (ANOVA) with Dunnett's posttest and a two-tailed t test was performed using GraphPad Prism, version 4.00, for Windows (GraphPad Software, San Diego, CA). P values of <0.05 were considered significant.
RESULTS
Polyanions suppress TLR-triggered cytokine production and release.
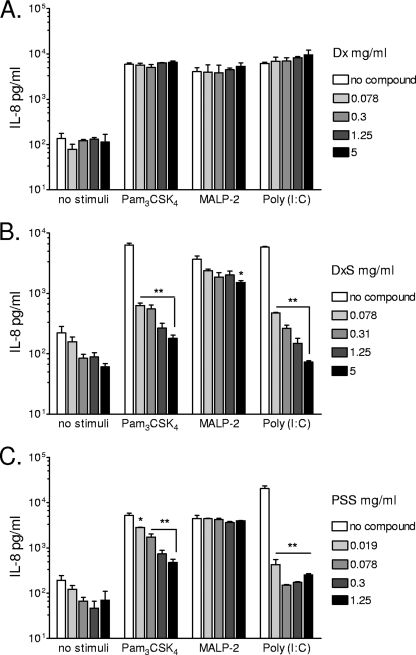
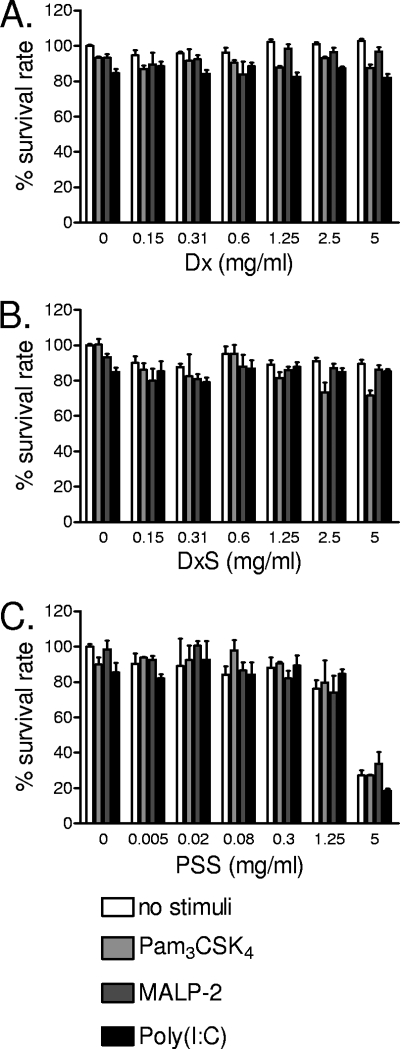
First, the effects of polyanionic microbicidal compounds on TLR-triggered signaling were assessed by measuring the extracellular and intracellular levels of IL-8 accumulated after 24 h of stimulation with TLR ligands. Extracellular levels were measured in cell culture supernatants, and intracellular levels were measured in cell lysates. IL-8 was chosen as the principal end point due to its established involvement in vaginal inflammation (22, 33). Figure 1 illustrates typical responses observed in immortalized endocervical epithelial cells stimulated with TLR ligands in the presence or absence of polyanions in a nontoxic dose range determined by MTT assay (Fig. 2). Unlike nonsulfated Dx (Fig. 1A), DxS (Fig. 1B) significantly (P < 0.01) inhibited, by a log magnitude, the extracellular IL-8 release in response to Pam3CSK4 and poly(I:C), in a dose-dependent manner. Within the 24-h stimulation period, DxS had a weaker effect on MALP-2-induced IL-8 release, which reached statistical significance (P < 0.05) only at the highest dose tested (5 mg/ml). Due to its higher cytotoxicity, PSS was tested at a lower dose range, starting at 1.25 mg/ml. Similar to the same doses of DxS, the tested PSS dose range (Fig. 1C) suppressed the IL-8 response to Pam3CSK4 (P < 0.01) and poly(I:C) (P < 0.01), by a log magnitude, and it had no significant effect on MALP-2-induced IL-8 (P > 0.05). The observed decrease in IL-8 concentration was not due to cytotoxicity, since none of the compounds and TLR ligands or their combinations were toxic in the immunosuppressive dose range of each compound (Fig. 2). Known concentrations of IL-8 spikes were fully recovered from compound solutions by the MSD ECL immunoassay (data not shown); thus, the observed changes in IL-8 responses were not due to assay interferences.
FIG. 1.
IL-8 levels measured by a MesoScale ECL assay in cell culture supernatants after 24 h of stimulation of endocervical epithelial cells with Pam3CSK4 (5 μg/ml), MALP-2 (100 nM), or poly(I:C) (25 μg/ml) in the presence of various doses of Dx (A), DxS (B), or PSS (C). Values represent means and standard errors of the means (SEM) for duplicate cultures from one of four experiments. *, P < 0.05; **, P < 0.01 (for comparison to medium-only [no compound] control by ANOVA with Dunnett's posttest).
FIG. 2.
Cell viability determined by MTT proliferation assay upon 24 h of treatment with broad dose ranges of Dx (A), DxS (B), and PSS (C) in the presence or absence of Pam3CSK4 (5 μg/ml), MALP-2 (100 nM), or poly(I:C) (25 μg/ml). Bars represent means and SEM for duplicate cultures from three experiments with the three epithelial cell lines (vaginal, endocervical, and ectocervical).
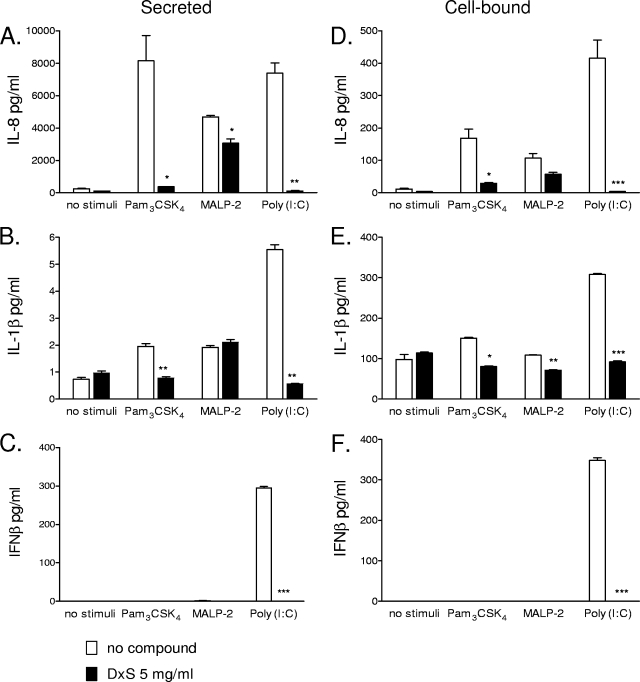
To determine whether the observed suppressive effects were specific for IL-8, which is released from cells as it is produced via a classical secretory pathway, we also measured levels of other cytokines, such as IL-1β, a major proinflammatory mediator with a nonclassical secretory pathway (4), and IFN-β, which also uses a classical secretory pathway (48) but is critical for protective responses to viral infections (9). Similar to IL-8 levels (Fig. 3A), IL-1β levels were reduced in the presence of DxS in response to Pam3CSK4 and poly(I:C) (P < 0.01) but not MALP-2 (Fig. 3B). Poly(I:C) induced significant IFN-β production, which was completely suppressed (P < 0.001) in the presence of DxS (Fig. 3C). As expected, MALP-2 and Pam3CSK4 did not induce IFN-β, which is a cytokine specific for TLR3 responses.
FIG. 3.
IL-8, IL-1β, and IFN-β levels measured by a MesoScale ECL assay in cell culture supernatants (A, B, and C) and cell lysates (D, E, and F) after 24 h of stimulation with Pam3CSK4 (5 μg/ml), MALP-2 (100 nM), or poly(I:C) (25 μg/ml) in the presence of DxS (5 mg/ml) or plain medium. Values represent means and SEM for duplicate measurements in one of two experiments with the endocervical epithelial cell line. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (for comparison to medium-only [no compound] control by two-tailed t test).
To determine if decreased cytokine production or impaired transmembrane cytokine transport was responsible for the observed compound-induced inhibition, cell-bound cytokine levels were measured in cell lysates in parallel with extracellular levels in cell-free culture supernatants. Simultaneously with reducing the extracellular levels of IL-8 (Fig. 3A), DxS also decreased levels of cell-associated IL-8 induced by Pam3CSK4 (P < 0.05) and poly(I:C) (P < 0.001) (Fig. 3D). The effect on MALP-2-induced IL-8 was not statistically significant. Cell-bound IL-1β was decreased by DxS regardless of which TLR pathway was stimulated (Fig. 3E). These data suggest that the suppressive mechanism was not limited to a specific cytokine secretion pathway and that it also involved cytokine production. DxS decreased the cell-bound levels of all cytokines triggered by poly(I:C) (P < 0.001) (Fig. 3D to F). These effects lasted for at least 6 h after compound washout (data not shown).
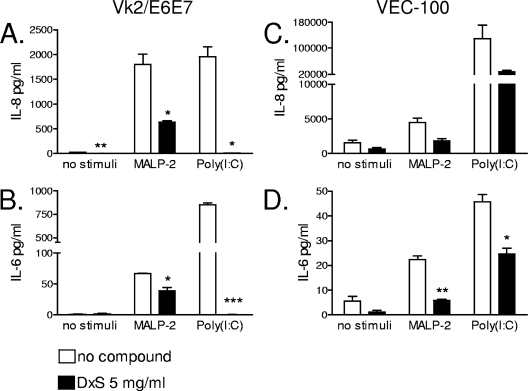
A side-by-side comparison between squamous immortalized and nontransformed epithelial cell cultures was performed to determine if the observed immunosuppressive phenomenon could be attributed to either cell type or immortalization status. For these experiments, Vk2/E6E7 monolayers and organotypic VEC-100 cultures were stimulated with TLR ligands in the presence or absence of 5 mg/ml DxS. IL-8 and IL-6 levels were measured in the Vk supernatants and in the basolateral (bottom chamber) compartment of the VEC-100 tissue. In the organotypic model, DxS was applied apically to mimic mucosal compound exposure. MALP-2 was also applied apically to mimic luminal bacterial exposure. Poly(I:C) was applied to the basolateral compartment to allow sufficient intracellular uptake independent of the physical presence of compound. Similar to results obtained with the simple columnar endocervical epithelial cell type (Fig. 1 and 3), DxS significantly suppressed the level of IL-8 secreted in response to MALP-2 (P < 0.05) and poly(I:C) (P < 0.05) in vaginal cells, representing the squamous nonkeratinized epithelial cell type, suggesting that the phenomenon was independent of cell type (Fig. 4A). IL-6, which is another in vivo validated marker of inflammatory responses in the cervicovaginal mucosa (22), was also significantly suppressed by DxS (Fig. 4B). A decrease in IL-8 and IL-6 in the presence of DxS was consistently observed in all four tested VEC-100 tissues, although the effect was statistically confirmed only for IL-6, possibly due to the small sample size, which was limited by the high cost of the organotypic model (Fig. 4C and D). These data demonstrate that the observed immunosuppressive effects were sustainable in a physiologic organotypic context and could not be attributed to the immortalization status of the vaginal and cervical epithelial cell lines.
FIG. 4.
IL-8 (A and C) and IL-6 (B and D) levels measured by a MesoScale ECL assay in cell culture supernatants from vaginal epithelial cells (Vk2/E6E7) (A and B) and VEC-100 tissue (C and D) after 24 h of stimulation with MALP-2 (100 nM) or poly(I:C) (25 μg/ml) in the presence of DxS (5 mg/ml) or plain medium. Values represent means and SEM for duplicate measurements in one of two experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (for comparison to medium-only [no compound] control by two-tailed t test).
Polyanions suppress TLR-triggered cytokine production at the mRNA level.
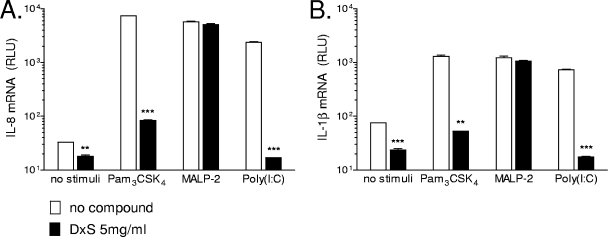
To determine whether the effects of the polyanionic compounds on TLR-induced cytokine gene expression were upstream or downstream of transcription, we measured the levels of IL-8 and IL-1β mRNAs by using a qNPA with vaginal epithelial cells stimulated for 2 hours with TLR ligands in the presence or absence of DxS. Within this time, DxS suppressed the mRNA expression of IL-1β and IL-8 in response to Pam3CSK4 and poly(I:C) (P < 0.01 and P < 0.001, respectively) and had no statistically significant effect on MALP-2-induced gene expression (Fig. 5). These data were consistent with the reduced protein levels of both cytokines observed at later time points (Fig. 1 and 3).
FIG. 5.
qNPA. IL-1β (Α) and IL-8 (B) mRNA levels were assessed in vaginal epithelial cells after 2 h of stimulation with TLR ligands in the presence or absence of 5 mg/ml DxS. The values were normalized to the levels of expression of two housekeeping genes (glyceraldehyde-3-phosphate dehydrogenase and β-actin) in each cellular extract as described in Materials and Methods. RLU, relative light units. Data are means and SEM for duplicate measurements representing two biological replicates. **, P < 0.01; ***, P < 0.001 (for comparison to medium-only [no compound] control by two-tailed t test).
Polyanions have different effects on NF-κB activation depending on the stimulated TLR pathway.
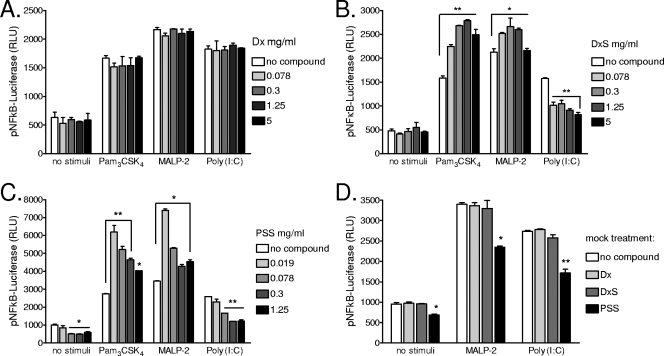
To determine whether the inhibitory effects of polyanions on TLR-stimulated cytokine/chemokine responses were exerted at the level of NF-κB, cells were transfected with an NF-κB reporter vector and NF-κB activity was measured by a luciferase reporter assay. Epithelial cells were stimulated with TLR ligands in the presence of DxS, PSS, or Dx for 6 and 24 hours. None of the test compounds induced significant NF-κB activation on its own (Fig. 6). After a short, 6-h stimulation in the presence of compound, DxS had a weak suppressive effect on Pam3CSK4 and poly(I:C) and no effect on MALP-2-triggered NF-κB activation, while PSS had no significant effect on TLR signaling (data not shown). After a 24-h stimulation, however, both DxS and PSS enhanced the NF-κB response to Pam3CSK4 (P < 0.01) and MALP-2 (P < 0.05) and suppressed the NF-κB response to poly(I:C) (P < 0.01) (Fig. 6).
FIG. 6.
NF-κB activity measured by firefly luciferase reporter assay after 24 h of stimulation of endocervical epithelial cells with Pam3CSK4 (5 μg/ml), MALP-2 (100 nM), or poly(I:C) (25 μg/ml) in the presence of various doses of Dx (A), DxS (B), or PSS (C). (D) Mock compound treatment was performed by adding the highest nontoxic dose of each compound (5 mg/ml of DxS and Dx or 1.25 mg/ml of PSS) immediately prior to lysis of TLR-stimulated cells for luciferase determination. RLU, relative light units. Values represent means and SEM for duplicate cultures in one of four experiments. *, P < 0.05; **, P < 0.01 (for comparison to medium-only [no compound] control by ANOVA with Dunnett's posttest).
To determine whether any of the compounds interfered with the luciferase assay, we performed a mock treatment in which compounds were added at the end of the TLR stimulation period, immediately prior to washing and cell lysis for luciferase activity assessment (Fig. 6D). Dx and DxS had no significant effect, while at the highest dose tested PSS reduced the luciferase assay readings ∼30%. Thus, the enhancement of the Pam3CSK4- and MALP-2-triggered NF-κB activity observed in the presence of DxS and PSS, as well as the significantly lower activity measured in response to poly(I:C) in the presence of all polyanionic compounds, was not due to compound interference with the luciferase assay. Endotoxin levels in DxS, Dx, and PSS preparations were undetectable (<0.05 endotoxin unit/ml), and thus bacterial contamination could not explain the NF-κB enhancement effects.
Polyanions exert their inhibitory effects downstream from receptor-ligand binding.
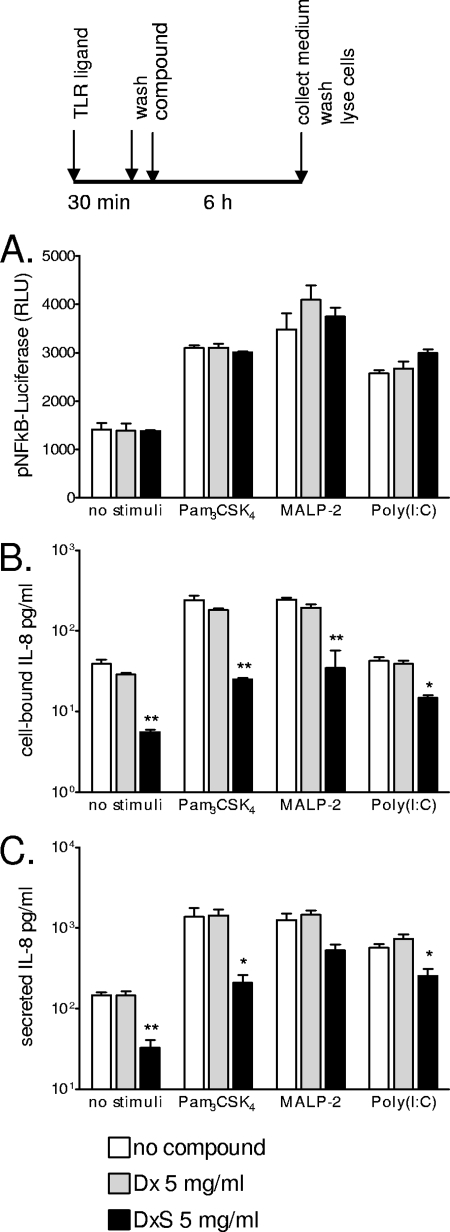
To determine whether the polyanions competed with TLR ligands at the receptor level or their effects occurred downstream from membrane receptor-ligand binding, we incubated epithelial cells with TLR agonists first, washed them off, and then added DxS, Dx, or plain medium for 6 h (Fig. 7). Preincubation of cells with Pam3CSK4, MALP-2, or poly(I:C) ligands, which ensured TLR-ligand binding in the absence of compound, resulted in NF-κB activation (Fig. 7A) but did not prevent the DxS-mediated reduction in both cell-bound and secreted IL-8 levels (Fig. 7B and C). These results indicate that cytokine inhibition involves cellular events that are NF-κB independent and/or occur downstream from NF-κB gene transactivation.
FIG. 7.
Immune responses measured in endocervical epithelial cells stimulated for 30 min with Pam3CSK4 (5 μg/ml), MALP-2 (100 nM), or poly(I:C) (25 μg/ml), followed by washing and 6 h of incubation with DxS (5 mg/ml), Dx (5 mg/ml), or plain medium. (A) NF-κB activation, measured in relative luminescent units (RLU) of luciferase reporter activity. (B) Levels of IL-8 in cell lysates. (C) IL-8 levels in cell culture supernatants. Values represent means and SEM for duplicate cultures in one of two experiments. *, P < 0.05; **, P < 0.01 (for comparison to medium-only [no compound] control by ANOVA with Dunnett's posttest).
DISCUSSION
This study shows that the negatively charged polyanionic microbicidal compounds DxS and PSS significantly modify the outcome of TLR stimulation in cervical and vaginal epithelial cells, including cytokine/chemokine responses and NF-κB activation.
TLR activation is critical for epithelial immune responses and the elimination of pathogen invasion. TLRs trigger innate epithelial defense mechanisms, including the production of antimicrobial peptides (64, 70) and antiviral cytokines, e.g., IFN-β, which is produced by virus-infected cells and increases bystander cell resistance to viral infection (9). In addition, TLR responses are needed for activation of adaptive immune responses via production of IL-1, IL-6, IL-8, tumor necrosis factor alpha, and IL-12 and upregulation of immune cell costimulatory molecules, e.g., CD80, CD86, and CD40 (43, 57). The production of IL-8 by epithelial cells in response to TLR stimulation serves to attract and activate innate immune cells, such as neutrophils, to the site of infection and inflammation, which is necessary for bacterial infection clearance (31, 37). Thus, the inhibition of cytokine/chemokine production and/or secretion could lead to impaired protective responses and increased risk of pathogen invasion.
The polyanionic microbicide compounds characterized in this study inhibited the production and release of IL-8 in response to the TLR1/2 agonist Pam3CSK4 while having no or only a weak impact on the TLR2/6 agonist MALP-2. These effects occurred in spite of the significantly enhanced NF-κB activation in the presence of both compounds, suggesting a pathway-specific modulation downstream from receptor-ligand interactions.
DxS and PSS radically suppressed multiple steps of the TLR3 pathway, including ligand uptake, NF-κB activation, and cytokine mRNA and protein production. In contrast to TLR1/2 and TLR2/6, TLR3 was inhibited both upstream and downstream from NF-κB activation. Because of the intracellular localization of TLR3, poly(I:C) must be internalized in order to initiate TLR signaling. The polyanionic compounds prevented poly(I:C) internalization and binding to TLR3, since the NF-κB inhibitory effect was seen only when the compound and the ligand were added simultaneously to the epithelial surface and not in experiments where epithelial cells were incubated with poly(I:C) prior to compound exposure. However, cytokine production was still reduced in the latter experiments, suggesting that the immunosuppressive mechanism also included events downstream from poly(I:C) uptake, TLR binding, and NF-κB activation. An additional proof for this hypothesis is that DxS inhibited the IL-8 and IL-6 responses to poly(I:C) in the VEC-100 model, where poly(I:C) was added to the basal surface and DxS was applied to the apical surface of the tissue. A recent study identified scavenger receptor class A (SR-A) as a novel epithelial cell surface receptor responsible for the internalization of poly(I:C) (40). Furthermore, the same study showed that polyanionic competitors of SR-A, such as DxS, inhibit poly(I:C)-SR-A binding, uptake, and proinflammatory response. Our data show that these upstream effects of the polyanionic compounds are different for poly(I:C) and for Pam3CSK4 and MALP-2. While the presence of polyanions inhibited the poly(I:C)-triggered NF-κB response, it potentiated responses to simultaneously added Pam3CSK4 and MALP-2, which bind cell surface TLR1/2 and TLR2/6, respectively. This enhancement of NF-κB activation might be due to potentiated TLR-ligand binding or might be a result of autocrine stimulation by cell membrane-retained cytokines.
It is of critical importance to understand the mechanisms underlying modified TLR-mediated responses in the presence of microbicidal agents, since they may be responsible for significant unwanted consequences during microbicide use for prevention of HIV transmission. The fact that both polyanionic compounds characterized in this study, DxS and PSS, had similar effects on TLR-mediated IL-8 secretion, while unsulfated Dx had no effect, suggests that their effects on TLR signaling are possibly related to their negative charge, which also underlies their main mechanism of anti-HIV-1 action. Both DxS and PSS exert anti-HIV-1 activity through binding to the positively charged sites in the V3 loop of the viral envelope glycoprotein gp120 and blocking its interaction with CD4 receptors of HIV-1 target cells (44, 49). Unsulfated Dx, which does not display antiviral activity, did not have any significant impact on TLR-mediated cytokine production and secretion.
The HIV-1 neutralization mechanism of DxS, PSS, and other sulfated polyanions also includes blocking the primary binding of HIV-1 gp120 to cell surface heparan sulfate (45). By competing with heparan sulfate on a larger scale, these compounds may interfere with a number of important immunoregulatory mechanisms. Sulfated disaccharides derived from heparin/heparan sulfate have been reported to downregulate the spontaneous and tumor necrosis factor alpha-stimulated secretion of IL-8 and IL-1β by intestinal epithelial cells (14). Their mechanism of action, however, has not been established fully (11, 32).
An increasing number of cytokines have been found to bind not only to their high-affinity receptors but also to cell surface glycosaminoglycans from the heparin/heparan sulfate family (47). The binding of a cytokine to heparan sulfate may have a number of functions, either facilitating or inhibiting the cytokine's biological activity. DxS and PSS may act as synthetic analogs of heparan sulfate and other sulfated glycosaminoglycans, which regulate the function of chemokines, e.g., IL-8 (59). The sequestration of proinflammatory cytokines at the cell surface has been shown to induce the synthesis and secretion of heparanase, an enzyme that cleaves heparan sulfate in epithelial and endothelial cells and has proinflammatory activity (13). Thus, the polyanion sequestration of cytokines may have anti-inflammatory but also proinflammatory consequences and deserves further investigation.
As a result of the interaction of polyanionic microbicides with the female genital tract epithelium, reduced levels of TLR-stimulated cytokines and chemokines may attenuate potential inflammatory reactions. Although the process of mucosal immune regulation is complex and requires further study, given the known HIV activation by inflammatory conditions and cytokines, the compound-induced reduction of the proinflammatory cytokine response in healthy individuals in the absence of bacterial infection may be regarded as beneficial. However, at the same time, the decreased production of cytokines would impair the protective and regulatory paracrine function of the cervicovaginal mucosa, rendering it more susceptible to bacterial and viral infections. Decreased levels of vaginal cytokines and certain microflora abnormalities have been reported in clinical studies upon application of polyanionic microbicides (10, 58). Our study offers a biological explanation for these observations and presents a model system for future assessment of other microbicides targeting host receptor interactions. The clinical significance of this in vitro model remains to be confirmed by future outcomes of microbicide trials.
Since TLR activation enhances HIV-1 replication (6, 18), it might be argued that inhibition of TLR by microbicidal compounds could be beneficial. However, women at higher risk of HIV-1 are also exposed to other sexually transmitted viral and bacterial pathogens, and inefficient stimulation of TLRs, which is the first innate immune response to infection, might increase the risk of microbial invasion, which in turn would lead to increased risk for HIV-1 infection. Thus, impaired TLR responses, especially those lasting after the microbicides are washed out and are no longer present in the vagina at a protective dose, may ultimately increase the risk of HIV-1 transmission. A recent study showed that TLR tolerance induced by TLR ligands enhances HIV-1 gene expression (5). Therefore, it seems safer for microbicide products to not interfere with epithelial TLR responses.
Our in vitro findings suggest that polyanionic microbicidal compounds may interfere with TLR responses and affect the ability of the cervicovaginal epithelium to mount normal responses against microbial pathogens. Given the diverse effects of TLR signaling on HIV-1 transmission, further studies are required to determine the clinical relevance of these interactions.
Acknowledgments
This work was supported by grant MSA-05-427 from CONRAD, Eastern Virginia Medical School, under a cooperative agreement with the U.S. Agency for International Development (USAID).
The views expressed by the authors do not necessarily reflect those of CONRAD or USAID.
We thank Mark Schwartz from HTGenomics Inc. for assistance with the mRNA multiplex array development and analysis.
Footnotes
Published ahead of print on 12 January 2009.
REFERENCES
- 1.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 2.Alfano, M., and G. Poli. 2005. Role of cytokines and chemokines in the regulation of innate immunity and HIV infection. Mol. Immunol. 42:161-182. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, J. M., D. Al-Khairy, and R. R. Ingalls. 2006. Innate immunity at the mucosal surface: role of Toll-like receptor 3 and Toll-like receptor 9 in cervical epithelial cell responses to microbial pathogens. Biol. Reprod. 74:824-831. [DOI] [PubMed] [Google Scholar]
- 4.Arend, W. P. 2002. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 13:323-340. [DOI] [PubMed] [Google Scholar]
- 5.Bafica, A., C. A. Scanga, O. Equils, and A. Sher. 2004. The induction of Toll-like receptor tolerance enhances rather than suppresses HIV-1 gene expression in transgenic mice. J. Leukoc. Biol. 75:460-466. [DOI] [PubMed] [Google Scholar]
- 6.Bafica, A., C. A. Scanga, M. L. Schito, S. Hieny, and A. Sher. 2003. Cutting edge: in vivo induction of integrated HIV-1 expression by mycobacteria is critically dependent on Toll-like receptor 2. J. Immunol. 171:1123-1127. [DOI] [PubMed] [Google Scholar]
- 7.Balzarini, J., and L. Van Damme. 2007. Microbicide drug candidates to prevent HIV infection. Lancet 369:787-797. [DOI] [PubMed] [Google Scholar]
- 8.Bauer, S., C. J. Kirschning, H. Hacker, V. Redecke, S. Hausmann, S. Akira, H. Wagner, and G. B. Lipford. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 98:9237-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biron, C. A. 1999. Initial and innate responses to viral infections—pattern setting in immunity or disease. Curr. Opin. Microbiol. 2:374-381. [DOI] [PubMed] [Google Scholar]
- 10.Bollen, L. J., K. Blanchard, P. H. Kilmarx, S. Chaikummao, C. Connolly, P. Wasinrapee, N. Srivirojana, J. Achalapong, J. W. Tappero, and J. M. McNicholl. 2008. No increase in cervicovaginal proinflammatory cytokines after Carraguard use in a placebo-controlled randomized clinical trial. J. Acquir. Immune Defic. Syndr. 47:253-257. [DOI] [PubMed] [Google Scholar]
- 11.Cahalon, L., O. Lider, H. Schor, A. Avron, D. Gilat, R. Hershkoviz, R. Margalit, A. Eshel, O. Shoseyev, and I. R. Cohen. 1997. Heparin disaccharides inhibit tumor necrosis factor-alpha production by macrophages and arrest immune inflammation in rodents. Int. Immunol. 9:1517-1522. [DOI] [PubMed] [Google Scholar]
- 12.Canny, G. O., R. T. Trifonova, D. W. Kindelberger, S. P. Colgan, and R. N. Fichorova. 2006. Expression and function of bactericidal/permeability-increasing protein in human genital tract epithelial cells. J. Infect. Dis. 194:498-502. [DOI] [PubMed] [Google Scholar]
- 13.Chen, G., D. Wang, R. Vikramadithyan, H. Yagyu, U. Saxena, S. Pillarisetti, and I. J. Goldberg. 2004. Inflammatory cytokines and fatty acids regulate endothelial cell heparanase expression. Biochemistry 43:4971-4977. [DOI] [PubMed] [Google Scholar]
- 14.Chowers, Y., O. Lider, H. Schor, I. Barshack, R. Tal, A. Ariel, S. Bar-Meir, I. R. Cohen, and L. Cahalon. 2001. Disaccharides derived from heparin or heparan sulfate regulate IL-8 and IL-1 beta secretion by intestinal epithelial cells. Gastroenterology 120:449-459. [DOI] [PubMed] [Google Scholar]
- 15.Doncel, G. 2007. Update on the CONRAD Cellulose Sulfate Trial: preclinical evaluation of cellulose sulfate. CROI, Los Angeles, CA.
- 16.Doncel, G., and C. Mauck. 2004. Vaginal microbicides: a novel approach to preventing sexual transmission of HIV. Curr. HIV/AIDS Rep. 1:25-32. [DOI] [PubMed] [Google Scholar]
- 17.Doncel, G. F. 2006. Exploiting common targets in human fertilization and HIV infection: development of novel contraceptive microbicides. Hum. Reprod. Update 12:103-117. [DOI] [PubMed] [Google Scholar]
- 18.Equils, O., M. L. Schito, H. Karahashi, Z. Madak, A. Yarali, K. S. Michelsen, A. Sher, and M. Arditi. 2003. Toll-like receptor 2 (TLR2) and TLR9 signaling results in HIV-long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells: implications of simultaneous activation of TLRs on HIV replication. J. Immunol. 170:5159-5164. [DOI] [PubMed] [Google Scholar]
- 19.Fazeli, A., C. Bruce, and D. O. Anumba. 2005. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum. Reprod. 20:1372-1378. [DOI] [PubMed] [Google Scholar]
- 20.Fichorova, R. N. 2004. Guiding the vaginal microbicide trials with biomarkers of inflammation. J. Acquir. Immune Defic. Syndr. 37(Suppl. 3):S184-S193. [PMC free article] [PubMed] [Google Scholar]
- 21.Fichorova, R. N., and D. J. Anderson. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol. Reprod. 60:508-514. [DOI] [PubMed] [Google Scholar]
- 22.Fichorova, R. N., M. Bajpai, N. Chandra, J. G. Hsiu, M. Spangler, V. Ratnam, and G. F. Doncel. 2004. Interleukin (IL)-1, IL-6, and IL-8 predict mucosal toxicity of vaginal microbicidal contraceptives. Biol. Reprod. 71:761-769. [DOI] [PubMed] [Google Scholar]
- 23.Fichorova, R. N., A. O. Cronin, E. Lien, D. J. Anderson, and R. R. Ingalls. 2002. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of Toll-like receptor 4-mediated signaling. J. Immunol. 168:2424-2432. [DOI] [PubMed] [Google Scholar]
- 24.Fichorova, R. N., J. G. Rheinwald, and D. J. Anderson. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 57:847-855. [DOI] [PubMed] [Google Scholar]
- 25.Fichorova, R. N., N. Richardson-Harman, M. Alfano, L. Belec, C. Carbonneil, S. Chen, L. Cosentino, K. Curtis, C. S. Dezzutti, B. Donoval, G. F. Doncel, M. Donaghay, J. C. Grivel, E. Guzman, M. Hayes, B. Herold, S. Hillier, C. Lackman-Smith, A. Landay, L. Margolis, K. H. Mayer, J. M. Pasicznyk, M. Pallansch-Cokonis, G. Poli, P. Reichelderfer, P. Roberts, I. Rodriguez, H. Saidi, R. R. Sassi, R. Shattock, and J. E. Cummins, Jr. 2008. Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal. Chem. 80:4741-4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fichorova, R. N., R. T. Trifonova, R. O. Gilbert, C. E. Costello, G. R. Hayes, J. J. Lucas, and B. N. Singh. 2006. Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infect. Immun. 74:5773-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fichorova, R. N., L. D. Tucker, and D. J. Anderson. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 184:418-428. [DOI] [PubMed] [Google Scholar]
- 28.Fichorova, R. N., F. Zhou, V. Ratnam, V. Atanassova, S. Jiang, N. Strick, and A. R. Neurath. 2005. Anti-human immunodeficiency virus type 1 microbicide cellulose acetate 1,2-benzenedicarboxylate in a human in vitro model of vaginal inflammation. Antimicrob. Agents Chemother. 49:323-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisette, P. L., S. Ram, J. M. Andersen, W. Guo, and R. R. Ingalls. 2003. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J. Biol. Chem. 278:46252-46260. [DOI] [PubMed] [Google Scholar]
- 30.Guillot, L., R. Le Goffic, S. Bloch, N. Escriou, S. Akira, M. Chignard, and M. Si-Tahar. 2005. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280:5571-5580. [DOI] [PubMed] [Google Scholar]
- 31.Harada, A., N. Sekido, T. Akahoshi, T. Wada, N. Mukaida, and K. Matsushima. 1994. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 56:559-564. [PubMed] [Google Scholar]
- 32.Hecht, I., R. Hershkoviz, S. Shivtiel, T. Lapidot, I. R. Cohen, O. Lider, and L. Cahalon. 2004. Heparin-disaccharide affects T cells: inhibition of NF-kappaB activation, cell migration, and modulation of intracellular signaling. J. Leukoc. Biol. 75:1139-1146. [DOI] [PubMed] [Google Scholar]
- 33.Hedges, S. R., W. W. Agace, and C. Svanborg. 1995. Epithelial cytokine responses and mucosal cytokine networks. Trends Microbiol. 3:266-270. [DOI] [PubMed] [Google Scholar]
- 34.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196-200. [DOI] [PubMed] [Google Scholar]
- 35.Herbst-Kralovetz, M. M., A. J. Quayle, M. Ficarra, S. Greene, W. A. Rose II, R. Chesson, R. A. Spagnuolo, and R. B. Pyles. 2008. Quantification and comparison of Toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am. J. Reprod. Immunol. 59:212-224. [DOI] [PubMed] [Google Scholar]
- 36.Hillier, S. L., T. Moench, R. Shattock, R. Black, P. Reichelderfer, and F. Veronese. 2005. In vitro and in vivo: the story of nonoxynol 9. J. Acquir. Immune Defic. Syndr. 39:1-8. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi, Y. 2008. The role of chemokines in neutrophil biology. Front. Biosci. 13:2400-2407. [DOI] [PubMed] [Google Scholar]
- 38.Krebs, F. C., S. R. Miller, B. J. Catalone, R. Fichorova, D. Anderson, D. Malamud, M. K. Howett, and B. Wigdahl. 2002. Comparative in vitro sensitivities of human immune cell lines, vaginal and cervical epithelial cell lines, and primary cells to candidate microbicides nonoxynol 9, C31G, and sodium dodecyl sulfate. Antimicrob. Agents Chemother. 46:2292-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levin, J., and F. B. Bang. 1968. Clottable protein in Limulus; its localization and kinetics of its coagulation by endotoxin. Thromb. Diath. Haemorrh. 19:186-197. [PubMed] [Google Scholar]
- 40.Limmon, G. V., M. Arredouani, K. L. McCann, R. A. Corn Minor, L. Kobzik, and F. Imani. 2008. Scavenger receptor class-A is a novel cell surface receptor for double-stranded RNA. FASEB J. 22:159-167. [DOI] [PubMed] [Google Scholar]
- 41.Martel, R. R., I. W. Botros, M. P. Rounseville, J. P. Hinton, R. R. Staples, D. A. Morales, J. B. Farmer, and B. E. Seligmann. 2002. Multiplexed screening assay for mRNA combining nuclease protection with luminescent array detection. Assay Drug Dev. Technol. 1:61-71. [DOI] [PubMed] [Google Scholar]
- 42.McGowan, I. 2006. Microbicides: a new frontier in HIV prevention. Biologicals 34:241-255. [DOI] [PubMed] [Google Scholar]
- 43.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 44.Mitsuya, H., D. J. Looney, S. Kuno, R. Ueno, F. Wong-Staal, and S. Broder. 1988. Dextran sulfate suppression of viruses in the HIV family: inhibition of virion binding to CD4+ cells. Science 240:646-649. [DOI] [PubMed] [Google Scholar]
- 45.Mondor, I., S. Ugolini, and Q. J. Sattentau. 1998. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 72:3623-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muhlradt, P. F., M. Kiess, H. Meyer, R. Sussmuth, and G. Jung. 1997. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J. Exp. Med. 185:1951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Najjam, S., B. Mulloy, J. Theze, M. Gordon, R. Gibbs, and C. C. Rider. 1998. Further characterization of the binding of human recombinant interleukin 2 to heparin and identification of putative binding sites. Glycobiology 8:509-516. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura, M., M. Maruyama, F. Yamashita, Y. Takakura, M. Hashida, and Y. Watanabe. 2004. Expression and visualization of a human interferon-beta-enhanced green fluorescent protein chimeric molecule in cultured cells. Biol. Pharm. Bull. 27:411-414. [DOI] [PubMed] [Google Scholar]
- 49.Neurath, A. R., N. Strick, and Y. Y. Li. 2002. Anti-HIV-1 activity of anionic polymers: a comparative study of candidate microbicides. BMC Infect. Dis. 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pioli, P. A., E. Amiel, T. M. Schaefer, J. E. Connolly, C. R. Wira, and P. M. Guyre. 2004. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect. Immun. 72:5799-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poli, G., A. L. Kinter, E. Vicenzi, and A. S. Fauci. 1994. Cytokine regulation of acute and chronic HIV infection in vitro: from cell lines to primary mononuclear cells. Res. Immunol. 145:578-582. [DOI] [PubMed] [Google Scholar]
- 53.Quinn, T. C. 1996. Association of sexually transmitted diseases and infection with the human immunodeficiency virus: biological cofactors and markers of behavioural interventions. Int. J. STD AIDS 7(Suppl. 2):17-24. [DOI] [PubMed] [Google Scholar]
- 54.Roberts, R. A., C. M. Sabalos, M. L. LeBlanc, R. R. Martel, Y. M. Frutiger, J. M. Unger, I. W. Botros, M. P. Rounseville, B. E. Seligmann, T. P. Miller, T. M. Grogan, and L. M. Rimsza. 2007. Quantitative nuclease protection assay in paraffin-embedded tissue replicates prognostic microarray gene expression in diffuse large-B-cell lymphoma. Lab. Investig. 87:979-997. [DOI] [PubMed] [Google Scholar]
- 55.Sawada, H., K. Taniguchi, and K. Takami. 2006. Improved toxicogenomic screening for drug-induced phospholipidosis using a multiplexed quantitative gene expression array plate assay. Toxicol. In Vitro 20:1506-1513. [DOI] [PubMed] [Google Scholar]
- 56.Schaefer, T. M., J. V. Fahey, J. A. Wright, and C. R. Wira. 2005. Migration inhibitory factor secretion by polarized uterine epithelial cells is enhanced in response to the TLR3 agonist poly(I:C). Am. J. Reprod. Immunol. 54:193-202. [DOI] [PubMed] [Google Scholar]
- 57.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947-950. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz, J. L., C. Mauck, J. J. Lai, M. D. Creinin, V. Brache, S. A. Ballagh, D. H. Weiner, S. L. Hillier, R. N. Fichorova, and M. Callahan. 2006. Fourteen-day safety and acceptability study of 6% cellulose sulfate gel: a randomized double-blind phase I safety study. Contraception 74:133-140. [DOI] [PubMed] [Google Scholar]
- 59.Selvan, R. S., N. S. Ihrcke, and J. L. Platt. 1996. Heparan sulfate in immune responses. Ann. N. Y. Acad. Sci. 797:127-139. [DOI] [PubMed] [Google Scholar]
- 60.Sharkey, D. J., A. M. Macpherson, K. P. Tremellen, and S. A. Robertson. 2007. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol. Hum. Reprod. 13:491-501. [DOI] [PubMed] [Google Scholar]
- 61.Steele, C., and P. L. Fidel, Jr. 2002. Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect. Immun. 70:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takeda, K., and S. Akira. 2004. TLR signaling pathways. Semin. Immunol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 63.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1-14. [DOI] [PubMed] [Google Scholar]
- 64.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 65.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 66.Till, B. J., C. Burtner, L. Comai, and S. Henikoff. 2004. Mismatch cleavage by single-strand specific nucleases. Nucleic Acids Res. 32:2632-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trifonova, R. T., J. M. Pasicznyk, and R. N. Fichorova. 2006. Biocompatibility of solid-dosage forms of anti-human immunodeficiency virus type 1 microbicides with the human cervicovaginal mucosa modeled ex vivo. Antimicrob. Agents Chemother. 50:4005-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Damme, L., R. Govinden, F. M. Mirembe, F. Guedou, S. Solomon, M. L. Becker, B. S. Pradeep, A. K. Krishnan, M. Alary, B. Pande, G. Ramjee, J. Deese, T. Crucitti, and D. Taylor. 2008. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N. Engl. J. Med. 359:463-472. [DOI] [PubMed] [Google Scholar]
- 69.Wasserheit, J. N. 1992. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex. Transm. Dis. 19:61-77. [PubMed] [Google Scholar]
- 70.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]