Abstract
Daptomycin is approved for treatment of Staphylococcus aureus bacteremia and right-sided endocarditis. Increases in daptomycin MICs have been associated with failure. A rabbit model of aortic valve endocarditis was used to determine whether MIC correlates with activity in vivo and whether a higher daptomycin dose can improve efficacy. Two related clinical S. aureus strains, one with a daptomycin MIC of 0.5 μg/ml and the other with a MIC of 2 μg/ml, were used to establish aortic valve endocarditis in rabbits. Daptomycin was administered once a day for 4 days at 12 mg/kg of body weight or 18 mg/kg to simulate doses in humans of 6 mg/kg and 10 mg/kg, respectively. Endocardial vegetations, spleens, and kidneys were harvested and quantitatively cultured. The strain with a MIC of 2 μg/ml had a survival advantage over the strain with a MIC of 0.5 μg/ml with >100 times more organisms of the former in endocardial vegetations at the 12-mg/kg dose in a dual-infection model. Both the 12-mg/kg dose and the 18-mg/kg dose completely eradicated the strain with a MIC of 0.5 from vegetations, spleens, and kidneys. The 12-mg/kg dose was ineffective against the strain with a MIC of 2 in vegetations; the 18-mg/kg dose produced a reduction of 3 log10 units in CFU in vegetations compared to the controls, although in no rabbit were organisms completely eliminated. Increasing the dose of daptomycin may improve its efficacy for infections caused by strains with reduced daptomycin susceptibility.
Daptomycin is FDA approved for treatment of bacteremia and right-sided endocarditis caused by methicillin-susceptible or methicillin-resistant strains of Staphylococcus aureus based on the results of a randomized controlled trial which showed that daptomycin was not inferior to standard therapy (4). In a subset analysis of patients with methicillin-resistant S. aureus infections, cure rates were higher for daptomycin-treated patients than for vancomycin-treated patients; on the other hand, the microbiologic failure rate was higher with daptomycin than it was with standard therapy. Neither of these differences were statistically significant.
Isolates with reduced susceptibility to daptomycin emerged during daptomycin therapy in 6 of the 19 patients with microbiologic failure and in 1 patient who was a clinical and microbiological success (4). The breakpoint for resistance to daptomycin is not formally defined, but a MIC of 2 μg/ml is considered “nonsusceptible.” Baseline MICs increased from 0.25 to 0.5 μg/ml to 2 μg/ml in six isolates and to 4 μg/ml in one isolate. Emergence of nonsusceptible isolates in many cases occurred in patients who did not receive surgical intervention for a deep-seated focus of infection. In the one infection caused by a nonsusceptible isolate (MIC = 2) in which daptomycin was successful, the patient underwent multiple debridement procedures. Persisting, undrained deep infection is an important and well-known cause of clinical and microbiological failure, which confounds determining the contribution of reduced in vitro susceptibility to the observed treatment failure.
The purpose of these studies was to determine whether there is a relationship between MIC and response to therapy, or more specifically, whether a daptomycin MIC of 2 μg/ml for S. aureus is associated with reduced bactericidal activity in vivo at therapeutically relevant doses and concentrations. There were two hypotheses: (i) daptomycin would be significantly less active in vivo against a strain with a MIC of 2 μg/ml compared to a strain with a MIC of 0.5 μg/ml and (ii) daptomycin activity against the strain with a MIC of 2 μg/ml would be dose dependent. The rabbit model of aortic valve endocarditis (2) was used to establish experimental infection either with the fully susceptible strain with a MIC of 0.5 μg/ml or with its nonsusceptible variant strain with a MIC of 2 μg/ml or with both strains in a competition, dual-infection model. Rabbits were treated with one of two doses of daptomycin, either 12 mg/kg of body weight once daily or 18 mg/kg, administered intravenously once daily. These doses were selected because they approximate the plasma AUCs (area under the concentration-time curve) achieved upon administration of 6 mg/kg and 10 mg/kg to humans. Daptomycin has concentration-dependent killing, and the 24-hour AUC/MIC ratio is strongly correlated with bactericidal activity in the neutropenic mouse thigh infection model (8, 11). At the end of therapy, the rabbits were sacrificed, and quantitative cultures were performed to determine bacterial counts in endocardial vegetations, kidneys, and spleens. The effects of daptomycin dose and strain susceptibility (as measured by MIC) on bacterial counts were assessed.
MATERIALS AND METHODS
Bacterial strains.
Two S. aureus clinical isolates were used. Strain A0.5 is a pretreatment blood isolate, and strain B2.0 is an on-therapy, day 20, blood isolate that arose in the patient from whom strain A0.5 was isolated and who experienced microbiological failure with daptomycin-plus-rifampin (rifampicin) combination therapy. The isolates had the same spa type, t002 (same as TJMBMDMGMK), common to multilocus sequence type 5, and an identical pulsed-field gel electrophoresis pattern, corresponding to the USA800 lineage of methicillin-resistant S. aureus (9, 12). Strain A0.5 is rifampin susceptible (MIC = 0.5 μg/ml) and has a daptomycin MIC of 0.5 μg/ml, as determined by Etest and by broth dilution with Ca2+-supplemented Mueller-Hinton broth (MHB). Strain B2.0 is rifampin resistant (MIC = 8 μg/ml) and has a daptomycin MIC of 2.0 μg/ml (nonsusceptible). Strain B2.0 has a leucine-to-phenylalanine substitution mutation in amino acid residue 826 of the mprF gene, which has been shown previously to be associated with daptomycin nonsusceptibility (5). The growth curves in tryptic soy broth for the two isolates were superimposable, and both had doubling times of 30 min in the exponential growth phase.
Pharmacokinetic studies.
The pharmacokinetics of daptomycin were determined after intravenous bolus; a single dose of 12 mg/kg or 18 mg/kg was infused over 1 to 2 min via the marginal vein to healthy New Zealand White rabbits (three rabbits per group). Blood was drawn at 0.25, 0.5, 1, 2, 4, and 6 h after injection. Daptomycin plasma concentrations were assayed by a validated high-performance liquid chromatography method (3). Standard noncompartmental analysis was used for the evaluation of pharmacokinetic parameters (WinNolin professional 5.2). The AUCinf was calculated by the log linear trapezoidal rule with extrapolation from 0 to infinity. The initial concentration was estimated by extrapolating from the plasma concentrations at 0.25 and 0.5 h back to the end of the infusion according to the log linear trapezoidal rule. The terminal elimination rate constant (kel) was determined from the slope of the terminal portion of the log concentration-time curve. The half-life, t1/2, was calculated as t1/2 = ln 2/kel.
Rabbit model of endocarditis.
New Zealand White rabbits (2.5 to 3 kg) were used. Endocarditis was established by standard methods (2). Briefly, a cutdown was made over the right carotid artery. A polyethylene catheter was introduced via carotid arteriotomy, positioned into the left ventricle, and sutured in place. Forty-eight to seventy-two hours later, a 1-ml suspension of ∼106 CFU of S. aureus in 0.9% NaCl was injected intravenously. In the dual-infection format, a standardized inoculum of strain A0.5 and strain B2.0 mixed at a ∼1:1 ratio was administered. On postinoculation day 1, approximately 16 to 18 h after infection, untreated control rabbits were sacrificed to determine pretreatment bacterial counts. The hearts, spleens, and kidneys were harvested. Aortic valves and endocardial vegetations and approximately 0.5-g samples of spleen and kidney were placed in 0.5 ml of 0.9% NaCl and homogenized with a tissue grinder. Tenfold serial dilutions of homogenate were prepared, and 0.1-ml volumes were inoculated onto blood agar medium, which was incubated for 24 to 48 h at 35°C. The remaining volume of homogenate was inoculated onto a blood agar plate. The numbers of CFUs were counted to determine the tissue burdens of organisms. The lower limit of detection is approximately 1 log10 CFU/g. For the dual-infection, competition model, the CFUs of strain A0.5 and strain B2.0 were differentiated by replica plating CFU growing on blood agar onto tryptic soy agar (TSA) plus 1 μg/ml of rifampin as a selective medium that allowed growth of strain B2.0 only.
Antibiotic-treated rabbits were administered daptomycin 12 mg/kg or 18 mg/kg intravenously once daily for 4 days. The first dose of drug was administered 18 to 20 h after inoculation. These rabbits were sacrificed on postinoculation day 5 approximately 18 to 24 h after the last dose of daptomycin. Bacterial burdens in endocardial vegetations, spleens, and kidneys were determined as described above for controls. Rabbits that died prior to sacrifice on day 5 or those sacrificed because of moribund condition had quantitative tissue cultures performed and were included in the data analysis only if they had received at least two doses of daptomycin.
Statistical analysis.
The number of organisms in tissues was expressed as log10 CFU/gram; tissues with no growth were assigned a value of 0. Statistically significant differences in bacterial burden in tissues among control and daptomycin (12-mg/kg/day and 8-mg/kg/day) groups were determined by analysis of variance. The Newman-Keuls test was used to determine statistically significant differences for pair-wise comparisons. The Fisher exact test was used to compare sterilization rates and ratios of strain A0.5 to strain B2.0 in tissues of untreated and daptomycin-treated animals. Statistically significant differences were defined as those with P < 0.05.
RESULTS
Pharmacokinetic studies.
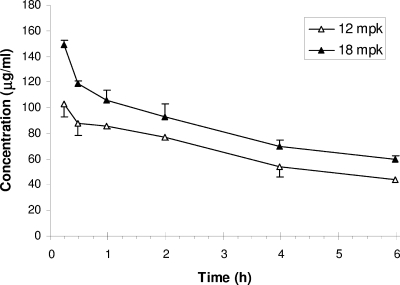
The plasma concentrations of daptomycin in rabbits given a single intravenous dose of 12 mg/kg or 18 mg/kg are shown in Fig. 1. The mean concentrations of plasma daptomycin 15 min after the end of infusion were 103.3 ± 6.4 μg/ml and 148.7 ± 17.9 μg/ml for the rabbits given 12-mg/kg and 18-mg/kg doses, respectively. The plasma half-life was approximately 5 h for both doses, and the mean AUCs were 719 ± 114 μg · h/ml and 965 ± 152 μg · h/ml for the rabbits given 12-mg/kg and 18-mg/kg doses, respectively. These mean AUCs were similar to those achieved at steady state in healthy humans administered a 6-mg/kg dose (AUC = 632 μg · h/ml) or a 10-mg/kg dose (AUC = 1,039 μg · h/ml) (1).
FIG. 1.
Plasma daptomycin pharmacokinetics in rabbits. Values of daptomycin plasma concentrations (mean ± standard error [error bar]) in uninfected rabbits given an intravenous dose of daptomycin, either 12 mg/kg (n = 3) or 18 mg/kg (n = 3), are shown. The concentration of daptomycin (in micrograms per milliliter) is given on the y axis.
Rabbit model of endocarditis.
Strain B2.0 had a survival advantage over strain A0.5 in the dual-infection model in rabbits treated with daptomycin at a dose of 12 mg/kg (roughly equivalent to a dose of 6 mg/kg in humans) (Table 1). Whereas strain A0.5 was 1.2 to 1.5 times more abundant than strain B2.0 in untreated controls, in daptomycin-treated rabbits, strain B2.0 was on the order of 100 times more abundant than strain A0.5. Daptomycin treatment significantly reduced bacterial counts in each of the infected tissues, although the effect was less for endocardial vegetations (mean decrease of 2.5 log10 units compared to the controls) than in other tissues (3.7 and 4.6 log10 units for spleen and kidney, respectively). None of the vegetation cultures was sterile; one spleen culture and three kidney cultures were sterile.
TABLE 1.
Bacterial tissue burdens and ratios of A0.5 CFU to strain B2.0 CFU in untreated control rabbits and in rabbits treated with daptomycin for 4 days in a dual-infection aortic valve endocarditis model
| Groupa | Endocardial vegetations
|
Spleens
|
Kidneys
|
|||
|---|---|---|---|---|---|---|
| Log10 CFU/gb | A0.5/B2.0 CFU ratioc | Log10 CFU/g | A0.5/B2.0 CFU ratio | Log10 CFU/g | A0.5/B2.0 CFU ratio | |
| No DAP (n = 6) | 7.8 ± 0.4 | 280:320 | 6.0 ± 0.3 | 333:267 | 6.3 ± 1.0 | 360:240 |
| DAP-12 (n = 7)d | 5.3 ± 2.1e | 1:604 | 2.3 ± 1.3f | 1:175 | 1.7 ± 2.2f | 0:156 |
The two groups were untreated control rabbits (no daptomycin [No DAP]) and rabbits treated with 12 mg of daptomycin/kg/day for 4 days (DAP-12).
Bacterial tissue burdens are shown as log10 CFU/g (means ± standard deviations).
The A0.5/B2.0 ratio is the total number of CFU for each tissue for all rabbits tested in the group that grew on blood agar minus the total number of CFU that grew on rifampin-containing agar (i.e., strain A0.5 CFU) to the total number of CFU that grew on rifampin-containing agar only (i.e., strain B2.0 CFU) (P < 0.0001 for the ratio for the no-DAP group versus the DAP-12 group by the Fisher exact test).
There were no deaths occurring in this group on therapy.
Significantly different from the value obtained for the no-DAP group (P < 0.05 by unpaired t test).
Significantly different from the value obtained for the no-DAP group (P < 0.001 by unpaired t test).
Both the 12-mg/kg and 18-mg/kg doses were highly effective for infection caused by the susceptible strain, strain A0.5 (Table 2). Not 1 CFU was recovered from any infected tissue at either dose.
TABLE 2.
Bacterial tissue burdens in rabbits infected with strain A0.5 and untreated or treated with two different doses of daptomycin in an aortic valve endocarditis modela
| Groupb | Bacterial tissue burden (log10 CFU/g) (mean ± SD) in:
|
||
|---|---|---|---|
| Endocardial vegetations | Spleens | Kidneys | |
| No DAP (n = 5) | 6.9 ± 0.7 | 6.1 ± 0.2 | 5.7 ± 0.7 |
| DAP-12 (n = 4)c | 0 ± 0e | 0 ± 0e | 0 ± 0e |
| DAP-18 (n = 5)d | 0 ± 0e | 0 ± 0e | 0 ± 0e |
A daptomycin dose of 12 mg/kg in rabbits produced an AUC of 719 μg · h/ml, comparable to the human 6-mg/kg dose (which produced an AUC of 632 μg · h/ml). A daptomycin dose of 18 mg/kg in rabbits produced an AUC of 965 μg · h/ml, comparable to the human 10-mg/kg dose (which produced an AUC of 1,039 μg · h/ml).
The three groups were untreated control rabbits (no daptomycin [No DAP]) and rabbits treated with 12 mg of daptomycin/kg/day for 4 days (DAP-12) or 18 mg of daptomycin/kg/day for 4 days (DAP-18).
Six rabbits were allocated to this arm. One rabbit was found dead on day 2 after receiving only one dose of daptomycin, and one rabbit was found moribund and euthanized on day 2 after receiving only one dose of daptomycin; neither of these rabbits was included in the data analysis.
Five rabbits were allocated to this arm. Two rabbits died on day 2 having received two doses of daptomycin, and both were included in the data analysis.
Significantly different from the value obtained for the no-DAP group (P < 0.001). All cultures from the daptomycin-treated animals were sterile.
Increasing the dose of daptomycin from 12 mg/kg/day to 18 mg/kg/day improved efficacy against the more resistant strain, strain B2.0 (Table 3). In the single-strain infection model, daptomycin at 12 mg/kg/day reduced the mean number of organisms by 1 log10 unit compared to the controls, not statistically significantly different. At a dose of 18 mg/kg/day, there was a reduction of 2.9 log10 units compared to the controls and a reduction of 1.9 log10 units compared to the 12-mg/kg/day dose, both of which were statistically significant. No rabbit in any group had sterile vegetations.
TABLE 3.
Bacterial tissue burdens in rabbits infected with strain B2.0 and untreated or treated with two different doses of daptomycin in an aortic valve endocarditis modela
| Groupb | Bacterial tissue burden (log10 CFU/g) (mean ± SD) in:
|
||
|---|---|---|---|
| Endocardial vegetations | Spleens | Kidneys | |
| No DAP (n = 5) | 7.3 ± 1.0 | 5.4 ± 0.2 | 5.1 ± 0.6 |
| DAP-12 (n = 9)c | 6.3 ± 1.8 | 3.2 ± 1.6e | 2.1 ± 1.9e |
| DAP-18 (n = 9)d | 4.4 ± 1.6e,f | 2.3 ± 1.6e | 2.5 ± 1.2e |
A daptomycin dose of 12 mg/kg in rabbits produced an AUC of 719 μg · h/ml, comparable to the human 6-mg/kg dose (which produced an AUC of 632 μg · h/ml). A daptomycin dose of 18 mg/kg in rabbits produced an AUC of 965 μg · h/ml, comparable to the human 10-mg/kg dose (which produced an AUC of 1,039 μg · h/ml).
The three groups were untreated control rabbits (no daptomycin [No DAP]) and rabbits treated with 12 mg of daptomycin/kg/day for 4 days (DAP-12) or 18 mg of daptomycin/kg/day for 4 days (DAP-18).
Eleven rabbits were allocated to this arm. Two rabbits, one found dead on day 1 and the other on day 2, received one dose of daptomycin each and were not included in the data analysis. Two euthanized rabbits were included in the analysis: one, given two doses of daptomycin, was found moribund on day 2 (endocardial vegetation titer of 6.48 log10 CFU/g); the other, given three doses of daptomycin, was found moribund on day 4 (vegetation titer of 8.28 log10 CFU/g).
Nine rabbits were allocated to this arm. One rabbit given two doses of daptomycin was found moribund and euthanized on day 2 (endocardial vegetation titer of 2.79 log10 CFU/g) and included in the data analysis.
Significantly different from the value obtained for the no-DAP group (P < 0.05).
Significantly different from the value obtained for the DAP-12 group (P < 0.05).
Both the 12-mg/kg dose and the 18-mg/kg dose were effective in reducing bacterial burdens in the spleens and kidneys of rabbits infected with strain B2.0, and the magnitude of reduction, 2 to 3 log10 units compared to the controls, was about the same for both doses. Of the nine rabbits treated with the 12-mg/kg dose, none had sterile spleen cultures and three had sterile kidney cultures. Of the nine rabbits treated with the 18-mg/kg dose, two had sterile spleen cultures and one had a sterile kidney culture. Sterilization rates for vegetations and tissues were significantly less for strain B2.0 daptomycin-treated rabbits versus strain A0.5 rabbits (P < 0.0001, the Fisher exact test).
DISCUSSION
A daptomycin MIC of 2 μg/ml compared to a MIC of 0.5 μg/ml was associated with reduced efficacy in the rabbit model of S. aureus aortic valve endocarditis. In the dual-infection model in which animals were administered daptomycin at a dose of 12 mg/kg/day to mimic the drug exposure in humans administered a dose of 6 mg/kg, the strain with a MIC of 2 μg/ml persisted, accounting for >99% of the CFU that were recovered from daptomycin-exposed animals. The susceptible strain (MIC = 0.5 μg/ml) was largely eliminated. Of the 937 colonies from daptomycin-treated rabbits that were tested, 935 were the nonsusceptible strain B2.0. In the untreated controls, 973 of 1,700 colonies were the susceptible strain, strain A0.5. Daptomycin was more efficacious against bacteria from the spleen and kidney, reducing counts by 4 log10 units compared to the controls, than against bacteria from endocardial vegetations in which there was a decrease in CFU of 2.5 log10 units.
Because the two strains that were tested were both clinical isolates and may differ in respects other than their susceptibility to daptomycin and rifampin, it is formally possible that such differences could have affected the results. However, there is little reason to doubt that the higher MIC to daptomycin was the main determinant of outcome. The two strains had identical genotypes, they exhibited indistinguishable growth curves in vitro, they were isolated from the same patient, the higher MIC strain was associated with treatment failure, and it had the iconic mutation associated with daptomycin nonsusceptibility in vitro and with treatment failure clinically. Resistance to rifampin in the isolate with the higher MIC is also unlikely to have much of an impact, although this cannot be definitively ruled out. In the absence of daptomycin selective pressure, the numbers of rifampin-susceptible CFU and rifampin-resistant CFU were similar in untreated controls, suggesting that the rifampin phenotype had little, if any, effect on fitness or conferred any advantage one way or the other. Thus, considering all of the evidence, it seems virtually certain that the outcome of the experiments was driven by the susceptibility or lack thereof to daptomycin.
Increasing the dose of daptomycin in rabbits from 12 mg/kg/day (equivalent to a 6-mg/kg dose in humans) to 18 mg/kg/day (equivalent to a 10-mg/kg dose in humans) improved efficacy against infection by the nonsusceptible strain, strain B2.0. The number of CFU in endocardial vegetations of rabbits treated with the 18-mg/kg dose was reduced by approximately ∼3 log10 units compared to the controls, a statistically significant difference, whereas the number of CFU in rabbits treated with the 12-mg/kg dose was not significantly different from the value for the controls. Efficacies of the 12-mg/kg and 18-mg/kg doses were similar for bacteria from the spleen and kidney, also achieving a reduction of ∼3 log10 units compared to the controls. Although the bacterial burdens were reduced, no vegetations and only a few kidneys and spleens were sterile at either dose.
Daptomycin was highly efficacious for infection caused by the susceptible strain (A0.5) exposed to either dose. In contrast to results with the strain with a MIC of 2 μg/ml, no colony of strain A0.5 was recovered from the tissues of any rabbit that received daptomycin.
Daptomycin exhibits concentration-dependent killing, and its efficacy has been correlated with two pharmacokinetic/pharmacodynamic parameters, AUC/MIC and maximum concentration of drug in serum/MIC, in various in vitro and in vivo infection models (8, 11). The results of the endocarditis experiments are consistent with predictions based on results in a neutropenic murine thigh infection model reported by Safdar et al. (11). In this model, a bacteriostatic effect was achieved at a 24-hour AUC/MIC mean ratio of 438, a 1-log10-unit killing effect was achieved at an AUC/MIC ratio of 666, and a 2-log10-unit killing effect was achieved at an AUC/MIC ratio of 1,061. These effect values for the peak plasma concentration/MIC ratio were 71, 129, and 255, respectively. In the case of infection with strain A0.5, the 12 mg/kg dose and the 18 mg/kg dose each exceeded the AUC/MIC threshold for a 2 log10 kill effect, with values of 1,438 and 1,930, respectively, and the concenttration at 15 min postinfusion to MIC [C(15′)/MIC] ratios of 206 and 297 were near the peak concentration/MIC threshold for a 2 log10 kill. For infection with strain B2.0, the AUC/MIC of 360 and C(15′)/MIC of 51 for the 12 mg/kg regimen both were below stasis thresholds. The AUC/MIC of 482 and C(15′)/MIC of 74 for the 18 mg/kg dose both fell between the stasis and 1 log10 kill thresholds. Accordingly, the higher dose was more effective than the lower dose in eradicating organisms from vegetations. Similar efficacies against both susceptible and nonsusceptible bacteria in the spleen and kidney regardless of dose likely reflects an intact and effective polymorphonuclear leukocyte response in these tissues but could also be due to higher local drug concentrations in these tissues.
These results suggest that daptomycin at a dose of 10 mg/kg/day (and perhaps higher) may be more effective than the currently approved 6-mg/kg/day dose for humans with serious S. aureus infections caused by nonsusceptible strains (i.e., those with MICs of >1 μg/ml). Data are limited concerning the safety of daptomycin at doses in excess of 6 mg/kg per day. Doses up to 12 mg/kg per day for 14 days were well tolerated in one study of healthy volunteers (1), but the safety profile with longer durations of exposure is unknown. There is also the question of whether a somewhat more effective dose is sufficiently efficacious to be clinically useful. Even with the higher daptomycin dose, attainment of target pharmacokinetic/pharmacodynamic parameters may be marginal and inadequate to effect a cure. This may explain why the nonsusceptible strain, strain B2.0, persisted in tissues even at the higher dose, whereas the susceptible strain was completely eradicated at both doses. Variability has been observed in the bactericidal activity of a simulated 10-mg/kg/day dose of daptomycin, alone or in combination with rifampin or gentamicin, against nonsusceptible strains in an in vitro fibrin clot model (10). Thus, while a dose higher than the recommended 6-mg/kg dose of daptomycin at this point remains an option for treatment of infection caused by daptomycin strains with MICs of >1, this MIC could also denote “true” resistance, i.e., resistance that is not amenable to being overcome simply by dose escalation and more drug exposure. Indeed, the nonsusceptible B2.0 strain has a mutation in mpfR that is associated with loss of daptomycin activity in vitro and treatment failure (6, 7). Also, in contrast to vancomycin, which retains potent bactericidal activity against vancomycin-intermediate strains at concentrations above the MIC (e.g., 10-fold), the loss of bactericidal activity against daptomycin-nonsusceptible strains persists at daptomycin concentrations well above its MIC (e.g., approximately 20-fold) (7).
A much more pressing and important clinical issue than treatment of infection caused by daptomycin-nonsusceptible S. aureus is how to prevent the emergence of resistance during therapy. While daptomycin doses of 10 to 12 mg/kg/day may be an effective strategy, these experiments did not directly test this. Because daptomycin so effectively eradicated the susceptible strain, nonsusceptible isolates were not selected from the susceptible population in this treatment model. Should a nonsusceptible mutant arise, the 3-log10-unit kill by daptomycin against the nonsusceptible strain in vegetations gives reason to believe that such a mutant might be eliminated. However, the numbers of organisms in humans with invasive S. aureus disease may be several orders of magnitude higher than those achieved in this rabbit model. The critical role of surgical debridement in management of invasive S. aureus infections cannot be overemphasized in order to reduce the bacterial load and the opportunity for resistance to emerge and to eliminate foci where nonsusceptible mutants can persist.
Acknowledgments
This research was supported by a grant from Cubist Pharmaceuticals.
Footnotes
Published ahead of print on 26 January 2009.
REFERENCES
- 1.Benvenuto, M., D. P. Benziger, S. Yankelev, and G. Vigliani. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers, H. F., C. J. Hackbarth, T. A. Drake, M. G. Rusnak, and M. A. Sande. 1984. Endocarditis due to methicillin-resistant Staphylococcus aureus in rabbits: expression of resistance to beta-lactam antibiotics in vivo and in vitro. J. Infect. Dis. 149:894-903. [DOI] [PubMed] [Google Scholar]
- 3.Dvorchik, B. H., D. Brazier, M. F. DeBruin, and R. D. Arbeit. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler, V. G., Jr., H. W. Boucher, G. R. Corey, E. Abrutyn, A. W. Karchmer, M. E. Rupp, D. P. Levine, H. F. Chambers, F. P. Tally, G. A. Vigliani, C. H. Cabell, A. S. Link, I. DeMeyer, S. G. Filler, M. Zervos, P. Cook, J. Parsonnet, J. M. Bernstein, C. S. Price, G. N. Forrest, G. Fatkenheuer, M. Gareca, S. J. Rehm, H. R. Brodt, A. Tice, and S. E. Cosgrove. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653-665. [DOI] [PubMed] [Google Scholar]
- 5.Friedman, L., J. D. Alder, and J. A. Silverman. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:2137-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Julian, K., K. Kosowska-Shick, C. Whitener, M. Roos, H. Labischinski, A. Rubio, L. Parent, L. Ednie, L. Koeth, T. Bogdanovich, and P. C. Appelbaum. 2007. Characterization of a daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus strain in a patient with endocarditis. Antimicrob. Agents Chemother. 51:3445-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemaire, S., K. Kosowska-Shick, K. Julian, P. M. Tulkens, F. Van Bambeke, and P. C. Appelbaum. 2008. Activities of antistaphylococcal antibiotics towards the extracellular and intraphagocytic forms of Staphylococcus aureus isolates from a patient with persistent bacteraemia and endocarditis. Clin. Microbiol. Infect. 14:766-777. [DOI] [PubMed] [Google Scholar]
- 8.Louie, A., P. Kaw, W. Liu, N. Jumbe, M. H. Miller, and G. L. Drusano. 2001. Pharmacodynamics of daptomycin in a murine thigh model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. 45:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose, W. E., S. N. Leonard, and M. J. Rybak. 2008. Evaluation of daptomycin pharmacodynamics and resistance at various dosage regimens against Staphylococcus aureus isolates with reduced susceptibilities to daptomycin in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 52:3061-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safdar, N., D. Andes, and W. A. Craig. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shopsin, B., M. Gomez, M. Waddington, M. Riehman, and B. N. Kreiswirth. 2000. Use of coagulase gene (coa) repeat region nucleotide sequences for typing of methicillin-resistant Staphylococcus aureus strains. J. Clin. Microbiol. 38:3453-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]