Abstract
A potential pathway linking hydroxyl radicals to antimicrobial lethality was examined by using mutational and chemical perturbations of Escherichia coli. Deficiencies of sodA or sodB had no effect on norfloxacin lethality; however, the absence of both genes together reduced lethal activity, consistent with rapid conversion of excessive superoxide to hydrogen peroxide contributing to quinolone lethality. Norfloxacin was more lethal with a mutant deficient in katG than with its isogenic parent, suggesting that detoxification of peroxide to water normally reduces quinolone lethality. An iron chelator (bipyridyl) and a hydroxyl radical scavenger (thiourea) reduced the lethal activity of norfloxacin, indicating that norfloxacin-stimulated accumulation of peroxide affects lethal activity via hydroxyl radicals generated through the Fenton reaction. Ampicillin and kanamycin, antibacterials unrelated to fluoroquinolones, displayed behavior similar to that of norfloxacin except that these two agents showed hyperlethality with an ahpC (alkyl hydroperoxide reductase) mutant rather than with a katG mutant. Collectively, these data are consistent with antimicrobial stress increasing the production of superoxide, which then undergoes dismutation to peroxide, from which a highly toxic hydroxyl radical is generated. Hydroxyl radicals then enhance antimicrobial lethality, as suggested by earlier work. Such findings indicate that oxidative stress networks may provide targets for antimicrobial potentiation.
Antimicrobials that actively kill pathogens are expected to cure disease more rapidly and restrict the emergence of resistance better than agents that are largely bacteriostatic. Consequently, considerable effort has been devoted to understanding mechanisms of lethal action. In general, antimicrobials are thought to kill microbes through interaction with specific intracellular targets, followed by corruption of particular cellular processes (22). However, Collins and associates recently proposed that many bactericidal antimicrobials also share a common lethal pathway that involves the generation/accumulation of hydroxyl radicals (13, 23). This hypothesis is supported by elevated hydroxyl radical levels associated with lethal antimicrobial treatment (23). How this occurs is largely unknown.
Three major reactive oxygen species, superoxide, hydrogen peroxide, and hydroxyl radical, are generated as by-products of normal aerobic respiration (17, 20). All three are cytotoxic, but they display different kinetics and levels of severity. For example, the effects of superoxide and hydrogen peroxide are probably less acute than those of hydroxyl radicals, since both superoxide and hydrogen peroxide can be detoxified by induced scavenging enzymes. In contrast, no enzyme can detoxify hydroxyl radicals, making them extremely toxic and acutely lethal. Hydroxyl radicals derive from hydrogen peroxide through the Fenton reaction (14), which makes the regulation of the intracellular peroxide concentration a starting point for exploring genetic pathways that affect hydroxyl radical-mediated killing. Peroxide is generated mainly from superoxide through the action of superoxide dismutase (SOD) (16). Dismutation of superoxide can also occur spontaneously; however, the rate is about 105 M−1 s−1, which is significant but about 4 orders of magnitude lower than enzymatic dismutation (15, 25). Consequently, disruption of SOD-mediated generation of peroxide is expected to reduce the accumulation of peroxide and thus the generation of hydroxyl radicals. Detoxification of hydrogen peroxide by conversion to water occurs via catalases/peroxidases at a rate of about 106 M−1 s−1, faster than spontaneous superoxide dismutation but significantly slower than SOD-mediated dismutation (9, 10, 29). Disruption of catalase-peroxidase genes is expected to raise peroxide levels, which in turn may raise hydroxyl radical levels and lower cell survival associated with antimicrobial treatment. Consequently, we were surprised by the report that a superoxide dismutase (sodB) mutation increased lethal susceptibility to norfloxacin (13) rather than lowering it or having no effect due to the presence of a second superoxide dismutase (sodA).
We began the present study by examining norfloxacin action with the sodB mutant used previously (13). In our hands, this mutant exhibited the same susceptibility as the wild-type strain, as was true with either a sodA or a sodB mutant in another genetic background. In contrast, a sodA sodB double mutant showed reduced rather than increased lethal susceptibility to norfloxacin treatment. Genetic inactivation of catalase-peroxidase increased quinolone susceptibility. Together, these results solidified a role for peroxide in quinolone lethality. Both an iron chelator (an inhibitor of the Fenton reaction) and a hydroxyl radical scavenger lowered norfloxacin lethality, consistent with conversion of peroxide to hydroxyl radical, contributing to cell death. Similar conclusions were reached with other antimicrobial classes, represented by kanamycin and ampicillin. These results help define an oxidative stress pathway that contributes to antimicrobial lethality.
MATERIALS AND METHODS
Bacterial strains, plasmid, and growth conditions.
Strains of Escherichia coli K-12 used in the work are listed in Table 1. E. coli was grown in LB broth or on LB agar (28) supplemented with the indicated concentrations of antimicrobials. Construction of mutant strains was performed by sequential kanamycin marker excision (11) and bacteriophage P1-mediated transduction (33). Plasmid DNA (pCP20) (8) was introduced into host strains by bacterial transformation for antibiotic marker excision as reported previously (11).
TABLE 1.
Bacterial strains used in this study
| Strain no. | Relevant genotype | Source/referencea |
|---|---|---|
| 3140 | BW25113 katE::kan | CGSC# 9453 (4) |
| 3144 | BW25113 ΔsodA | This work, by antibiotic marker excision from CGSC# 10798 |
| 3145 | BW25113 ΔsodB | This work, by antibiotic marker excision from CGSC# 9402 |
| 3149 | BW25113 sodA::kan ΔsodB | This work, by P1-medicated transduction from CGSC# 10798 to 3145 |
| 3156 | BW25113 ΔsodA ΔsodB | This work, by antibiotic marker excision from 3149 |
| 3157 | BW25113 ΔkatG | This work, by antibiotic marker excision from CGSC# 10827 |
| 3163 | BW25113 ΔkatG katE::kan | This work, by P1-medicated transduction from 3140 to 3157 |
| 3200 | BW25113 ΔahpC | This work, by antibiotic marker excision from CGSC# 8713 |
| 3201 | BW25113 ΔkatG ΔkatE | This work, by antibiotic marker excision from 3163 |
| 3202 | BW25113 ΔkatE | This work, by antibiotic marker excision from 3140 |
| BW25113 | Wild type | CGSC# 7636 (4) |
| CSH7 | Wild type | Richard D'Ari (26) |
| CSH57A | Wild type | Richard D'Ari (26) |
| DKsodB | BW25113 ΔsodB | James Collins (13) |
| GC4468 | Wild type | Richard D'Ari (7) |
| QC772 | GC4468F sodA::Cm | Richard D'Ari (7) |
| QC773 | GC4468F sodB::Kan | Richard D'Ari (7) |
| QC774 | GC4468F sodA::Cm sodB::Kan | Richard D'Ari (7) |
| UM1 | CSH7 katE1 katG14b | Richard D'Ari (26) |
| UM2 | CSH57A katE2 katG15b | Richard D'Ari (26) |
CGSC#, Coli Genetic Stock Center strain number. All CGSC strains were obtained from Yale University Coli Genetic Stock Center.
katG14 encodes a protein that is deficient in catalase but proficient in peroxidase activity, while katG15 encodes a protein deficient in both enzymatic activities.
Chemicals and reagents.
Oxolinic acid, norfloxacin, rifampin (rifampicin), 2,2′-bipyridyl, and thiourea were purchased from Sigma Chemical Co. (St. Louis, MO). Ciprofloxacin was from Bayer AG (Wuppertal, Germany). Chloramphenicol and tetracycline were purchased from EMD Chemicals, Inc. (Gibbstown, NJ). Kanamycin was from Fisher Scientific (Pittsburgh, PA). Ampicillin was purchased from Roche Diagnostics (Indianapolis, IN).
Antimicrobial susceptibility assays.
Growth inhibition (MIC) was determined by broth dilution with visual inspection of a series of tubes each containing about 105 bacteria in 1 ml of LB medium supplemented with concentrations of drug increasing by 50% increments. Following an overnight incubation at 37°C, the lowest concentration that prevented visible growth was defined as the MIC.
Lethal activity was measured in two ways. Slow killing was expressed by the minimal bactericidal concentration (MBC) in which survival was reduced by 1,000-fold following an 18-h incubation of a series of 1-ml broth cultures each containing about 106 CFU bacteria and twofold incremental concentrations of antimicrobial. For rapid killing, overnight cultures were grown at 37°C with shaking at 250 rpm, diluted by 200-fold into fresh medium, and regrown to early- to mid-exponential phase (approximately 5 × 108 CFU/ml) before exposure to various concentrations of drug for a fixed time or to various times for a fixed drug concentration. Before and after treatment, cells were serially diluted and applied to drug-free agar plates for determination of viable counts. After 16 to 24 h of incubation at 37°C, bacterial colonies were counted and the percent survival was calculated relative to values taken at the time of antimicrobial addition. Rapid killing measurements with stationary- or lag-phase cultures were made as with exponentially growing cultures except that overnight cultures were either used directly (stationary phase) or diluted by 50-fold (lag phase) before being treated with the antimicrobials.
Effect of iron chelator and hydroxyl radical scavenger on antimicrobial lethality.
Subinhibitory concentrations of 2,2′-bipyridyl (250 μM; 50% MIC) and thiourea (100 mM; 50% MIC) were added to bacterial cultures 10 min prior to initiation of antimicrobial treatment. The cultures were then processed as for the antimicrobial susceptibility assays described above.
RESULTS
Involvement of superoxide dismutase in rapid killing by norfloxacin.
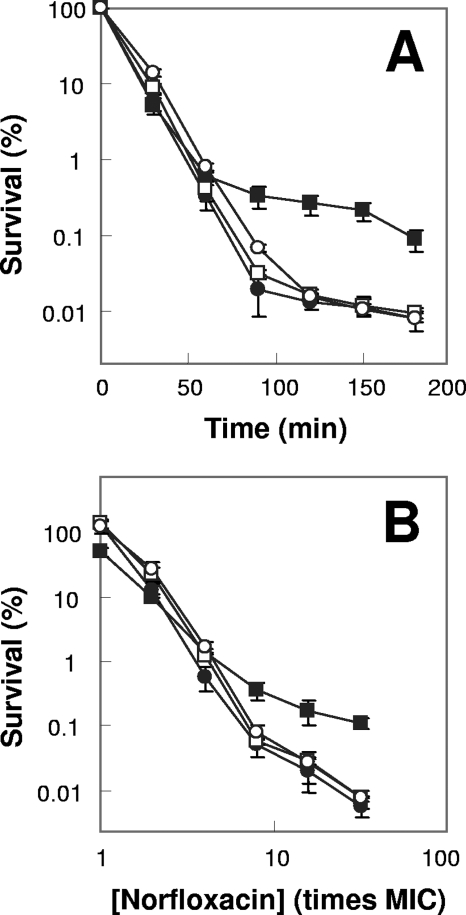
When we examined the ability of norfloxacin to block growth of an E. coli strain deficient in sodB (strain DKsodB), little effect was observed for the norfloxacin MIC (Table 2). Likewise, little effect was observed on lethal activities measured at various incubation times and norfloxacin concentrations (Fig. 1). The sodB mutation also had little effect on either the MIC or the lethal action of oxolinic acid or ciprofloxacin measured at various times and concentrations (not shown). When we examined a sodA mutant and another sodB mutant constructed with the same genetic background (strains 3144 and 3145), we also observed little effect on the norfloxacin MIC or on the ability of the drug to kill E. coli, measured at various times and norfloxacin concentrations (Table 2 and Fig. 1; also not shown). Similar data were obtained for oxolinic acid and ciprofloxacin (not shown). Deficiencies of sodA (strain QC772) or sodB (strain QC773) in a different genetic background also showed little effect on the MICs or lethal susceptibilities for the three quinolones tested (not shown). Thus, we conclude that deficiencies of either sodA or sodB have little or no effect on quinolone lethality.
TABLE 2.
Bacteriostatic activities of compounds investigated
| Bacterial strain | Relevant genotype | MIC of compounda
|
||||
|---|---|---|---|---|---|---|
| Thiourea (mM) | Bipyridyl (μM) | Norfloxacin | Ampicillin | Kanamycin | ||
| BW25113 | Wild type | 200 | 500 | 0.04 | 15 | 0.6 |
| DKsodB | ΔsodB | 200 | 500 | 0.04 | 15 | 0.6 |
| 3144 | ΔsodA | 200 | 500 | 0.04 | 15 | 0.6 |
| 3145 | ΔsodB | 200 | 500 | 0.04 | 15 | 0.6 |
| 3156 | ΔsodA ΔsodB | 200 | 500 | 0.04 | 15 | 0.6 |
| 3157 | ΔkatG | 200 | 500 | 0.04 | 15 | 0.6 |
| 3200 | ΔahpC | 150 | 500 | 0.04 | 15 | 2.4 |
| 3201 | ΔkatG ΔkatE | 200 | 500 | 0.08 | 30 | 1.2 |
| 3202 | ΔkatE | 200 | 500 | 0.04 | 15 | 0.6 |
Expressed in μg/ml unless otherwise indicated.
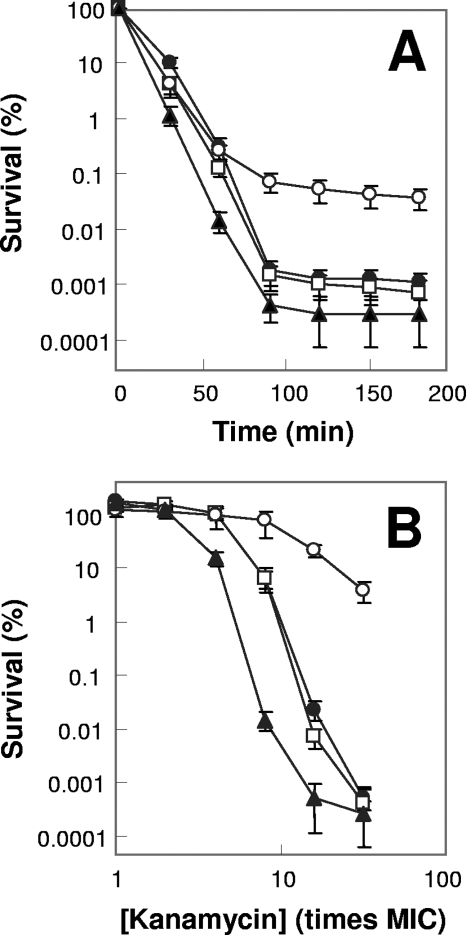
FIG. 1.
Effects of superoxide dismutase deficiency on norfloxacin lethality. Exponentially growing E. coli cells were treated with 0.4 μg/ml (10 times the MIC) norfloxacin for various times (A) or with the indicated concentrations of norfloxacin (B) for 2 h. Symbols: filled circles, wild type; filled squares, sodA sodB mutant; open circles, sodA mutant; open squares, sodB mutant. Error bars indicate standard deviations for replicate samples. At least three replicate experiments were performed, and each had results similar to those shown.
Since the two sod gene products may have redundant activities, we examined the effect of mutations in both sod genes (strain 3156). No effect was observed on the MIC (Table 2). However, norfloxacin lethality, measured either at various incubation times or at various drug concentrations, was 10-fold lower with the double mutant (Fig. 1A and B). The protective effect seen with the sod double mutant occurred only with treatment times of >50 min and norfloxacin concentrations of >4 times the MIC. Cells recovered from treatment in which protection was evident (e.g., 10 times the MIC, 120-min incubation with the sodA sodB double mutant) were not enriched for norfloxacin-resistant mutants (not shown). A 10- to 100-fold protective effect was also observed with a sodA sodB double mutant in a different genetic background (strain QC774), either when growing exponentially or when grown overnight, diluted by 50-fold, and then treated with norfloxacin or ciprofloxacin (not shown). Thus, a deficiency of superoxide dismutase activity partially protects from quinolone-mediated cell death.
Involvement of catalase in rapid killing by norfloxacin.
If the protective effect of a sodA sodB deficiency is due to restricted conversion of superoxide to peroxide, then catalase/peroxidase-deficient mutants should cause an increase in peroxide accumulation and render cells more easily killed. We first measured bacteriostatic activity. Deficiencies of katG (strain 3157) and katE (strain 3202) had little effect on the norfloxacin MIC; the MIC of a katG katE double mutant (strain 3201) was only twice that of wild-type cells (Table 2). MICs showed a similar behavior with two other quinolones, oxolinic acid and ciprofloxacin (not shown). Thus, bacteriostatic activity is insensitive to catalase/peroxidase deficiencies.
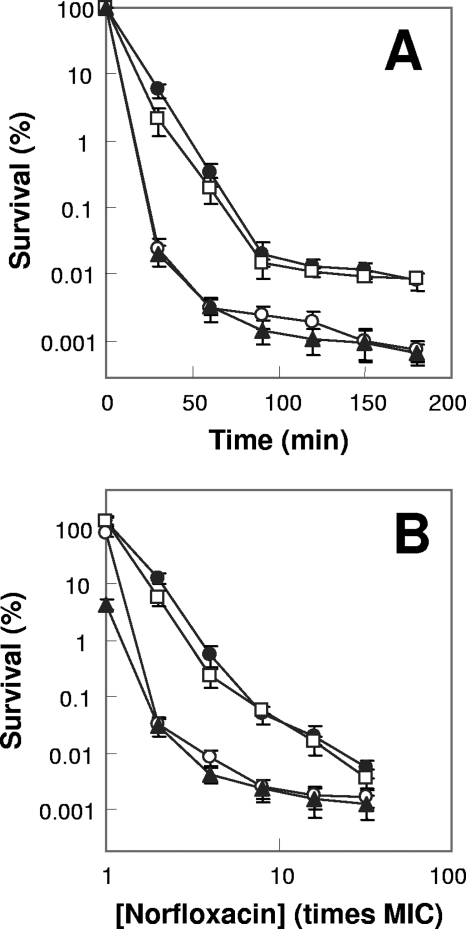
In contrast to bacteriostatic activity being unaffected by catalase/peroxidase deficiency, lethal action of norfloxacin was increased 10- to 100-fold by a katG single mutation and by a katG katE double mutation when measured at various incubation times or at various drug concentrations as a function of the MIC (Fig. 2). The enhancement of killing by catalase/peroxidase deficiency was more pronounced at shorter incubation times (≤60) min and lower norfloxacin concentrations (e.g., two times the MIC gave the largest difference, while 32 times the MIC showed the smallest difference). Increased lethality was also observed for oxolinic acid and ciprofloxacin (not shown); similar results were obtained with a different genetic background (strains UM2 and CSH57A [not shown]). A katE single mutant failed to exhibit hyperlethality, and the katG katE double mutant showed the same lethal susceptibility as the katG single mutant (Fig. 2). Since KatG possesses both catalase and peroxidase activity, a katG mutant (strain UM1) deficient in catalase but proficient in peroxidase activity and a katG mutant (strain UM2) deficient in both enzymatic activities were also tested. The catalase deficiency alone (UM1) failed to cause the hyperlethal phenotype, while a katG mutant (UM2) lacking both catalase and peroxidase activity, showed hyperlethality (not shown). Collectively, these data allow us to conclude that catalase-peroxidase activity behaves in an opposite way to that of superoxide dismutase and that type I hydroperoxidase (KatG), not type II hydroperoxidase (KatE), is primarily responsible for removing excess hydrogen peroxide accumulated upon lethal quinolone exposure.
FIG. 2.
Effects of catalase/peroxidase deficiency on norfloxacin lethality. Exponentially growing E. coli cells were treated with 10 times the MIC of norfloxacin (0.8 μg/ml for the katG katE double mutant and 0.4 μg/ml for other strains) for various times (A) or with the indicated concentrations of norfloxacin (B) for 2 h as in Fig. 1. Symbols: filled circles, wild type; open circles, katG mutant; open squares, katE mutant; triangles, katG katE mutant. Error bars indicate standard deviations for replicate samples. At least three replicate experiments were performed, and each had results similar to those shown.
Effect of thiourea and ferrous chelator on norfloxacin lethality.
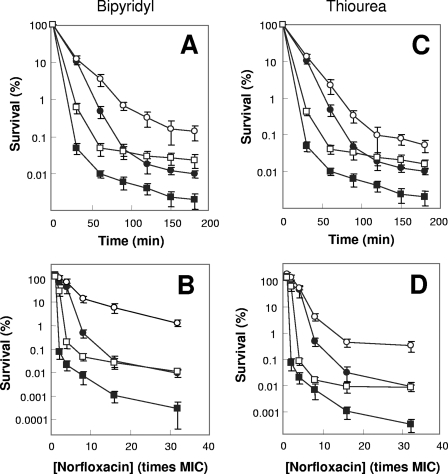
We next examined the possibility that the increased lethality of norfloxacin associated with peroxide was due, at least in part, to an increase in the hydroxyl radical concentration by treating wild-type cells with an iron chelator (bipyridyl) that interferes with the Fenton reaction or with thiourea, a hydroxyl radical scavenger. Cotreatment with subinhibitory concentrations (e.g., 50% MIC) of either bipyridyl or thiourea failed to affect the norfloxacin MIC (not shown). However, the same cotreatments reduced norfloxacin-mediated killing by 10- to 100-fold when either the treatment time (Fig. 3A and C) or the norfloxacin concentration (Fig. 3B and D) was varied.
FIG. 3.
Effects of a ferrous chelator and a hydroxyl radical scavenger on norfloxacin lethality. Exponentially growing E. coli cells were preincubated with 250 μM bipyridyl (A and B) or 100 mM thiourea (C and D) for 10 min before they were treated with 10 times the MIC of norfloxacin (0.4 μg/ml) for various times (A and C) or with the indicated concentrations of norfloxacin (B and D) for 2 h as in Fig. 1. Symbols: filled circles, wild type; open circles, wild type plus bipyridyl or thiourea; filled squares, katG mutant; open squares, katG mutant plus bipyridyl or thiourea. Error bars indicate standard deviations for replicate samples. At least three replicate experiments were performed, and each had results similar to those shown.
We also measured the effects of thiourea and bipyridyl separately on the sodA sodB (strain 3156) and katG (strain 3157) mutants. Both thiourea and bipyridyl had less protective effect with the sodA sodB strain than with wild-type cells (not shown), but they conferred similar overall protective effects with the katG mutant and wild-type E. coli (Fig. 3). The lesser protection with the sodA sodB mutant may derive from slower/less accumulation of peroxide and hydroxyl radical in the mutant than in the wild-type strain. The failure of thiourea and bipyridyl to restore killing of a katG mutant to wild-type levels is probably due to our use of subinhibitory scavenger/chelator concentrations that were unable to completely eliminate hydroxyl radical accumulation. Cotreatment with bipyridyl, which inhibits generation of hydroxyl radicals, was slightly more protective than scavenging of hydroxyl radicals with thiourea when each was present at 50% MIC. Such a difference is possibly because hydroxyl radicals, once generated, are too reactive to allow thiourea enough time to scavenge them before they damage nearby macromolecules.
Involvement of superoxide dismutase and catalase-peroxidase in ampicillin- and kanamycin-mediated cell death.
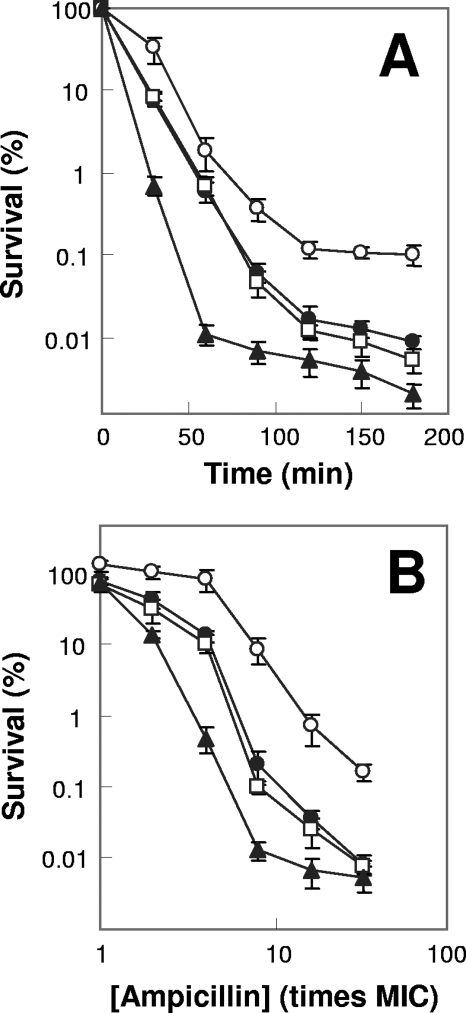
To determine whether peroxide-hydroxyl radical aspects of quinolone-mediated cell death also apply to the lethal action of β-lactams and aminoglycosides, we examined ampicillin- and kanamycin-mediated killing with sod and kat mutants. A deficiency of superoxide dismutatase (sodA sodB; strain 3156) conferred protection (Fig. 4 and 5); thiourea and bipyridyl were also protective (not shown), consistent with hydroxyl radical formation contributing to ampicillin and kanamycin lethality. However, a deficiency of catalase-peroxidase (katG; strain 3157) conferred wild-type susceptibility to ampicillin and kanamycin (Fig. 4 and 5), unlike the situation observed with fluoroquinolones.
FIG. 4.
Effects of katG, sodA sodB, and ahpC deficiencies on ampicillin lethality. Exponentially growing E. coli cells were treated with five times the MIC of ampicillin (150 μg/ml for the katG katE mutant and 75 μg/ml for other strains) for various times (A) or with the indicated concentrations of ampicillin (B) for 90 min. Symbols: filled circles, wild type; squares, katG mutant; open circles, sodA sodB mutant; triangles, ahpC mutant. Error bars indicate standard deviations for replicate samples. At least three replicate experiments were performed, and each had results similar to those shown.
FIG. 5.
Effects of katG, sodA sodB, and ahpC deficiencies on kanamycin lethality. Exponentially growing E. coli cells were treated with five times the MIC of kanamycin (12 μg/ml for the ahpC mutant and 3 μg/ml for other strains) for various times (A) or with the indicated concentrations of kanamycin (B) for 45 min. Symbols: filled circles, wild type; squares, katG mutant; open circles, sodA sodB mutant; triangles, ahpC mutant. Error bars indicate standard deviations for replicate samples. At least three replicate experiments were performed, and each had results similar to those shown.
We next considered the possibility that failure to observe hyperlethality in the katG mutant arose from ampicillin/kanamycin-mediated production/accumulation of hydrogen peroxide being below the Km of KatG. To test this idea, we examined alkyl hydroperoxide reductase (encoded by ahpCF), which is thought to act as the primary scavenger of peroxides at low substrate concentrations due to a higher affinity (lower Km) than KatG (31). When alkyl hydroperoxide reductase was deficient (ΔahpC; strain 3200), E. coli was hypersensitive to ampicillin and kanamycin (Fig. 4 and 5). These data are consistent with ampicillin and kanamycin causing a lower accumulation of peroxide than norfloxacin, which results in AhpCF being the primary scavenger for hydrogen peroxide. A katG ahpC double mutant was not tested, since it grows very poorly under aerobic conditions and since the antimicrobials investigated have poor lethal activity with nongrowing cells.
Absence of involvement of catalase-peroxidase in activities of bacteriostatic antibacterials.
To test the suggestion that the effects of hydroxyl radical formation are restricted to bactericidal agents (23), we examined the effects of katG, ahpC, and katG katE mutations on the activity of chloramphenicol, a bacteriostatic agent with E. coli. Mutant and wild-type MICs were identical (not shown), and even at 100 times the MIC (200 μg/ml), chloramphenicol failed to kill E. coli. No enhancement of killing was observed with katG, katG katE, and ahpC mutants (not shown). A similar conclusion was reached with another static agent, rifampin (not shown). These data support the assertion (23) that enhancement of hydroxyl radical action occurs only with bactericidal agents.
Effect of superoxide dismutase and catalase-peroxidase deficiencies on antimicrobial lethalities under various growth and treatment conditions.
The phenomena described above were observed with rapid killing of exponentially growing cells. To test whether slow killing is also affected by oxidative stress pathway mutations, MBCs were measured with the three compound classes using the same set of isogenic strains (Table 3). Little difference in MBCs was observed among wild-type and mutant strains except for the ahpC mutant and the sodA sodB double mutant (both exhibited a two- to fourfold reduction in the MBC [strains 3200 and 3156; Table 3]). The slight decrease in the MBC seen with the sodA sodB mutant seems contradictory to the protective effect exhibited for this mutant in rapid killing assays. This discrepancy may derive from superoxides being chronically toxic, so that long-term exposure to elevated levels of superoxide is detrimental to cells, which would override short-term protective effects conferred by deficiencies in SodA and SodB. Such a possibility is currently under investigation. Rapid killing with lag-phase cells (stationary-phase culture diluted by 50-fold) by kanamycin and norfloxacin gave results similar to those seen with exponentially growing cells except for diminished protection from a sodA sodB deficiency for kanamycin (not shown). No strain tested was killed by ampicillin when in lag phase. Likewise, no killing was observed with wild-type and mutant strains when stationary cultures were directly treated with any of the three compounds tested (not shown). Thus, oxidative stress-stimulated antimicrobial lethality is absent in stationary-phase cells, but it is present at similar levels during the exponential and lag phases of growth when lethality is observed.
TABLE 3.
MBCs of compounds investigated
| Bacterial strain | Relevant genotype | MBC of compound (μg/ml)
|
||
|---|---|---|---|---|
| Norfloxacin | Ampicillin | Kanamycin | ||
| BW25113 | Wild type | 0.32 | 80 | 20 |
| DKsodB | ΔsodB | 0.32 | 80 | 20 |
| 3144 | ΔsodA | 0.32 | 80 | 20 |
| 3145 | ΔsodB | 0.32 | 80 | 20 |
| 3156 | ΔsodA ΔsodB | 0.16 | 20 | 10/20a |
| 3157 | ΔkatG | 0.16/0.32a | 80 | 20 |
| 3200 | ΔahpC | 0.16 | 40 | 10 |
| 3201 | ΔkatG ΔkatE | 0.32 | 80 | 20 |
| 3202 | ΔkatE | 0.16/0.32a | 80 | 20 |
Different values were obtained in replicate assays; in all other cases, replicate values were identical.
DISCUSSION
Antimicrobial-mediated killing of some bacteria appears to involve oxidative stress, as suggested by the accumulation of superoxide anions following exposure to at least three antimicrobial classes (1, 5) and by reactive oxygen species being associated with ciprofloxacin susceptibility (19). The possibility was recently raised that hydroxyl radical overproduction may serve as a common path to antimicrobial-mediated cell death (13, 23). The present work provides support for a possible lethal pathway from antimicrobial treatment to hydroxyl radical accumulation via superoxides and peroxides (Fig. 6). A deficiency in superoxide dismutase (sodA sodB double mutation) reduced the lethal action of norfloxacin (Fig. 1), ampicillin (Fig. 4), and kanamycin (Fig. 5). Single mutations were insufficient, as indicated by experiments with norfloxacin (Fig. 1). Superoxide dismutases are expected to greatly accelerate the conversion of superoxide to peroxide (15, 25), which serves as a substrate for generation of highly toxic hydroxyl radicals through the Fenton reaction (14). Although spontaneous dismutation of superoxide to hydrogen peroxide can occur, the rate is 4 orders of magnitude lower than that of enzymatic dismutation (15, 25). Thus, in a sodA sodB mutant, catalase/peroxidase is expected to detoxify enough hydrogen peroxide to explain protection from norfloxacin-mediated killing. A deficiency of KatG catalase/peroxidase, which is expected to allow accumulation of peroxide, rendered cells hypersusceptible to the lethal action of quinolones (Fig. 2). A deficiency of alkyl hydroperoxide reductase (AhpCF) played that role for ampicillin and kanamycin (Fig. 4 and 5). An iron chelator expected to block the generation of hydroxyl radicals and a hydroxyl radical scavenger both reduced antimicrobial-mediated cell death (Fig. 3 and data not shown). Thus, part of the lethal activity for members of at least three antimicrobial classes involves a surge in hydroxyl radical accumulation (13, 23) mediated by enzymes of superoxide and peroxide metabolism (Fig. 6).
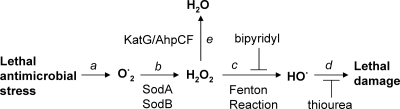
FIG. 6.
Scheme depicting pathway by which bactericidal antimicrobial stress modulates lethal oxidative damage. Lethal stress from antimicrobial treatment causes an undefined redox imbalance, such that intracellular superoxides accumulate (step a). Upon conversion to hydrogen peroxide by dismutases (step b), hydroxyl radicals are generated from elevated levels of hydrogen peroxide via the Fenton reaction (step c). Hydroxyl radical species cause cell death (step d). Hydrogen peroxide is normally decomposed/detoxified by catalase/peroxidase (step e). Suppression of the generation of peroxide by a deficiency of both sodA and sodB protects cells from antimicrobial lethality, while inhibition of detoxification of peroxide via deficiencies in katG (in the case of norfloxacin) or ahpC (in the case of ampicillin and kanamycin) enhances cell death. Both an iron chelator (bipyridyl), which inhibits the Fenton reaction, and thiourea, a potent hydroxyl radical scavenger, protect cells from death.
Deficiencies in sod and kat conferred distinct phenotypes that were most pronounced at different antimicrobial exposures. For example, the protective effect of a sodA sodB double mutation was evident only at long treatment times and high antimicrobial concentrations (Fig. 1, 4, and 5). In contrast, the katG defect increased lethality and the increase was most obvious at short treatment times and low drug concentrations (Fig. 2). These observations are consistent with the kinetic properties of the enzymes. Long incubation at a high drug concentration is expected to substantially elevate the superoxide concentration, which may not be acutely toxic, since the superoxide half-life in aqueous phase is long and since superoxide can be scavenged by both SODs and many types of antioxidant. However, if superoxide is enzymatically converted to H2O2 at a rate of >109 M−1 s−1, as expected with wild-type cells (15, 25), catalase/peroxidase may become saturated, since its rate constant is only about 106 M−1 s−1 (29). The buildup of peroxide would then lead to production of hydroxyl radicals via the Fenton reaction and result in cell death. But when SOD is absent, the spontaneous rate (about 105 M−1 s−1) (15, 25) may be insufficient to challenge the ability of catalase to detoxify H2O2. Then, little hydroxyl radical would be generated for enhancement of antimicrobial lethality, resulting in lower norfloxacin-mediated killing with a sodA sodB double-deficient strain (Fig. 1). An alternative explanation for the sodA sodB-mediated protection is that spontaneous elevation of the intracellular superoxide concentration in the sodA sodB double mutant stimulates generation of “persisters”—a subpopulation of cells that tolerate a variety of lethal stresses (24). Such a possibility is currently under investigation.
At mild antimicrobial exposure, superoxide levels would not generate enough H2O2 to overwhelm catalase activity, regardless of how superoxide undergoes dismutation to H2O2. Thus, the absence/presence of SOD would have little effect on killing under mild antimicrobial conditions (Fig. 1). When katG is deficient, even low levels of hydrogen peroxide generated by mild antimicrobial exposure cannot be detoxified, thereby increasing lethality (Fig. 2). In this situation, the additional removal of SOD (sodA sodB katG triple mutant) would confer protection (compared with results for the katG single mutant) at mild antimicrobial exposures, as observed (not shown). We speculate that the reduced hyperlethality of the katG mutant relative to results for the wild type at high drug exposures (Fig. 2) is due to the net effect of two factors: saturation of hydroxyl radical-mediated killing in the katG mutant and enhanced hydroxyl radical-mediated killing in wild-type cells when H2O2 accumulation exceeds the capacity of KatG-mediated detoxification.
The scheme presented in Fig. 6 and the protective effects seen in the sodA sodB double mutant (Fig. 1, 4, and 5) are consistent with several other reports. For example, detrimental rather than protective effects are observed with SOD overexpression in E. coli cells treated with a superoxide generator, paraquat (6, 30). Since paraquat treatment increases superoxide levels (21), as does antimicrobial treatment (1, 5), rapid conversion of excessive superoxide to H2O2 by overexpressed SOD is likely to trigger lethal hydroxyl radical generation. Similar toxic effects have been observed when E. coli cells are treated with SOD mimetics (27), which leads to elevated levels of superoxide and H2O2 (27). Moreover, in cultured mouse epidermal cells, SOD overexpression causes sensitization to rather than protection against oxidants; such sensitization is corrected by concurrent overexpression of catalase or glutathione peroxidase (2, 3). Thus, the balance between SOD and catalase/peroxidase activity may determine how much and how rapidly intracellular H2O2 is generated, which in turn determines hydroxyl radical production.
Although the present work supports the general conclusion that antimicrobial lethality is tied to intracellular hydroxyl radical accumulation (13, 23), two aspects of recent studies merit attention. First, a sodB mutant was reported to be more readily killed by the fluoroquinolones than wild-type cells (13, 23). We were unable to confirm that observation for norfloxacin (Fig. 1) and several other fluoroquinolones with several E. coli strains (not shown). Instead, a sodA sodB double mutant exhibited reduced lethal susceptibility to norfloxacin (Fig. 1), the result opposite to that expected from previous work (13). Second, the protective activities of thiourea and bipyridyl were previously noted using these two agents at concentrations that inhibit E. coli growth. Since growth inhibition blocks quinolone and ampicillin lethality (12, 18, 32), the protective effect of thiourea and bipyridyl could have been unrelated to hydroxyl radical accumulation. To reduce this possibility, we showed that subinhibitory concentrations of hydroxyl radical scavenger or iron chelator lowered norfloxacin lethality (Fig. 3). Subinhibitory scavenger and chelator treatments also reduced norfloxacin-mediated killing with a katG mutant. However, bacterial survival was not restored to levels observed with the wild type plus a scavenger/chelator or even with the wild type alone (Fig. 3). Incomplete protection is probably due to our use of subinhibitory concentrations of the scavenger and chelator being insufficient to completely prevent hydroxyl radical accumulation in mutants.
While the scheme shown in Fig. 6 applies to β-lactam and aminoglycoside action, kanamycin- and ampicillin-mediated killing was insensitive to a katG deficiency. Instead, it was enhanced by ahpC depletion. These data are consistent with peroxide accumulation being stimulated by kanamycin or ampicillin at lower levels than by a quinolone, since the lower levels would allow AhpCF, an enzyme having a greater affinity for hydrogen peroxide but a lower scavenging capacity (31), to serve as the primary peroxide scavenger after treatment with ampicillin and kanamycin.
In summary, oxidative stress pathways are involved in fluoroquinolone, β-lactam, and aminoglycoside lethality. Characterization of these pathways may provide new targets for small-molecule potentiators of antimicrobial lethality. The next step is to determine how these stress pathways distinguish between bacteriostatic and bactericidal events.
Acknowledgments
We thank Karl Drlica, Marila Gennaro, David Perlin, and Richard Pine for critical comments on the manuscript.
The work was supported by NIH grants AI 068014 and AI 073491.
Footnotes
Published ahead of print on 17 February 2009.
REFERENCES
- 1.Albesa, I., M. C. Becerra, P. C. Battan, and P. L. Paez. 2004. Oxidative stress involved in the antibacterial action of different antibiotics. Biochem. Biophys. Res. Commun. 317:605-609. [DOI] [PubMed] [Google Scholar]
- 2.Amstad, P., R. Moret, and P. Cerutti. 1994. Glutathione peroxidase compensates for the hypersensitivity of Cu,Zn-superoxide dismutase overproducers to oxidant stress. J. Biol. Chem. 269:1606-1609. [PubMed] [Google Scholar]
- 3.Amstad, P., A. Peskin, G. Shah, M. E. Mirault, R. Moret, I. Zbinden, and P. Cerutti. 1991. The balance between Cu,Zn-superoxide dismutase and catalase affects the sensitivity of mouse epidermal cells to oxidative stress. Biochemistry 30:9305-9313. [DOI] [PubMed] [Google Scholar]
- 4.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becerra, M. C., and I. Albesa. 2002. Oxidative stress induced by ciprofloxacin in Staphylococcus aureus. Biochem. Biophys. Res. Commun. 297:1003-1007. [DOI] [PubMed] [Google Scholar]
- 6.Bloch, C. A., and F. M. Ausubel. 1986. Paraquat-mediated selection for mutations in the manganese-superoxide dismutase gene sodA. J. Bacteriol. 168:795-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlioz, A., and D. Touati. 1986. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 5:623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9-14. [DOI] [PubMed] [Google Scholar]
- 9.Claiborne, A., and I. Fridovich. 1979. Purification of the o-dianisidine peroxidase from Escherichia coli B. Physicochemical characterization and analysis of its dual catalatic and peroxidatic activities. J. Biol. Chem. 254:4245-4252. [PubMed] [Google Scholar]
- 10.Claiborne, A., D. P. Malinowski, and I. Fridovich. 1979. Purification and characterization of hydroperoxidase II of Escherichia coli B. J. Biol. Chem. 254:11664-11668. [PubMed] [Google Scholar]
- 11.Datsenko, K., and B. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deitz, W. H., T. M. Cook, and W. A. Goss. 1966. Mechanism of action of nalidixic acid on Escherichia coli. 3. Conditions required for lethality. J. Bacteriol. 91:768-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwyer, D. J., M. A. Kohanski, B. Hayete, and J. J. Collins. 2007. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol. Syst. Biol. 3:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenton, H. J. H. 1894. Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 65:899-911. [Google Scholar]
- 15.Fridovich, I. 1989. Superoxide dismutases. An adaptation to a paramagnetic gas. J. Biol. Chem. 264:7761-7764. [PubMed] [Google Scholar]
- 16.Fridovich, I. 1983. Superoxide dismutases: regularities and irregularities. Harvey Lect. 79:51-75. [PubMed] [Google Scholar]
- 17.Fridovich, I. 1983. Superoxide radical: an endogenous toxicant. Annu. Rev. Pharmacol. Toxicol. 23:239-257. [DOI] [PubMed] [Google Scholar]
- 18.Goss, W. A., W. H. Deitz, and T. M. Cook. 1964. Mechanism of action of nalidixic acid on Escherichia coli. J. Bacteriol. 88:1112-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goswami, M., S. H. Mangoli, and N. Jawali. 2006. Involvement of reactive oxygen species in the action of ciprofloxacin against Escherichia coli. Antimicrob. Agents Chemother. 50:949-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan, H. M., and I. Fridovich. 1979. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch. Biochem. Biophys. 196:385-395. [DOI] [PubMed] [Google Scholar]
- 21.Hassan, H. M., and I. Fridovich. 1978. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J. Biol. Chem. 253:8143-8148. [PubMed] [Google Scholar]
- 22.Keren, I., D. Shah, A. Spoering, N. Kaldalu, and K. Lewis. 2004. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J. Bacteriol. 186:8172-8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohanski, M. A., D. J. Dwyer, B. Hayete, C. A. Lawrence, and J. J. Collins. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797-810. [DOI] [PubMed] [Google Scholar]
- 24.Lewis, K. 2008. Multidrug tolerance of biofilms and persister cells. Curr. Top. Microbiol. Immunol. 322:107-131. [DOI] [PubMed] [Google Scholar]
- 25.Liochev, S. I., and I. Fridovich. 2007. The effects of superoxide dismutase on H2O2 formation. Free Radic. Biol. Med. 42:1465-1469. [DOI] [PubMed] [Google Scholar]
- 26.Loewen, P. C., B. L. Triggs, C. S. George, and B. E. Hrabarchuk. 1985. Genetic mapping of katG, a locus that affects synthesis of the bifunctional catalase-peroxidase hydroperoxidase I in Escherichia coli. J. Bacteriol. 162:661-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthijssens, F., P. Back, B. P. Braeckman, and J. R. Vanfleteren. 2008. Prooxidant activity of the superoxide dismutase (SOD)-mimetic EUK-8 in proliferating and growth-arrested Escherichia coli cells. Free Radic. Biol. Med. 45:708-715. [DOI] [PubMed] [Google Scholar]
- 28.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 29.Moore, R. L., L. J. Powell, and D. C. Goodwin. 2008. The kinetic properties producing the perfunctory pH profiles of catalase-peroxidases. Biochim. Biophys. Acta 1784:900-907. [DOI] [PubMed] [Google Scholar]
- 30.Scott, M. D., S. R. Meshnick, and J. W. Eaton. 1987. Superoxide dismutase-rich bacteria. Paradoxical increase in oxidant toxicity. J. Biol. Chem. 262:3640-3645. [PubMed] [Google Scholar]
- 31.Seaver, L. C., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tipper, D. J., and J. L. Strominger. 1965. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc. Natl. Acad. Sci. USA 54:1133-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wall, J. D., and P. D. Harriman. 1974. Phage P1 mutants with altered transducing abilities for Escherichia coli. Virology 59:532-544. [DOI] [PubMed] [Google Scholar]