Abstract
Mycoplasma pneumoniae is one of the causative agents of atypical community-acquired pneumonia. Tigecycline belongs to a new class of glycylcycline antimicrobials that have activity against a wide range of microorganisms, including in vitro activity against M. pneumoniae. We investigated the effect of tigecycline on microbiologic, histologic, and immunologic indices in a murine model of M. pneumoniae pneumonia. BALB/c mice were inoculated intranasally with M. pneumoniae and treated subcutaneously with tigecycline or placebo for 6 days. Outcome variables included quantitative bronchoalveolar lavage (BAL) M. pneumoniae culture, lung histopathologic score (HPS), BAL cytokine and chemokine concentrations (tumor necrosis factor alpha [TNF-α], gamma interferon [IFN-γ], interleukin 1β [IL-1β], IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 [p40/p70], granulocyte-macrophage colony-stimulating factor, MIP-1α, MIG, KC, MCP-1, and IP-10). BAL M. pneumoniae concentrations in mice treated with tigecycline (MpTige) tended to be reduced compared with mice treated with placebo (MpPl); however this did not reach statistical significance. The lung HPS was significantly lower, as well as the parenchymal-pneumonia subscore, in the MpTige mice than in the MpPl mice. MpTige mice had significantly lower BAL cytokine concentrations of IL-1β, IL-12 (p40/p70), IFN-γ, and TNF-α; of the chemokines, MIG, MIP-1α, and IP-10 were statistically lower in MpTige mice. While tigecycline treatment demonstrated a modest microbiologic effect, it significantly improved lung histologic inflammation and reduced pulmonary cytokines and chemokines.
Antimicrobials have been used successfully for the treatment of many infectious diseases; however, the continuous increase in resistance among many bacteria in the community, as well as in the hospital setting, has led to research for new classes of antibiotics with a broader range of activity against pathogenic microorganisms (2, 6, 17, 18). Tigecycline is a novel antibiotic, a 9-t-butylglycylamido derivative of minocyline, which inhibits protein synthesis in the presence of tetracycline-resistant, tet(M)-protected ribosomes (30); it belongs to a new semisynthetic class of antimicrobials named glycylcyclines (37). It has been shown to have activity against a wide range of microorganisms, both gram-positive and gram-negative bacteria, as well as anaerobes (16, 29, 39). Moreover tigecycline has activity in vitro against rapidly growing mycobacteria, such as Mycobacterium chelonae and Mycobacterium fortuitum, as well as atypical respiratory pathogens, such as Mycoplasma pneumoniae (20, 21, 41). In animal pneumonia models, tigecycline has been shown to have effective activity against Legionella pneumophila, Pseudomonas aeruginosa, and Streptococcus pneumoniae pulmonary infections (33).
M. pneumoniae is an “atypical” bacterium that lacks a cell wall and plays an important role in acute lower respiratory tract infections in children and adults; approximately 20 to 30% of community-acquired pneumonia in the general population is due to M. pneumoniae (1, 3, 27, 42). In the last few decades, M. pneumoniae respiratory infection has drawn increasing attention for its association with reactive airway disease and asthma (22-24). Clinical studies have shown that therapy with macrolides or tetracyclines, which are considered the drugs of choice for M. pneumoniae (11), is able to reduce the morbidity of pneumonia, shorten the duration of symptoms, and decrease the recurrence of wheezing (5, 7, 8, 12, 25, 26). However, even though treatment with macrolide, ketolide, and peptide deformylase inhibitor antimicrobials significantly improves pulmonary inflammation in animal M. pneumoniae pneumonia investigations, M. pneumoniae is not eradicated from the lungs in these in vivo investigations (10, 15, 31, 32). Of note, in these experimental investigations examining the effects of antimicrobials for M. pneumoniae pneumonia, a disassociation is often found between the microbiologic response and the significant improvement observed in other markers of disease severity.
While tigecycline demonstrates in vitro activity against M. pneumoniae, tigecycline therapy in vivo for M. pneumoniae pneumonia has not been evaluated. In the present study, we investigate the effect of tigecycline on microbiologic, histologic, and immunologic (pulmonary cytokine and chemokine) parameters in our established murine model of M. pneumoniae pneumonia (9, 10, 14, 15).
MATERIALS AND METHODS
Organism and growth conditions.
M. pneumoniae (ATCC 29342) was reconstituted in SP4 broth and subcultured after 24 to 48 h in a flask containing 20 ml of SP4 medium at 37°C. Approximately 72 h later, the broth turned orange, the supernatant was decanted, and 2 ml of fresh SP4 broth was added to the flask. A cell scraper was used to harvest the adherent mycoplasmas from the bottom of the flask. This achieved an M. pneumoniae concentration in the range of 108 to 109 CFU/ml (determined by plating dilutions on SP4 agar). Aliquots were stored at −80°C. All SP4 media contained nystatin (50 units/ml) and ampicillin (1.0 mg/ml) to inhibit the growth of potential contaminants.
Animals and inoculation.
Animal guidelines were followed in accordance with the Institutional Animal Care and Research Advisory Committee. BALB/c mice were obtained from Charles River Laboratories, who confirmed their mycoplasma- and murine-virus-free status. The mice were housed in the animal care facility of our institution in filter-top cages and allowed to acclimate to their new environment for 1 week. Isofluorane, an inhaled anesthetic, was used for sedation during inoculation. Two-month-old female BALB/c mice were intranasally inoculated to achieve aspiration once (day zero) with 107 CFU of M. pneumoniae in 50 μl SP4 broth. All mice were housed in the same animal room and received identical daily care for the duration of the experiment.
Tigecycline administration, experimental design, and sample collection.
Starting 1 day after M. pneumoniae inoculation, the mice were treated subcutaneously once daily for six consecutive days with tigecycline or placebo (sterile 0.9% sodium chloride). Tigecycline (Wyeth Pharmaceuticals, Madison, NJ) was reconstituted with sterile 0.9% sodium chloride. Tigecycline was administered at a dosage of 10 mg/kg of body weight (0.2 mg in 0.2 ml per mouse) on the first day of treatment, followed by 5 mg/kg of body weight (0.1 mg in 0.2 ml per mouse) for the remaining 5 days of therapy. Groups of mice were evaluated after 1, 3, and 6 days of therapy. Samples were obtained from a total of 10 mice per treatment group (two treatment groups: mice treated with tigecycline and mice treated with placebo) at each time point from repeated identical experiments.
The mice were euthanized for sample collection by cardiac puncture after being anesthetized with an intraperitoneal injection of 75 mg of ketamine per kg and 5 mg of acepromazine per kg. Bronchoalveolar lavage (BAL) specimens were obtained by infusing 500 μl of SP4 broth through a 25-gauge needle into the lungs via the trachea, followed by aspiration of this fluid into a syringe. Whole-lung specimens (including the trachea and both lungs) were collected and fixed with a 10% buffered formalin solution for histologic evaluation. Samples were taken approximately 24 h after the last tigecycline dose.
Culture.
Twenty-five microliters of undiluted BAL sample and serial 10-fold dilutions of BAL fluid in SP4 broth (50 μl of undiluted BAL sample in 450 μl SP4 broth was used for the initial dilution; subsequent dilutions were done in similar fashion) were immediately cultured on SP4 agar plates at 37°C, while the remaining undiluted BAL fluid specimens were stored at −80°C. Quantification was performed by counting colonies on plated specimens and expressed as log10 CFU per milliliter. Our laboratory has previously compared BAL and homogenized whole-lung cultures (unpublished data). The two sites follow the same kinetics time course or “curve” over time. Although there is an absolute difference between the two sites in terms of colonies counted, the absolute numbers of colonies counted at the two sites differ by a constant factor over time. In addition, homogenized lung tissue is known to have inhibitors of M. pneumoniae growth, which makes the detection and quantification of low concentrations of M. pneumoniae impossible in homogenized lung tissue.
Histopathology.
Lung tissue was fixed in buffered formalin and whole-mount sections of paraffin-embedded lungs were stained with hematoxylin and eosin. The histopathologic score (HPS) was determined by a single pathologist who was unaware of the treatment status of the animals from which specimens were taken. The HPS was based on individual grading of peribronchiolar/bronchial infiltrate (percentage of sites and quality), bronchiolar/bronchial luminal exudate, perivascular infiltrate (percentage of sites), and parenchymal pneumonia (neutrophilic alveolar infiltrate); after each of these was graded, the subscores were compiled for the HPS. This HPS system assigned values from 0 to 26: the higher the score, the greater the inflammatory changes in the lung (4). The parenchymal-pneumonia subscore was based on grading of the neutrophilic alveolar infiltrate (scoring: 0, no parenchymal pneumonia; 3, patchy parenchymal pneumonia; 5, patchy and confluent parenchymal pneumonia).
Measurement of cytokines in BAL fluid.
Concentrations of cytokines and chemokines in BAL specimens were assessed using Multiplex Bead Immunoassays (BioSource International, Camarillo, CA) in conjunction with the Luminex LabMAP system, following the manufacturer's instructions. The cytokines and chemokines examined and their levels of sensitivity were as follows: tumor necrosis factor alpha (TNF-α), 5 pg/ml; gamma interferon (IFN-γ), 1 pg/ml; interleukin 1β (IL-1β), 10 pg/ml; IL-2, 15 pg/ml; IL-4, 5 pg/ml; IL-5, 10 pg/ml; IL-6, 10 pg/ml; IL-10, 15 pg/ml; IL-12 (p40/p70), 15 pg/ml; granulocyte-macrophage colony-stimulating factor, 10 pg/ml; MIP-1α, <15 pg/ml; MIG, <15 pg/ml; KC, <15 pg/ml; MCP-1, <5 pg/ml; and IP-10, <40 pg/ml.
Statistics.
For all statistical analysis, Sigma Stat 2003 software (SPSS Science, San Rafael, CA) was used. The t test was used to compare the values of the different groups of animals treated with tigecycline versus placebo at the same time point if the data were normally distributed. In the instances where the data were not normally distributed, the Mann-Whitney rank sum test was used for this comparison. Two-way analysis of variance (ANOVA) was used to compare values for the different groups of animals treated with tigecycline versus placebo over 1, 3, and 6 days of treatment if the data were normally distributed. A comparison was considered statistically significant if the P value was <0.05.
RESULTS
BAL culture.
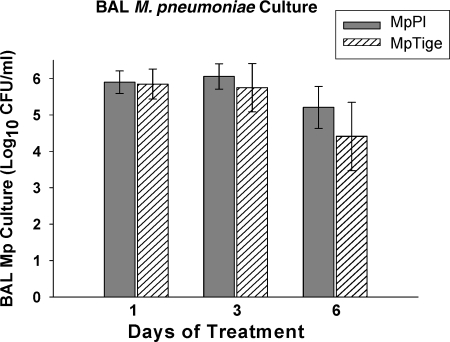
Quantitative cultures were positive in all mice infected with M. pneumoniae. M. pneumoniae concentrations in mice treated with tigecycline (MpTige) tended to be lower than in mice treated with placebo (MpPl) after 6 days of therapy (t test; P = 0.07); however, these results did not reach statistical significance by t test or two-way ANOVA (Fig. 1).
FIG. 1.
Quantitative M. pneumoniae cultures of BAL fluid specimens from MpTige or MpPl mice. The values shown are means ± standard deviations (error bars). The data represent results from two separate experiments with 10 mice in each group at each time point.
Histopathology.
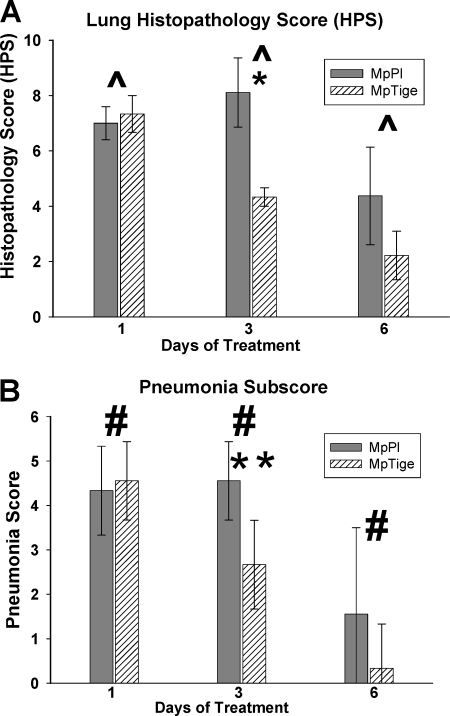
MpTige mice had significantly reduced lung HPS (P = 0.035) and parenchymal-pneumonia subscores (P = 0.004) compared with MpPl mice over 1, 3, and 6 days of treatment by two-way ANOVA (Fig. 2A and B). In addition, MpTige mice had significantly reduced lung HPS (t test; P = 0.002) compared with MpPl mice after 3 days of therapy (Fig. 2A). The parenchymal-pneumonia subscore (scoring of neutrophilic alveolar infiltrate alone) of the overall HPS was also significantly lower in MpTige mice than in MpPl mice (t test; P = 0.004) after 3 days of therapy (Fig. 2B). All other subscores of the overall HPS were not significantly different between the treatment groups.
FIG. 2.
Lung HPS (A) and pneumonia subscore (B) of MpTige or MpPl mice. The values shown are means ± standard deviations (error bars). The data represent the results from two separate experiments with 10 mice in each group at each time point. ^, P = 0.035 by two-way ANOVA; #, P = 0.004 by two-way ANOVA; *, P = 0.002 for HPS between MpPl mice by t test; **, P = 0.004 for pneumonia subscore between MpTige and MpPl mice by t test.
BAL cytokines and chemokines.
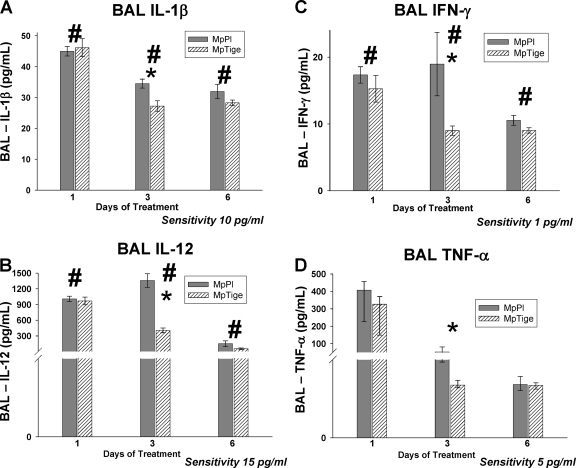
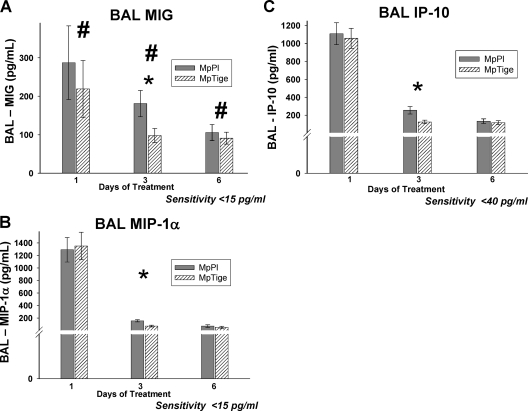
MpTige mice had significantly reduced concentrations of IL-1β (P = 0.043), IL-12 (P < 0.001), IFN-γ (P = 0.016), and MIG (P = 0.044) compared with MpPl mice over 1, 3, and 6 days of treatment by two-way ANOVA (Fig. 3A, B, and C and 4A). By t test, MpTige mice had significantly reduced concentrations of IL-12 (p40/p70) (P < 0.001), IFN-γ (P = 0.004), TNF-α (P < 0.001), and IL-1β (P = 0.005) compared with MpPl mice after 3 days of therapy (Fig. 3A, B, C, and D). No significant differences were found for the BAL concentrations of IL-2, IL-4, IL-5, IL-6, IL-10, and granulocyte-macrophage colony-stimulating factor in the MpTige mice compared with the MpPl mice. By t test, among the chemokines after 3 days of therapy, concentrations of MIG (P = 0.047), MIP-1α (P = 0.002), and IP-10 (P = 0.004) were significantly lower in MpTige mice than in the MpPl mice (Fig. 4A, B, and C); no significant differences in the BAL fluid concentrations of KC and MCP-1 were found in the MpTige mice compared with the MpPl mice.
FIG. 3.
Cytokine concentrations in BAL fluid specimens from MpTige or MpPl mice. The values shown are means ± standard deviations (error bars). The data represent results from two separate experiments with 10 mice in each group at each time point. (A) #, P = 0.043 by two-way ANOVA; *, P = 0.005 by t test. (B) #, P < 0.001 by two-way ANOVA; *, P < 0.001 by t test. (C) #, P = 0.016 by two-way ANOVA; *, P = 0.004 by t test. (D) *, P < 0.001 by t test.
FIG. 4.
Chemokine concentrations in BAL fluid specimens from MpTige or MpPl mice. The values shown are means ± standard deviations (error bars). The data represent results from two separate experiments with 10 mice in each group at each time point. (A) #, P = 0.044 by two-way ANOVA; *, P < 0.047 by t test. (B) *, P = 0.002 by t test. (C) *, P = 0.004 by t test.
DISCUSSION
This investigation demonstrates that therapy with tigecycline in an experimental model of M. pneumoniae lower respiratory tract infection significantly lessens disease severity by reducing histologic pulmonary inflammation, as well as cytokines and chemokines. Although quantitative M. pneumoniae cultures tended to be smaller in tigecycline-treated mice, there was not a significant reduction in quantitative M. pneumoniae cultures for mice treated with tigecycline compared to those treated with placebo. This discrepancy observed for tigecycline therapy between quantitative microbiologic culture results and pulmonary inflammation, as measured by histopathology scores/cytokines/chemokines has also been observed with other antimicrobial classes (macrolide, ketolide, and peptide deformylase inhibitor; all microbial protein synthesis inhibitors) in the treatment of M. pneumoniae experimental infection (10, 15, 32). Although it has been shown in randomized, double-blind, placebo-controlled clinical trials that tetracycline class antimicrobials significantly improve markers of diseases severity in M. pneumoniae pneumonia, a recent clinical investigation found that persistent M. pneumoniae carriage following acute infection is not affected by antibiotic therapy (26, 28). Thus, it is a known characteristic of M. pneumoniae pneumonia that with antimicrobial therapy a disassociation is often seen between microbiologic outcome and disease severity outcome.
The exact mechanism for this disassociation is not known, but it may be related to effects of therapy on microbial protein synthesis or other virulence factors or is perhaps due to a host immunomodulating action. Tigecycline, as well as macrolides, ketolides, and peptide deformylase inhibitors, are microbial protein synthesis inhibitors. Our laboratory hypothesizes that these antimicrobials primarily act to reduce pulmonary inflammation by interfering with the production of M. pneumoniae virulence proteins (such as the recently described ADP-ribosylating, vacuolating toxin of M. pneumoniae [19]) and not primarily by reduction of bacterial numbers, although there is certainly a strong association between the two effects. For instance, in the current investigation, quantitative M. pneumoniae culture results were not statistically different between the tigecycline and placebo treatment groups; however, treatment with tigecycline likely affects the replication frequency of M. pneumoniae, which could alter activation of the host immune system. In addition, direct host immunomodulation by these protein synthesis inhibitor antimicrobials could be hypothesized to be the mechanism for the observed dissociation; however, our laboratory's previous investigations with clarithromycin do not support this hypothesis (15). The discrepancy observed in our study between the antimycoplasmal outcome and the anti-inflammatory outcome illustrates the likely importance of factors (host factors and microbial factors) beyond solely the presence of the microorganism in the pathogenesis of M. pneumoniae respiratory infection.
The differences between tigecycline-treated mice and placebo-treated mice were most pronounced at 3 days of therapy, while at 6 days of therapy the differences between tigecycline-treated mice and placebo-treated mice were not significant by t test. Our mouse model is not a lethal model, just as M. pneumoniae infection is generally not a lethal infection in humans. However, in the mouse model, similar to clinical investigations of M. pneumoniae pneumonia in humans, antimicrobial therapy statistically significantly reduces the severity of pulmonary disease at early time points compared with placebo. It is unusual for children and adults to die from M. pneumoniae pneumonia, although it does rarely occur, with resultant case reports. In placebo-controlled, double-blind, randomized clinical investigations of antimicrobial agents for M. pneumoniae in adults, appropriate antimicrobial therapy significantly reduces the duration of fever, rales, cough, malaise, and abnormal chest radiographs. Additionally, it has been shown that appropriate antimicrobial therapy significantly reduces the duration of hospitalization (26). The chronic persistence of low-level M. pneumoniae infection in the airway after resolution of the acute respiratory infection, with or without appropriate therapy, has been demonstrated in animal models, as well as in humans (36), and it has been hypothesized that this persistence of the microorganism has a major role in the pathogenesis of M. pneumoniae-associated asthma and reactive airway disease (13).
It is possible that a higher dosage or a longer duration of treatment with tigecycline in our investigations would have demonstrated a microbiological effect, along with improvements in markers of disease severity. The approved adult tigecycline dosage is an initial dose of 100 mg intravenously followed by 50 mg every 12 h, which with a 60-min infusion gives a serum maximum concentration of 0.63 μg/ml with an AUC0-24 (area under the curve from 0 to 24 h) of 4.70 μg · h/ml. Tigecycline concentrations in lung tissue have been found to be 8.6-fold higher than serum concentrations in adult studies, and the AUC in alveolar cells has been found to be approximately 78-fold higher than the AUC in serum (Tygacil [tigecycline] prescribing information; Wyeth Pharmaceuticals Inc.). For our investigation, we chose to administer tigecycline subcutaneously at a dosage of 10 mg/kg of body weight on the first day of treatment, followed by 5 mg/kg of body weight daily for the remaining 5 days of therapy. In a neutropenic mouse S. pneumoniae thigh infection model, a tigecycline dosage of 3 mg/kg given subcutaneously resulted in a serum maximum concentration of 0.42 μg/ml (40). Based on data from Wyeth Pharmaceuticals, 5 mg/kg of tigecycline in a mouse pneumonia model resulted in an AUC of 2.9 μg · h/ml (personal communication from Wyeth Pharmaceuticals). Given the above-mentioned mouse and human pharmacokinetic data, it is likely that the tigecycline pharmacokinetic values achieved in the mice in this investigation were lower than those achieved with the tigecycline dosage recommended in adults. Future studies comparing higher and lower doses of tigecycline may help to determine the pharmacodynamics of tigecycline in relationship to the noted reductions in disease severity. In addition, the dose frequency may be an important factor in the pharmacodynamics of tigecycline, as tigecycline is dosed twice a day in people but was only dosed once daily in this investigation in mice. The MIC of tigecycline for M. pneumoniae has been found to range from 0.06 to 0.25 μg/ml (20).
To our knowledge, this is the first report that investigates the effect of tigecycline on cytokines and chemokines. The significantly reduced concentrations of IL-12 (p40/p70), IFN-γ, TNF-α, IL-1β, MIG, MIP-1α, and IP-10 with tigecycline therapy is similar to what our laboratory has observed with therapy with other antimicrobial classes (macrolides, ketolides, and peptide deformylase inhibitors) in the treatment of M. pneumoniae experimental infection (10, 15, 31, 32). The ability of antimicrobials to reduce pulmonary cytokines and chemokines in M. pneumoniae infection does not appear to be specific to a particular protein synthesis inhibitor class of antimicrobials. The sum of our investigations of experimental M. pneumoniae respiratory infection indicates that pulmonary disease severity correlates directly with T-helper 1, proinflammatory, and chemotatic cytokines and not with T-helper 2 cytokines (9, 14, 34, 35, 38).
In conclusion, this investigation demonstrated that tigecycline therapy has a significant effect on pulmonary inflammation in an experimental model of M. pneumoniae respiratory infection, resulting in decreased T-helper 1, proinflammatory, and chemotatic cytokines, as well as histologic lung inflammation. The significant reduction in pulmonary inflammation occurred without a corresponding significant antimicrobial effect. Future studies will further characterize and explore the mechanisms responsible for this discrepancy between the anti-inflammatory effects and the antimicrobial effects observed with various classes of protein synthesis inhibitor antimicrobials.
Acknowledgments
This work was funded by a research grant from Wyeth Pharmaceuticals (Hardy).
Footnotes
Published ahead of print on 12 January 2009.
REFERENCES
- 1.Block, S., J. Hedrick, M. R. Hammerschlag, G. H. Cassell, and J. C. Craft. 1995. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr. Infect. Dis. J. 14:471-477. [DOI] [PubMed] [Google Scholar]
- 2.Canton, R., S. Unal, and D. J. Farrell. 2007. Antibacterial resistance patterns in Streptococcus pneumoniae isolated from elderly patients: PROTEKT years 1-5 (1999-2004). Int. J. Antimicrob. Agents 30:546-550. [DOI] [PubMed] [Google Scholar]
- 3.Cassell, G. H., and B. C. Cole. 1981. Mycoplasmas as agents of human disease. N. Engl. J. Med. 304:80-89. [DOI] [PubMed] [Google Scholar]
- 4.Cimolai, N., G. P. Taylor, D. Mah, and B. J. Morrison. 1992. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol. Immunol. 36:465-478. [DOI] [PubMed] [Google Scholar]
- 5.Denny, F. W., W. A. Clyde, Jr., and W. P. Glezen. 1971. Mycoplasma pneumoniae disease: clinical spectrum, pathophysiology, epidemiology, and control. J. Infect. Dis. 123:74-92. [DOI] [PubMed] [Google Scholar]
- 6.Doern, G. V., and S. D. Brown. 2004. Antimicrobial susceptibility among community-acquired respiratory tract pathogens in the USA: data from PROTEKT US 2000-01. J. Infect. 48:56-65. [DOI] [PubMed] [Google Scholar]
- 7.Esposito, S., F. Blasi, C. Arosio, L. Fioravanti, L. Fagetti, R. Droghetti, P. Tarsia, L. Allegra, and N. Principi. 2000. Importance of acute Mycoplasma pneumoniae and Chlamydia pneumoniae infections in children with wheezing. Eur. Respir. J. 16:1142-1146. [DOI] [PubMed] [Google Scholar]
- 8.Esposito, S., S. Bosis, N. Faelli, E. Begliatti, R. Droghetti, E. Tremolati, A. Porta, F. Blasi, and N. Principi. 2005. Role of atypical bacteria and azithromycin therapy for children with recurrent respiratory tract infections. Pediatr. Infect. Dis. J. 24:438-444. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca-Aten, M., A. M. Rios, A. Mejias, S. Chavez-Bueno, K. Katz, A. M. Gomez, G. H. McCracken, Jr., and R. D. Hardy. 2005. Mycoplasma pneumoniae induces host-dependent pulmonary inflammation and airway obstruction in mice. Am. J. Respir. Cell Mol. Biol. 32:201-210. [DOI] [PubMed] [Google Scholar]
- 10.Fonseca-Aten, M., C. M. Salvatore, A. Mejias, A. M. Rios, S. Chavez-Bueno, K. Katz, A. M. Gomez, G. H. McCracken, Jr., and R. D. Hardy. 2005. Evaluation of LBM415 (NVP PDF-713), a novel peptide deformylase inhibitor, for treatment of experimental Mycoplasma pneumoniae pneumonia. Antimicrob. Agents Chemother. 49:4128-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gendrel, D. 1997. Antibiotic treatment of Mycoplasma pneumoniae infections. Pediatr. Pulmonol. Suppl. 16:46-47. [PubMed] [Google Scholar]
- 12.Gendrel, D., J. Raymond, F. Moulin, J. L. Iniguez, S. Ravilly, F. Habib, P. Lebon, and G. Kalifa. 1997. Etiology and response to antibiotic therapy of community-acquired pneumonia in French children. Eur. J. Clin. Microbiol. Infect. Dis. 16:388-391. [DOI] [PubMed] [Google Scholar]
- 13.Hardy, R. D., H. S. Jafri, K. Olsen, J. Hatfield, J. Iglehart, B. B. Rogers, P. Patel, G. Cassell, G. H. McCracken, and O. Ramilo. 2002. Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection-associated chronic reactive airway disease. Infect. Immun. 70:649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy, R. D., H. S. Jafri, K. Olsen, M. Wordemann, J. Hatfield, B. B. Rogers, P. Patel, L. Duffy, G. Cassell, G. H. McCracken, and O. Ramilo. 2001. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect. Immun. 69:3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy, R. D., A. M. Rios, S. Chavez-Bueno, H. S. Jafri, J. Hatfield, B. B. Rogers, G. H. McCracken, and O. Ramilo. 2003. Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae-induced pneumonia. Antimicrob. Agents Chemother. 47:1614-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoellman, D. B., G. A. Pankuch, M. R. Jacobs, and P. C. Appelbaum. 2000. Antipneumococcal activities of GAR-936 (a new glycylcycline) compared to those of nine other agents against penicillin-susceptible and -resistant pneumococci. Antimicrob. Agents Chemother. 44:1085-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, A. P., C. L. Sheppard, S. J. Harnett, A. Birtles, T. G. Harrison, N. P. Brenwald, M. J. Gill, R. A. Walker, D. M. Livermore, and R. C. George. 2003. Emergence of a fluoroquinolone-resistant strain of Streptococcus pneumoniae in England. J. Antimicrob. Chemother. 52:953-960. [DOI] [PubMed] [Google Scholar]
- 18.Jones, R. N. 2001. Resistance patterns among nosocomial pathogens: trends over the past few years. Chest 119:397S-404S. [DOI] [PubMed] [Google Scholar]
- 19.Kannan, T. R., and J. B. Baseman. 2006. ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc. Natl. Acad. Sci. USA 103:6724-6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenny, G. E., and F. D. Cartwright. 2001. Susceptibilities of Mycoplasma hominis, M. pneumoniae, and Ureaplasma urealyticum to GAR-936, dalfopristin, dirithromycin, evernimicin, gatifloxacin, linezolid, moxifloxacin, quinupristin-dalfopristin, and telithromycin compared to their susceptibilities to reference macrolides, tetracyclines, and quinolones. Antimicrob. Agents Chemother. 45:2604-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenny, G. E., and F. D. Cartwright. 1993. Susceptibilities of Mycoplasma hominis, Mycoplasma pneumoniae, and Ureaplasma urealyticum to a new quinolone, OPC 17116. Antimicrob. Agents Chemother. 37:1726-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraft, M. 2000. The role of bacterial infections in asthma. Clin. Chest Med. 21:301-313. [DOI] [PubMed] [Google Scholar]
- 23.Kraft, M., G. H. Cassell, J. E. Henson, H. Watson, J. Williamson, B. P. Marmion, C. A. Gaydos, and R. J. Martin. 1998. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am. J. Respir. Crit. Care Med. 158:998-1001. [DOI] [PubMed] [Google Scholar]
- 24.Kraft, M., and Q. Hamid. 2006. Mycoplasma in severe asthma. J. Allergy Clin. Immunol. 117:1197-1198. [DOI] [PubMed] [Google Scholar]
- 25.Marc, E., M. Chaussain, F. Moulin, J. L. Iniguez, G. Kalifa, J. Raymond, and D. Gendrel. 2000. Reduced lung diffusion capacity after Mycoplasma pneumoniae pneumonia. Pediatr. Infect. Dis. J. 19:706-710. [DOI] [PubMed] [Google Scholar]
- 26.McCracken, G. H., Jr. 1986. Current status of antibiotic treatment for Mycoplasma pneumoniae infections. Pediatr. Infect. Dis. 5:167-171. [DOI] [PubMed] [Google Scholar]
- 27.Michelow, I. C., K. Olsen, J. Lozano, N. K. Rollins, L. B. Duffy, T. Ziegler, J. Kauppila, M. Leinonen, and G. H. McCracken, Jr. 2004. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics 113:701-707. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson, A. C., P. Björkman, and K. Persson. 2008. Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiol. 8:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nord, C. E., A. Lindmark, and I. Persson. 1993. In vitro activity of DMG-Mino and DMG-DM Dot, two new glycylcyclines, against anaerobic bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 12:784-786. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen, B. A., Y. Gluzman, and F. P. Tally. 1994. Inhibition of protein synthesis occurring on tetracycline-resistant, TetM-protected ribosomes by a novel class of tetracyclines, the glycylcyclines. Antimicrob. Agents Chemother. 38:1658-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rios, A. M., M. Fonseca-Aten, A. Mejias, S. Chavez-Bueno, K. Katz, A. M. Gomez, G. H. McCracken, Jr., O. Ramilo, and R. D. Hardy. 2005. Microbiologic and immunologic evaluation of a single high dose of azithromycin for treatment of experimental Mycoplasma pneumoniae pneumonia. Antimicrob. Agents Chemother. 49:3970-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rios, A. M., A. Mejias, S. Chavez-Bueno, M. Fonseca-Aten, K. Katz, J. Hatfield, A. M. Gomez, H. S. Jafri, G. H. McCracken, Jr., O. Ramilo, and R. D. Hardy. 2004. Impact of cethromycin (ABT-773) therapy on microbiological, histologic, immunologic, and respiratory indices in a murine model of Mycoplasma pneumoniae lower respiratory infection. Antimicrob. Agents Chemother. 48:2897-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubinstein, E., and D. Vaughan. 2005. Tigecycline: a novel glycylcycline. Drugs 65:1317-1336. [DOI] [PubMed] [Google Scholar]
- 34.Salvatore, C. M., M. Fonseca-Aten, K. Katz-Gaynor, A. M. Gomez, and R. D. Hardy. 2008. Intranasal interleukin-12 therapy inhibits Mycoplasma pneumoniae clearance and sustains airway obstruction in murine pneumonia. Infect. Immun. 76:732-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvatore, C. M., M. Fonseca-Aten, K. Katz-Gaynor, A. M. Gomez, A. Mejias, C. Somers, S. Chavez-Bueno, G. H. McCracken, and R. D. Hardy. 2007. Respiratory tract infection with Mycoplasma pneumoniae in interleukin-12 knockout mice results in improved bacterial clearance and reduced pulmonary inflammation. Infect. Immun. 75:236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, C. B., W. T. Friedewald, and R. M. Chanock. 1967. Shedding of Mycoplasma pneumoniae after tetracycline and erythromycin therapy. N. Engl. J. Med. 276:1172-1175. [DOI] [PubMed] [Google Scholar]
- 37.Sum, P. E., V. J. Lee, R. T. Testa, J. J. Hlavka, G. A. Ellestad, J. D. Bloom, Y. Gluzman, and F. P. Tally. 1994. Glycylcyclines. 1. A new generation of potent antibacterial agents through modification of 9-aminotetracyclines. J. Med. Chem. 37:184-188. [DOI] [PubMed] [Google Scholar]
- 38.Tagliabue, C., C. M. Salvatore, C. Techasaensiri, A. Mejias, J. P. Torres, K. Katz, A. M. Gomez, S. Esposito, N. Principi, and R. D. Hardy. 2008. The impact of steroids given with macrolide therapy on experimental Mycoplasma pneumoniae respiratory infection. J. Infect. Dis. 198:1180-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Testa, R. T., P. J. Petersen, N. V. Jacobus, P. E. Sum, V. J. Lee, and F. P. Tally. 1993. In vitro and in vivo antibacterial activities of the glycylcyclines, a new class of semisynthetic tetracyclines. Antimicrob. Agents Chemother. 37:2270-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Ogtrop, M. L., D. Andes, T. J. Stamstad, B. Conklin, W. J. Weiss, W. A. Craig, and O. Vesga. 2000. In vivo pharmacodynamic activities of two glycylcyclines (GAR-936 and WAY 152,288) against various gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 44:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallace, R. J., Jr., B. A. Brown-Elliott, C. J. Crist, L. Mann, and R. W. Wilson. 2002. Comparison of the in vitro activity of the glycylcycline tigecycline (formerly GAR-936) with those of tetracycline, minocycline, and doxycycline against isolates of nontuberculous mycobacteria. Antimicrob. Agents Chemother. 46:3164-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wubbel, L., L. Muniz, A. Ahmed, M. Trujillo, C. Carubelli, C. McCoig, T. Abramo, M. Leinonen, and G. H. McCracken, Jr. 1999. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr. Infect. Dis. J. 18:98-104. [DOI] [PubMed] [Google Scholar]