Abstract
Plectasin is the first defensin-type antimicrobial peptide isolated from a fungus and has potent activity against gram-positive bacteria. By using an experimental meningitis model, the penetration of plectasin into the cerebrospinal fluid (CSF) of infected and uninfected rabbits and the bactericidal activities in CSF of the plectasin variant NZ2114 and ceftriaxone against a penicillin-resistant Streptococcus pneumoniae strain (NZ2114 and ceftriaxone MICs, 0.25 and 0.5 μg/ml, respectively) were studied. Pharmacokinetic analysis showed that there was a significantly higher level of CSF penetration of NZ2114 through inflamed than through noninflamed meninges (area under the concentration-time curve for CSF/area under the concentration-time curve for serum, 33% and 1.1%, respectively; P = 0.03). The peak concentrations of NZ2114 in purulent CSF were observed ∼3 h after the infusion of an intravenous bolus of either 20 or 40 mg/kg of body weight and exceeded the MIC >10-fold for a 6-h study period. Treatment with NZ2114 (40 and 20 mg/kg at 0 and 5 h, respectively; n = 11) caused a significantly higher reduction in CSF bacterial concentrations than therapy with ceftriaxone (125 mg/kg at 0 h; n = 7) at 3 h (median changes, 3.7 log10 CFU/ml [interquartile range, 2.5 to 4.6 log10 CFU/ml] and 2.1 log10 CFU/ml [interquartile range, 1.7 to 2.6 log10 CFU/ml], respectively; P = 0.001), 5 h (median changes, 5.2 log10 CFU/ml [interquartile range, 3.6 to 6.1 log10 CFU/ml] and 3.1 log10 CFU/ml [interquartile range, 2.6 to 3.7 log10 CFU/ml], respectively; P = 0.01), and 10 h (median changes, 5.6 log10 CFU/ml [interquartile range, 5.2 to 5.9 log10 CFU/ml] and 4.2 log10 CFU/ml [interquartile range, 3.6 to 5.0 log10 CFU/ml], respectively; P = 0.03) after the start of therapy as well compared to the CSF bacterial concentrations in untreated rabbits with meningitis (n = 7, P < 0.05). Also, significantly more rabbits had sterile CSF at 5 and 10 h when they were treated with NZ2114 than when they were treated with ceftriaxone (67% [six of nine rabbits] and 0% [zero of seven rabbits], respectively, at 5 h and 75% [six of eight rabbits] and 14% [one of seven rabbits], respectively, at 10 h; P < 0.05). Due to its excellent CSF penetration and potent bactericidal activity in CSF, the plectasin variant NZ2114 could be a promising new option for the treatment of CNS infections caused by gram-positive bacteria, including penicillin-resistant pneumococcal meningitis.
Streptococcus pneumoniae is the leading cause of bacterial meningitis, and pneumococcal meningitis still remains a disease with high rates of mortality and morbidity (8). The cornerstone of treatment for bacterial meningitis is the prompt initiation of adequate antibiotic therapy (12), and the rapid sterilization of the cerebrospinal fluid (CSF) with antibiotic therapy has been associated with a better clinical outcome (4). The worldwide emergence of antibiotic-resistant strains of S. pneumoniae still demands the development of new antimicrobial agents for the treatment of pneumococcal disease, including meningitis.
Antimicrobial peptides are evolutionarily ancient molecules with antimicrobial effects that are widely distributed in plants and animals and that are used by the innate immune system to control infections. They are a new class of antimicrobial agents, but their mechanisms of action have not yet been determined. Most antimicrobial peptides are cationic and readily bind to the negatively charged bacterial membrane. Their activity may be promoted by their ability to compromise bacterial membranes (e.g., by fatal depolarization of the bacterial membrane, degradation of the cell wall by hydrolases, or disturbance of membrane function), or they may interact with critical intracellular targets (for a review, see reference 13).
Plectasin was the first defensin-like antimicrobial peptide isolated from a black saprophytic ascomycete (Pseudoplectania nigrella) and showed potent activity against gram-positive bacteria in vitro and in vivo (5). NZ2114 was identified in a high-throughput mutation and screening campaign aimed at identifying variants of plectasin with improved potency against staphylococci and streptococci (10). NZ2114 exhibited improved in vitro activity compared with the activities of other plectasin variants against staphylococci, including Staphylococcus aureus, as well as S. pneumoniae and hemolytic streptococcal strains resistant to clinically used antibiotics (11). Little is known about the distribution of antimicrobial peptides into various body tissues, including their penetration across the blood-brain barrier, which forms a tight membrane that limits the entry of many antimicrobial agents into the central nervous system. Therefore, when a new antimicrobial agent is introduced, it is important to study its penetration into the brain and to determine the bactericidal activity of the drug in this compartment.
Correspondingly, we studied the CSF penetration of NZ2114 as well as its bactericidal activity in CSF against a penicillin-resistant S. pneumoniae strain in an experimental meningitis model.
MATERIALS AND METHODS
Test organism.
A penicillin-resistant but ceftriaxone-sensitive Streptococcus pneumoniae type 9V strain originally isolated from the CSF of a 78-year-old woman was used for all meningitis experiments. The MICs of NZ2114, ceftriaxone, and penicillin were 0.25 mg/liter, 0.5 mg/liter, and 1 mg/liter, respectively, as determined by the microdilution broth method according to the guidelines of the CLSI (formerly the NCCLS) (6).
Antimicrobial agents.
The NZ2114 plectasin variant was produced, purified, and formulated at Novozymes A/S, Bagsværd, Denmark. In short, Aspergillus oryzae MT3343 was selected as the production host, and the fermentation conditions were optimized at Novozymes. The fermented broth was pretreated, filtered, and centrifuged before the recovered product was further processed by two chromatographic purifications by use of a cation exchanger and hydrophobic interaction, followed by filtration with a 30-kDa cutoff and final diafiltration. The active pharmaceutical ingredient was diluted into the final formulation buffer (10 mM sodium acetate, 0.9% NaCl, pH 5.0) after diafiltration and germ filtered before filled in vials.
Stock solutions were made by dissolving NZ2114 (batch PSI6012; purity, 95%) in potassium-natrium-glucose infusion liquid (06105C03; Fresenius Kabi, Uppsala, Sweden) to a final concentration of 18.3 g/liter (vehicle pH, 5). Ceftriaxone (no. C-5793; Sigma Chemical Co., St. Louis, MO) was dissolved in sterile water to a final concentration of 50 g/liter. The antibiotics were administered intravenously as a bolus infusion over 5 to 10 min.
Determination of NZ2114 concentrations in CSF and serum.
The NZ2114 concentrations in CSF and serum were determined by high-pressure liquid chromatography. The lower limit of detection was 50 μg/liter.
Determination of bacterial concentrations.
Bacterial concentrations in CSF were determined by plating 140 μl of undiluted CSF and 10-fold serial dilutions of CSF (in duplicate) on 5% horse blood agar plates (Statens Serum Institute, Copenhagen, Denmark). Thus, the lowest detectable bacterial count was 7 CFU/ml. The bacterial counts in different dilutions of CSF were compared to exclude the possibility of the presence of significant carryover phenomena.
Meningitis model.
The experimental protocols were approved by the Danish Animal Experiments Inspectorate (Dyreforsøgstilsynet). A rabbit meningitis model previously described in detail was used (9). New Zealand White rabbits (weight, 2.5 to 3.0 kg) were anesthetized with midazolam (Dormicum; F. Hoffmann-La Roche AG, Basel, Switzerland) at 0.5 mg/kg of body weight subcutaneously and a combination of fentanyl-fluanisone (Hypnorm; Janssen Pharmaceutica N.V., Beerse, Belgium) at 0.35 ml/kg intramuscularly. A dental helmet in which a half turnbuckle was embedded was then attached to the skull. The rabbits were returned to their cages, after buprenorphin (Nycomed, Roskilde, Denmark) at 0.1 mg/kg was given as analgesia.
The rabbits that were to become infected with pneumococci were reanesthetized with midazolam and fentanyl-fluanisone 10 h later, followed by intracisternal inoculation of the test organism (∼1 × 106 CFU, as confirmed by quantitative cultures). Buprenorphin was again given, before the infected rabbits were returned to their cages. After another 12 h, all rabbits (both rabbits with meningitis and uninfected controls) were reanesthetized with ethyl carbamate (urethane; Fluka Kemi AG, Buchs, Switzerland) at 1.75 g/kg subcutaneously and pentobarbital (Mebumal; Nycomed) at 10 mg/kg and immobilized in a stereotaxic frame. A 25-gauge spinal needle was introduced into the cisterna magna for repetitive CSF sampling. Blood samples were taken from a central ear artery of the right ear, whereas antibiotics and pentobarbital were administered intravenously into the left ear. After the CSF and blood samples were tested for their bacterial concentrations, they were centrifuged, and the supernatants were immediately stored at −20°C for subsequent analysis.
Pharmacokinetics of NZ2114.
Three different doses (10, 20, and 40 mg/kg) were tested in uninfected rabbits and two different doses (20 and 40 mg/kg) were tested in infected rabbits (n = 2 for each group). CSF and blood samples were taken at 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, and 6 h after antibiotic challenge. The following pharmacokinetic parameters were determined for each rabbit by using Prism (version 4.01) software (GraphPad Software, Inc., La Jolla, CA): the maximum concentration in serum (Cmax), half-life (t1/2; estimated by the expression −log10 2/β, where β is the slope of the elimination regression line), the area under the concentration-time curve (AUC) from time zero to 6 h (AUC0-6), and the time at which the concentration remained above the MIC (T > MIC).
Efficacy of NZ2114 and ceftriaxone against meningitis caused by a penicillin-resistant pneumococcus.
The efficacy of NZ2114 administered at 40 mg/kg at 0 h and 20 mg/kg at 5 h (n = 11) was compared to that of ceftriaxone administered at 125 mg/kg at 0 h (n = 7). Seven untreated rabbits were reserved as a control group. CSF and blood samples were taken at 0, 1, 3, 5, 6, and 10 h after the start of antibiotic treatment and were analyzed for CSF bacterial concentrations at 0, 3, 5, and 10 h as well as concentrations of NZ2114 in CSF and blood at 1, 5, 6, and 10 h. The dose of NZ2114 was chosen to mimic the CSF pharmacokinetic profile after a bolus infusion with ceftriaxone (125 mg/kg), as previous experiments evaluating treatment with ceftriaxone for meningitis showed that CSF concentrations remained at concentrations 10× the MIC (∼5 mg/liter) for the test organism at 10 h after the start of therapy (9).
Statistical analysis.
All results are provided as medians and interquartile ranges. For calculation of the reduction in CSF bacterial concentrations (e.g., the change in the log10 CFU/ml from 0 to 3 h), a CSF concentration under the detection limit was assigned a value of 1 CFU/ml. Comparison between groups was performed by the nonparametric Mann-Whitney test for continuous data and by the Fisher exact test for categorical data. P values less than 0.05 were considered statistically significant.
RESULTS
Pharmacokinetics of NZ2114.
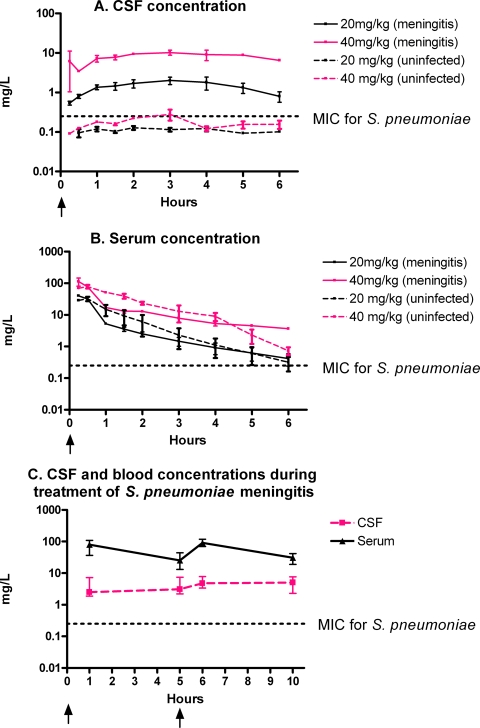
CSF and serum concentration-time curves and the values of the pharmacokinetic indices after the administration of intravenous bolus infusions of NZ2114 are shown in Fig. 1 and Table 1, respectively. Whereas the distribution of NZ2114 in the systemic compartment was comparable for both infected and uninfected rabbits (one-compartment model with a t1/2 of 54 min [interquartile range, 49 to 56 min]), the CSF penetration (ratio of the AUC for CSF/AUC for serum) was significantly higher in rabbits with meningitis than in the uninfected controls by use of the current dosing regimens (one dose of 20 and 40 mg/kg; Fig. 1A and B): 33% (interquartile range, 24 to 50%) and 1.1% (interquartile range, 0.7 to 1.9%), respectively (P = 0.03). The Cmax was 20 to 30 times higher in rabbits with meningitis than in uninfected rabbits (e.g., 10.4 mg/liter [interquartile range, 8.8 to 11.7 mg/liter] and 0.3 mg/liter [interquartile range, 0.2 to 0.4 mg/liter], respectively, after administration of a 40-mg/kg bolus infusion) and was observed ∼3 h after the bolus infusion. The CSF concentration did not decrease significantly after the time to Cmax (t1/2 was presumably more than 10 times longer in the CSF than in serum) and remained above the MIC for the 6-h study period for rabbits with meningitis, whereas it was below the MIC for the uninfected controls most of the time. During the treatment efficacy studies (40 and 20 mg/kg at 0 and 5 h, respectively), NZ2114 concentrations in CSF and blood remained at concentrations above 10× the MIC during the 10-h study period (Fig. 1C).
FIG. 1.
CSF and serum concentrations of NZ2114 in infected and uninfected rabbits. (A and B) NZ2114 was administered as an intravenous bolus infusion over 5 to 10 min at 0 h (arrows) (n = 2 for each group); (C) NZ2114 was administered at 0 h (40 mg/kg) and at 5 h (20 mg/kg) (arrows) (n = 11). Data are shown as medians and ranges (A and B) or interquartile ranges (C).
TABLE 1.
Pharmacokinetics of NZ2114 in CSF and blood of rabbits with and without pneumococcal meningitisa
| Disease state and NZ2114 dose (mg/kg) | t1/2 (min) in bloodb |
Cmax (μg/ml)c
|
AUC0-6 (mg·h/ml)
|
CSF penetration (%) | ||
|---|---|---|---|---|---|---|
| CSF | Blood | CSF | Blood | |||
| With meningitis | ||||||
| 20 | 55 (55-55) | 2.0 (1.6-2.4) | 40.2 (39.9-40.4) | 8.6 (6.4-10.8) | 31.4 (30.3-32.5) | 27 (21-33) |
| 40 | 68 (49-87) | 10.2 (8.8-11.7) | 113 (80.8-145) | 42.3 (34.2-50.4) | 97.0 (90.0-104) | 44 (33-56) |
| Without meningitis | ||||||
| 10 | 63 (61-64) | 0.10 (0.08-0.11) | 11.6 (11.4-11.7) | 0.55 (0.36-0.74) | 11.4 (8.5-14.3) | 4.7 (4.2-5.1) |
| 20 | 50 (45-54) | 0.12 (0.11-0.14) | 32.3 (27.2-37.3) | 0.58 (0.57-0.60) | 40.0 (26.8-53.1) | 1.6 (1.1-2.1) |
| 40 | 53 (49-57) | 0.28 (0.19-0.37) | 77.8 (68.0-87.5) | 1.0 (0.9-1.2) | 134 (111-156) | 0.8 (0.6-1.1) |
Data are shown as medians (ranges) (n = 2 for each group). T > MIC values were ∼100% and ∼0% for infected and uninfected CSF, respectively.
The t1/2 in CSF was not determined.
The times to Cmax were ∼15 to 30 min and ∼3 h in blood and CSF, respectively.
Treatment efficacy of NZ2114 in rabbits with meningitis caused by a penicillin-resistant pneumococcal strain.
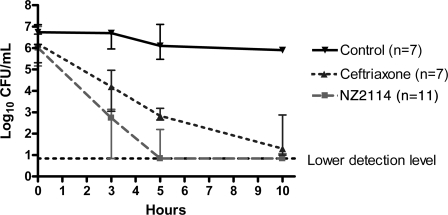
CSF bacterial concentrations are shown in Fig. 2. Before the initiation of antibiotic therapy (at 0 h), no significant differences in the CSF bacterial concentrations were observed among the three experimental groups: for NZ2114-treated rabbits (n = 11), 6.0 log10 CFU/ml (interquartile range, 5.2 to 7.0 log10 CFU/ml); for ceftriaxone-treated rabbits (n = 7), 6.2 log10 CFU/ml (interquartile range, 5.3 to 6.7 log10 CFU/ml); and for untreated rabbits with meningitis (n = 7), 6.7 log10 CFU/ml (interquartile range, 6.2 to 7.1 log10 CFU/ml) (P > 0.05). The NZ2114-treated rabbits had significantly lower CSF bacterial concentrations than the ceftriaxone-treated rabbits at 3 h (2.7 log10 CFU/ml [interquartile range, 0.8 to 3.1 log10 CFU/ml] and 4.2 [interquartile range, 3.1 to 5.0 log10 CFU/ml], respectively; P = 0.01), 5 h (0.8 log10 CFU/ml [interquartile range, 0.8 to 2.2 log10 CFU/ml] and 2.8 log10 CFU/ml [interquartile range, 2.6 to 3.2 log10 CFU/ml], respectively; P = 0.005), and 10 h (0.8 log10 CFU/ml [0.8 to 1.1 log10 CFU/ml] and 1.3 log10 CFU/ml [1.0 to 2.9], respectively; P = 0.05) after the start of antibiotic therapy, whereas antibiotic-treated rabbits had significantly lower CSF bacterial counts than untreated rabbits with meningitis at all three time points (P < 0.02) (Fig. 2). This was a significantly higher reduction in the numbers of CFU for rabbits treated with NZ2114 than for rabbits treated with ceftriaxone: at 3 h, the reductions were 3.7 log10 CFU/ml/h (interquartile range, 2.5 to 4.6 log10 CFU/ml/h) and 2.1 log10 CFU/ml/h (interquartile range, 1.7 to 2.6 log10 CFU/ml/h), respectively (P = 0.001); at 5 h, the reductions were 5.2 log10 CFU/ml/h (interquartile range, 3.6 to 6.1 log10 CFU/ml/h) and 3.1 log10 CFU/ml/h (interquartile range, 2.6 to 3.7 log10 CFU/ml/h), respectively (P = 0.01); and at 10 h, the reductions were 5.6 log10 CFU/ml/h (interquartile range, 5.2 to 5.9 log10 CFU/ml/h) and 4.2 log10 CFU/ml/h (interquartile range, 3.6 to 5.0 log10 CFU/ml/h), respectively (P = 0.03). In addition, significantly more NZ2114-treated rabbits than ceftriaxone-treated rabbits had sterile CSF: at 5 h, 67% (six of nine rabbits) and 0% (zero of seven rabbits), respectively, and at 10 h, 75% (six of eight rabbits) and 14% (one of seven rabbits), respectively (P < 0.05).
FIG. 2.
CSF bacterial concentrations after start of antibiotic therapy in experimental pneumococcal meningitis. Data are shown as medians and interquartile ranges. Significant differences were as follows: NZ2114-treated rabbits (n = 11) versus ceftriaxone-treated rabbits (n = 7) or untreated rabbits (controls; n = 7) and ceftriaxone-treated rabbits versus uninfected rabbits (controls) at 3, 5, and 10 h, P < 0.05 (Mann-Whitney test). Among the NZ2114- and ceftriaxone-treated rabbits, 67% (six of nine rabbits) and 0% (zero of seven rabbits), respectively, had sterile CSF at 5 h and 75% (six of eight rabbits) and 14% (one of seven rabbits), respectively, had sterile CSF at 10 h (Fisher exact test, P < 0.05). The lower limit of detection of bacteria was 7 CFU/ml.
DISCUSSION
To our knowledge, this is the first study to describe the CSF penetration and bactericidal activity in CSF of an antimicrobial peptide. The plectasin variant NZ2114 had a CSF penetration of 33% through inflamed meninges, which resulted in CSF concentrations above the MICs for most gram-positive pathogens, including S. pneumoniae. Interestingly, this level of CSF penetration was higher than the levels for most other antimicrobial agents except fluoroquinolones (e.g., ceftriaxone, ∼15%; vancomycin, 13%; moxifloxacin, 81%) (9). This indicates that even relatively large and water-soluble molecules like NZ2114 (a peptide of 40 amino acids in length) (5), which has a molecular mass (4.4 kDa) ∼10 times larger than the molecular masses of most other antimicrobial agents (e.g., the ceftriaxone molecular mass is 0.6 kDa) (7), readily enter the CSF when the blood-brain barrier is inflamed. In contrast, the level of CSF penetration of NZ2114 through an intact blood-brain barrier was poor (1.1%), and the concentration in CSF hardly reached the MIC after the administration of an intravenous dose of 40 mg/kg, but its level of penetration through noninflamed meninges was still comparable to that of ceftriaxone (∼2%) (1). Besides alterations in blood-brain barrier permeability, the lipid solubility, and the molecular mass of the drug, the level of protein binding and whether it is a substrate of various active influx and efflux pumps of the brain endothelium also influence the CSF penetration of antibiotics (for a review, see reference 2) and peptides (for a review, see reference 3). The passage of NZ2114 across an intact blood-brain barrier in part showed saturable features, since the CSF penetration decreased with higher doses; however, this was not the case for an inflamed blood-brain barrier.
The bactericidal activity of NZ2114 against penicillin-resistant pneumococci in CSF was high, resulting in a rapid killing rate in CSF (∼1 log10 CFU/ml/h) during experimental pneumococcal meningitis in rabbits, and this killing rate was significantly more rapid than that achieved with therapy with ceftriaxone, a recommended treatment for pneumococcal meningitis (12). Moreover, sterilization of the CSF was rapid and was observed for the majority of rabbits within 5 h of therapy with NZ2114, and no regrowth was observed during the following 5 h of therapy. A previous study investigated the in vivo activity of the parent wild-type molecule against pneumococci by using a mouse peritonitis model and a mouse pneumonia model and found, in accordance with the findings of the present study, that plectasin had excellent killing rates that were comparable to those achieved with therapy with other antibiotics (5).
Most antimicrobial peptides are positively charged and readily bind to the negatively charged phospholipid of the bacterial cytoplasmic membrane, which promotes the rapid killing of microorganisms at concentrations equivalent or close to the MIC. In addition, contrary to the case when conventional antibiotics are used, the development of resistance to antimicrobial peptides seems to be surprisingly rare. It has been hypothesized that antimicrobial peptides primarily work by compromising bacterial membranes (e.g., by fatal depolarization of the bacterial membrane, degradation of the cell wall by hydrolase, and the disturbance of membrane functions [for a review, see reference 13]). However, preliminary results showed that the mechanism by which NZ2114 kills bacteria is different from membrane lysis (H.-H. Kristensen, personal communication). In addition, no cross-resistance to other classes of antibiotics has yet been observed (Kristensen, personal communication). Thus, due to the emergence of resistant pneumococci and the potent bactericidal activity of NZ2114 in CSF, NZ2114 could be an interesting option for the treatment of pneumococcal meningitis and warrants further clinical and experimental evaluations.
In conclusion, NZ2114 could be a promising new option for the treatment of central nervous system infections, including penicillin-resistant pneumococcal meningitis, due to its excellent CSF penetration and potent bactericidal activity in CSF.
Acknowledgments
We thank Jytte Mark Andersen and Flemming Pedersen for skillful technical assistance.
Footnotes
Published ahead of print on 2 February 2009.
REFERENCES
- 1.Andersen, C. O. 2007. Streptococcus penumoniae meningitis. Clinical and experimental studies. Dan. Med. Bull. 54:189-209. [PubMed] [Google Scholar]
- 2.Andes, D. R., and W. A. Craig. 1999. Pharmacokinetics and pharmacodynamics of antibiotics in meningitis. Infect. Dis. Clin. N. Am. 13:595-618. [DOI] [PubMed] [Google Scholar]
- 3.Banks, W. A., and A. J. Kastin. 1996. Passage of peptides across the blood-brain barrier: pathophysiological perspectives. Life Sci. 59:1923-1943. [DOI] [PubMed] [Google Scholar]
- 4.Lebel, M. H., and G. H. McCracken, Jr. 1989. Delayed cerebrospinal fluid sterilization and adverse outcome of bacterial meningitis in infants and children. Pediatrics 83:161-167. [PubMed] [Google Scholar]
- 5.Mygind, P. H., R. L. Fischer, K. M. Schnorr, M. T. Hansen, C. P. Sonksen, S. Ludvigsen, D. Raventos, S. Buskov, B. Christensen, L. De Maria, O. Taboureau, D. Yaver, S. G. Elvig-Jorgensen, M. V. Sorensen, B. E. Christensen, S. Kjaerulff, N. Frimodt-Moller, R. I. Lehrer, M. Zasloff, and H. H. Kristensen. 2005. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 437:975-980. [DOI] [PubMed] [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed.; Approved standard, p.10-13. NCCLS document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 7.Nau, R., F. Sorgel, and H. W. Prange. 1994. Lipophilicity at pH 7.4 and molecular size govern the entry of the free serum fraction of drugs into the cerebrospinal fluid in humans with uninflamed meninges. J. Neurol. Sci. 122:61-65. [DOI] [PubMed] [Google Scholar]
- 8.Østergaard, C., H. B. Konradsen, and S. Samuelsson. 2005. Clinical presentation and prognostic factors of Streptococcus pneumoniae meningitis according the focus of infection. BMC Infect. Dis. 5:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Østergaard, C., T. K. Sørensen, J. D. Knudsen, and N. Frimodt-Møller. 1998. Evaluation of moxifloxacin, a new 8-methoxyquinolone, for treatment of meningitis caused by a penicillin-resistant pneumococcus in rabbits. Antimicrob. Agents Chemother. 42:1706-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raventos, D., O. Taboureau, P. H. Mygind, J. D. Nielsen, C. P. Sonksen, and H. H. Kristensen. 2005. Improving on nature's defenses: optimization & high throughput screening of antimicrobial peptides. Comb. Chem. High Throughput. Screen. 8:219-233. [DOI] [PubMed] [Google Scholar]
- 11.Sandvang, D., P. H. Mygind, M. E. Jones, D. F. Sahm, and H.-H. Kristensen. 2007. In vitro activity and characterization of NZ2114, an improved variant of plectasin, abstr. F1-1663. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother.
- 12.Tunkel, A. R., B. J. Hartman, S. L. Kaplan, B. A. Kaufman, K. L. Roos, W. M. Scheld, and R. J. Whitley. 2004. Practice guidelines for the management of bacterial meningitis. Clin. Infect. Dis. 39:1267-1284. [DOI] [PubMed] [Google Scholar]
- 13.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]