Abstract
A population pharmacokinetic model of cefepime was constructed from data from adult critical care patients with ventilator-associated pneumonia (VAP). A total of 32 patients treated with high-dose cefepime, 2 g every 8 h (3-h infusion) or a renal function-adjusted equivalent dose, were randomized into two groups—26 for the initial model and 6 for model validation. Serum samples of cefepime were collected at steady state. Nonparametric adaptive grid population modeling was employed using a two-compartment Kslope pharmacokinetic model relating the elimination rate constant (K10) to renal function, as defined by creatinine clearance (CLCR), and central distribution volume (V1) to total body weight (TBW). The final model was described by the following equations: K10 = 0.0027 × CLCR + 0.071 h−1 and V1 = TBW × 0.21 liter/kg. The median intercompartmental transfer constants K12 and K21 were 0.780 h−1 and 0.472 h−1, respectively. Using these median parameter estimates, the bias, precision, and coefficient of determination for the initial model were 11.3 μg/ml, 24.0 μg/ml, and 26%, respectively. The independent validation group displayed a bias, precision, and coefficient of determination of −1.64 μg/ml, 17.1 μg/ml, and 62%, respectively. Time-concentration profiles were assessed for various dosing regimens, using 5,000-patient Monte Carlo simulations. Among the regimens, the likelihoods of 2 g every 8 h (3-h infusion) achieving free drug concentrations above the MIC for 50% of the dosing interval were 91.8%, 78.1%, and 50.3% for MICs of 8, 16, and 32 μg/ml, respectively. This study provides a pharmacokinetic model capable of predicting cefepime concentrations in critically ill patients with VAP.
Cefepime is a fourth-generation parenteral cephalosporin with activity against gram-positive and gram-negative organisms, including Pseudomonas aeruginosa and Acinetobacter baumannii (1, 6). Because of its broad coverage and favorable adverse event profile, cefepime is extensively used as an empirical antimicrobial therapy for serious infections in intensive care units (ICU). In patients with normal renal function, cefepime is typically dosed according to the manufacturer's recommendations: 1 g every 12 h in mild to moderate infections, 2 g every 12 h in severe infections, and 2 g every 8 h in neutropenic patients, all utilizing a 30-min infusion time.
With the rise of multidrug-resistant, gram-negative bacilli (10, 20), there is the potential for poor infection-related outcomes, particularly in high-risk patients, such as the critically ill whom are receiving mechanical ventilation. Moreover, various studies have illustrated that the manufacturer's recommended doses may fall short against less-susceptible gram-negative pathogens (2, 17, 23). This has led some investigators to explore or suggest alternative cefepime dosing strategies, including prolonged and continuous infusions (3, 4, 8, 22, 24). Like other β-lactams, cefepime displays time-dependent bactericidal activity whereby efficacy is optimized when free drug concentrations exceed the MIC for at least 50% of the dosing interval (50% fT>MIC) (19). As a result of these pharmacodynamic characteristics, prolonging the infusion duration from the standard 30 min to 3 to 4 h or administering β-lactam antibiotics as continuous infusions over 24 h will increase the probability of pharmacodynamic target attainment at higher MICs (9, 11, 13, 18, 21, 24).
Recently at our 840-bed tertiary care hospital, the high prevalence of resistant organisms, including P. aeruginosa, as a cause of ventilator-associated pneumonia (VAP) led to the development of a clinical pathway incorporating high-dose, prolonged-infusion antibiotic regimens (14). In this pathway, cefepime is empirically administered as a 2-g dose every 8 h, with each dose infused over 3 h, or a renal function-adjusted equivalent dose. The current population pharmacokinetic analysis was conducted to demonstrate that this new cefepime dosing regimen was effectively achieving the intended concentration-time profiles in patients treated for VAP at our institution, with the goal of empirically achieving 50% fT>MIC in the majority of patients infected with organisms harboring cefepime MICs of up to 32 μg/ml. The utility of this model not only allowed confirmation of dosing regimens in our patient population but also provided a means of estimating pharmacokinetic parameters for those patients receiving cefepime for the treatment of VAP who do not have concentration data available.
MATERIALS AND METHODS
Patient population and setting.
Blood sample collection was performed on patients who were admitted to the medical, surgical, or neurotrauma ICU at Hartford Hospital between April 2007 and December 2007 and received cefepime as part of the VAP clinical pathway (approved by the Pharmacy and Therapeutics and Medical Executive Committees) (14). Hartford Hospital, located in Hartford, CT, is a 840-bed tertiary care hospital, consisting of two 12-bed medical ICUs, a 12-bed surgical ICU, a 12-bed cardiothoracic ICU, and an 18-bed neurotrauma ICU. When placed on the clinical pathway, patients empirically received vancomycin, tobramycin (or a fluoroquinolone if tobramycin was contraindicated), and high-dose cefepime (2 g every 8 h, infused over 3 h). Doses were adjusted for renal function by using the Cockcroft-Gault equation, without the application of weight for estimating creatinine clearance (CLCR) (7). Cefepime doses were originally developed based on a 5,000-patient Monte Carlo simulation, applying previously published pharmacokinetic data from patients with various degrees of renal function (23). A waiver of consent for the collection of blood samples was granted by Hartford Hospital's Institutional Review Committee, since these data were part of an ongoing quality assurance assessment of the VAP pathway. All information was kept confidential and secured by the Center for Anti-Infective Research and Development, Hartford, CT, in compliance with the Health Insurance Portability and Accountability Act of 1996, and patient identifiers were destroyed after data analyses were complete. Inclusion criteria consisted of adult patients (≥18 years old) in the ICU, who were placed on the VAP clinical pathway and prescribed cefepime. Patients with severely impaired renal function requiring dialysis and those deemed poor candidates for blood collection were excluded.
Blood sampling.
Blood samples (2 to 3 per patient) were collected from an in situ venous line in a nonanticoagulant tube after at least three consecutive doses of cefepime in order to ensure steady-state concentrations. The blood samples were collected immediately after infusion, at 3 to 7 h after the start of infusion, and prior to the next dose, when possible. Once collected, blood samples were immediately centrifuged, and the serum was stored at −80°C until drug analysis.
Analytic methods.
Cefepime concentrations in human serum were determined using a validated high-performance liquid chromatography assay (4). Intraday and interday coefficients of variation for the low (2 mg/liter) and high (40 mg/liter) quality control samples were all <6%.
Pharmacokinetic analysis.
Population modeling of cefepime concentrations were performed using the nonparametric adaptive grid program in the MM-USC*PACK collection (5, 15). A two-compartment pharmacokinetic model with zero-order infusion and first-order elimination, applying creatinine clearance (CLCR) as a function, was chosen based on log-likelihood values and Akaike's information criterion (25). The following parameters were estimated for each patient: volume of distribution in the central compartment (V1 [liter/kg]), elimination rate constant (K10 [h−1]), and intercompartmental transfer constants (K12, K21 [h−1]). Total body clearance (CLT [liter/kg/h and liter/h]) was then derived from the above-described estimates. Demographic variables were used to determine correlation with pharmacokinetic parameters. These variables included age, gender, ethnicity, body weight, APACHE II (acute physiology and chronic health evaluation) score on the day of cefepime sampling (12), and CLCR. A Kslope population pharmacokinetic analysis was performed with CLCR for the elimination rate parameter (K10) according to the following equation: K10 = Ki + KS × CLCR, where Ki is the intercept, KS is the slope parameter, and CLCR was calculated using an adjusted Cockcroft-Gault equation that excluded weight from the numerator and denominator [CLCR = (140 − age)/serum creatinine; the result of this equation is multiplied by 0.85 for females]. Body weight was considered a function of V1. The overall assay error variance model with a gamma function (γ) was determined by fitting a first-order polynomial to the plot of the assay standard deviations (SD) versus the measured cefepime concentrations on an interday basis, generating the following formula: SD = γ(0. 0224 + 0. 056 × C), where C was concentration and γ was identified to be 1.07. The modeling procedure weighted the individual concentrations in the serum by the reciprocal of the assay error variance pattern, giving more influence to the precisely measured cefepime concentrations and less weight to the less-precise values. Measures of predictive performance and coefficients of determination were applied to observed-predictive plots. An independent group of randomly selected patients (n = 6) was withheld from the initial model-building process in order to test model bias and precision.
Pharmacodynamic analysis.
A 5,000-patient Monte Carlo simulation (Crystal Ball version 2000; Decisioneering Inc., Denver, CO) using an open two-compartment model was conducted using the pharmacokinetic parameter median estimates, dispersion, and a lower triangular covariance matrix acquired from the final model to generate steady-state concentration-time profiles for various cefepime dosing regimens. Protein binding of 15% was applied by multiplying the cefepime dose by the fraction unbound before performing each simulation. The probability of target attainment (PTA) was calculated for each dosing regimen as a function of increasing MIC dilutions, using 50% fT>MIC as the pharmacodynamic target (19). Cefepime dosage regimens, including package insert-recommended dosing as well as higher-dose prolonged 3-h infusion regimens, were simulated for three categories of renal function (based on CLCR ranges), as follows: 50 to 120 ml/min, 30 to 49 ml/min, and 10 to 29 ml/min, using the K10 equation described above.
Statistical analysis.
Dichotomous variables (e.g., gender, combination therapy, type of infection) were compared using a chi-square test. Continuous variables were compared using Student's t test or the Mann-Whitney U test, where appropriate. An a priori P value of <0.05 was statistically significant. All statistical tests were conducted on SigmaStat statistical software version 2 (SPSS Inc., Chicago, IL).
RESULTS
Population demographics.
Of the 32 total patients (88 collected serum samples), 26 patients (72 serum samples) were used to develop the cefepime population pharmacokinetic model (experimental group). The six remaining patients (16 collected serum samples) were used to validate the model (validation group). Not all patients had three blood samples collected, due to nursing shift changes or deterioration in the patient's clinical status. No significant differences were observed among the patient characteristics (Table 1). APACHE II scores were similarly high, suggesting that both groups of patients were severely ill at time of blood sample collection. One patient placed on the VAP pathway actually had an intra-abdominal infection, but the pharmacokinetics of this patient was similar to that of the others and, therefore, was kept in the analysis. High-dose cefepime of 2 g every 8 h (3-h infusion) or a dose consistent with renal adjustment was administered to 29 of the 32 critically ill patients (24 in the experimental group and 5 in the validation group); the remaining patients received package insert-recommended doses. In the experimental group, renal function was normal (CLCR, 50 to 120 ml/min) in 22 patients and poor in four patients (three patients with CLCR values of 30 to 49 ml/min and one patient with a CLCR of 10 to 29 ml/min). Within the validation group, five patients had normal renal function, while one patient had a CLCR between 30 and 49 ml/min. In addition to cefepime, tobramycin or a fluoroquinolone was used concurrently in 28 of the 32 patients. Most patients (n = 24) received tobramycin concomitantly with cefepime. No patient experienced adverse events attributed to the high-dose prolonged-infusion cefepime dosages.
TABLE 1.
Comparative demographics of patients receiving cefepime for VAP between the experimental and validation groups
| Characteristica | Experimental group (n = 26)
|
Validation group (n = 6)
|
||||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (range) | No. (%) of patients | Mean ± SD | Median (range) | No. (%) of patients | |
| Continuous variables | ||||||
| Age | 57.0 ± 21.3 | 60.5 (19-91) | 62.0 ± 8.3 | 63.5 (49-72) | ||
| Wt (kg) | 84.0 ± 23.2 | 78.6 (50.4-158.4) | 86.9 ± 15.7 | 85.4 (70.5-106.8) | ||
| APACHE II scoreb | 19.5 ± 4.6 | 19 (11-29) | 18.3 ± 5.0 | 16.5 (15-28) | ||
| SCr (mg/dl) | 0.9 ± 0.4 | 0.8 (0.4-2.3) | 1.0 ± 0.5 | 0.9 (0.4-1.8) | ||
| CLCR (ml/min)c | 100.5 ± 40.7 | 97.5 (26.1-186.7) | 93.7 ± 56.2 | 80.7 (40.6-193.4) | ||
| Dichotomous variables | ||||||
| Male gender | 17 (65) | 4 (67) | ||||
| ICU type | ||||||
| SICU | 13 (50) | 3 (50) | ||||
| NTICU | 13 (50) | 3 (50) | ||||
| Concomitant treatment with: | ||||||
| Tobramycin | 19 (73) | 5 (83) | ||||
| Vancomycin | 22 (85) | 5 (83) | ||||
| Fluoroquinolone | 4 (15) | 0 (0) | ||||
| Tobramycin + vancomycin | 18 (69) | 5 (83) | ||||
SICU, surgical intensive care unit; NTICU, neurotrauma intensive care unit; SCr, serum creatinine; CLCR, creatinine clearance.
APACHE II score was measured at the time of the first blood sample from the patient.
CLCR was calculated using the Cockcroft-Gault equation, independent of weight.
Pharmacokinetic parameters.
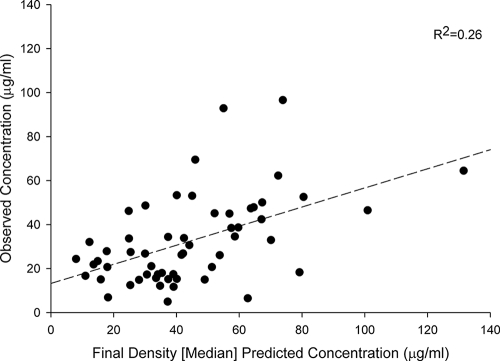
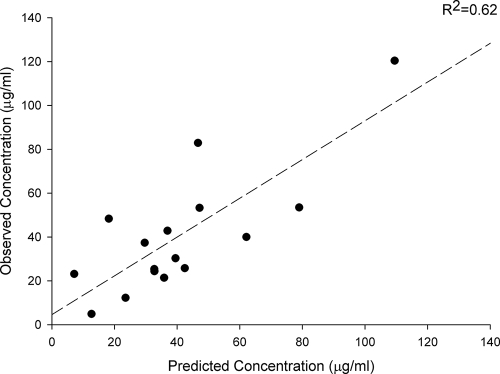
Population pharmacokinetic parameters for cefepime for the 26 subjects are provided in Tables 2 and 3. Model building using log-likelihood values and Akaike's information criterion identified the optimal model, with K10 as a function of CLCR, whereby K10 = 0.071 + 0.0027 × CLCR, and with V1 as a function of total body weight (TBW), whereby V1 = TBW × 0.21 liter/kg. Other tested covariates had no identifiable influence on the pharmacokinetic parameters. Using these median parameter estimates, the bias, precision, and coefficient of determination for the initial model were 11.3 μg/ml, 24.0 μg/ml, and 26%, respectively (Fig. 1). Examining the data using the maximum a posteriori Bayesian estimation step as a reference, the bias, precision, and coefficient of determination for the model were 0.28 μg/ml, 7.39 μg/ml, and 98%, respectively. The independent validation group displayed a bias, precision, and coefficient of determination of −1.64 μg/ml, 17.1 μg/ml, and 62%, respectively (Fig. 2). This model was considered acceptable for predicting cefepime concentrations in our population. For comparisons with other research in the field, we also examined CLT as a function of CLCR and identified CLT by using the formula 0.048 × CLCR + 1.2. Our mean (SD) V1 was 22.1 liters (6.1 liters), and our mean CLT was 7.6 liters/h (3.3 liters/h). The clearance parameter was calculated as the product of the elimination rate parameter and V1.
TABLE 2.
Pharmacokinetic parameters of the cefepime population modela
| Parameter | Mean | Median | SD |
|---|---|---|---|
| Ki (h−1) | 0.094 | 0.071 | 0.06 |
| KS (h−1) | 0.006 | 0.003 | 0.011 |
| K12 (h−1) | 1.337 | 0.78 | 1.023 |
| K21 (h−1) | 1.046 | 0.472 | 1.082 |
| V1 (liter/kg) | 0.263 | 0.206 | 0.187 |
K10 = Ki + (KS × CLCR). CLT, total body clearance; Ki, y-intercept constant; KS, slope constant; K12, intercompartmental transfer rate constant from the central to the peripheral compartment; K21, intercompartmental transfer rate constant from the peripheral to the central compartment; V1, volume of distribution of the central compartment.
TABLE 3.
Covariance matrix in lower triangular form
| Parameter | Covariance
|
||||
|---|---|---|---|---|---|
| Ki | KS | K12 | K21 | V1 | |
| Ki | 0.0037 | ||||
| KS | −0.0001 | 0.0001 | |||
| K12 | −0.0370 | 0.0008 | 1.0466 | ||
| K21 | −0.0136 | −0.0015 | 0.7208 | 1.1717 | |
| V1 | 0.0026 | −0.0011 | −0.0455 | 0.0346 | 0.0348 |
Ki, the y intercept of the elimination rate constant; KS, the slope of the elimination rate constant; K12, intercompartmental transfer rate constant from the central to the peripheral compartment; K21, intercompartmental transfer rate constant from the peripheral to the central compartment; V1, volume of distribution of the central compartment.
FIG. 1.
Scatter plot of observed versus predicted cefepime concentrations, using the population pharmacokinetic model with covariates.
FIG. 2.
Scatter plot of observed and predicted cefepime concentrations of the validation group, in accordance with the median parameter values of the pharmacokinetic population model.
Pharmacodynamic analysis.
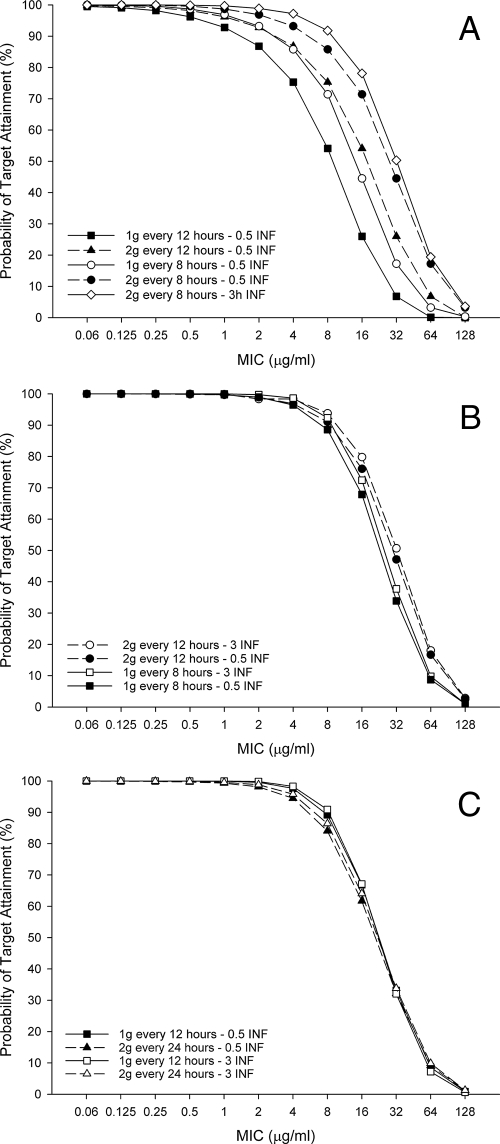
The probabilities of achieving a target of 50% fT>MIC for cefepime with various dosing regimens in three groups of critically ill patients with various renal functions (CLCR of 50 to 120, 30 to 49, and 10 to 29 ml/min) are shown in Fig. 3A to C. In patients with CLCR of 50 to 120 ml/min, the high-dose, prolonged-infusion regimen (2 g every 8 h, as a 3-h infusion) achieved 91.8%, 78.1%, and 50.3% PTAs at MICs of 8 μg/ml (susceptibility breakpoint), 16 μg/ml (intermediate), and 32 μg/ml (resistance breakpoint), respectively (Fig. 3A). Traditional 0.5-h infusion dosing regimens achieved significantly lower PTAs at these respective MICs. Among simulated patients with a CLCR of 30 to 49 ml/min, a 2-g dose every 12 h infused over 3 h achieved PTAs of 93.8%, 79.8%, and 50.7% at MICs of 8 μg/ml, 16, μg/ml, and 32 μg/ml, respectively (Fig. 3B). The same dose administered as a 0.5-h infusion had slightly lower probabilities in this MIC range. In patients with CLCR of 10 to 29 ml/min, all regimens achieved similar PTAs across the tested MIC range (Fig. 3C); however, no regimen achieved a PTA against MICs of 16 μg/ml and 32 μg/ml as high as those of the other CLCR groups.
FIG. 3.
PTA for cefepime regimens achieving 50% fT>MIC at various CLCR levels: 50 to 120 ml/min (A), 30 to 49 ml/min (B), 10 to 29 ml/min (C). 0.5 INF, 0.5-h (30-min) infusion; 3h INF and 3 INF, 3-h infusion.
DISCUSSION
Herein, we created a population pharmacokinetic model to describe the concentration data obtained in patients receiving a high-dose, prolonged-infusion cefepime dosing regimen (2 g every 8 h, infused over 3 h) according to our hospital's VAP pathway. The model was generated from critically ill patients predominately diagnosed with VAP. CLCR and body weight were shown to be the covariates most influential of K10 and V1, thus enabling for the prediction of individual cefepime serum concentrations. A unique characteristic of our model-building process was the use of a randomly selected independent population of VAP patients for validation. Compared with the population pharmacokinetic model that influenced the development of our hospital's VAP clinical pathway (23), our model had similar intercompartmental transfer and elimination rate constants. Although the intercept values and slopes are slightly different, the final regression equation published by Tam and colleagues (CLT = 0.055 × CLCR + 0.329; median values) (23) produced values of drug clearance at 50 ml/min and 100 ml/min that were virtually identical to ours: 3.1 versus 3.6 liters/h and 5.8 versus 6.0 liters/h, respectively.
Although there have been several cefepime population pharmacokinetic models developed in the literature (16, 23), there are few that specifically observe an adult critically ill patient population (8, 22), and only one has explored the utilization of a prolonged infusion of cefepime (24). The importance of observing this patient population is centered upon the patient heterogeneity and the large interindividual pharmacokinetic parameters within the ICU populations. Additionally, our population model is believed to be the first generated using a prolonged-infusion cefepime dose. Recently, another cefepime model with a similar population utilized doses of 4 g administered continuously over 24 h (8). Despite receiving a different dosing strategy and using a different parameter-covariant relationship (CLT to serum creatinine), V1 and CLT rates were comparable to those in our assessment. Other cefepime pharmacokinetic studies have observed mean V1 and CLT rates in critical care patients in the ranges of 23 to 27 liters and 6 to 7 liters/h, respectively, results which are similar to our mean values of 22.1 liters and 7.6 liters/h, respectively (17, 22, 23).
Because of the high prevalence of multidrug-resistant, gram-negative organisms as a cause of VAP in critically ill patients, the need for appropriate antibiotic therapy and optimal drug dosing is paramount. Although P. aeruginosa is considered susceptible to cefepime when the MIC is ≤8 μg/ml, a recent report noted increased mortality at this MIC in patients with bacteremia who were treated with standard cefepime dosages (2). Based on these data and the frequency of isolates nonsusceptible to this antibiotic at our institution, we employed a high-dose, prolonged-infusion (2 g every 8 h, with each dose infused over 3 h) aimed at achieving 50% fT>MIC for pathogens with cefepime MICs of up to 32 μg/ml (i.e., resistant). The actual pharmacokinetics observed in our patient population demonstrated that this dosage regimen did indeed achieve this pharmacodynamic exposure, with a high likelihood at MICs of 8 μg/ml, 16 μg/ml, and to a lower probability, 32 μg/ml. As shown in this study (Fig. 3A), as well as with other β-lactams, the prolonged-infusion regimen increased the fT>MIC against organisms with higher MICs compared to that of traditional 30-min infusion (9, 11, 13, 18). The prolonged-infusion strategy also permits ample time for other drugs to be administered through the same intravenous line during the breaks in infusion time.
We also evaluated different dosing regimens based on CLCR ranges to confirm that optimal exposure was maintained when doses were adjusted for renal dysfunction. Among simulated patients with a CLCR of 30 to 49 ml/min, 2-g doses administered every 12 h as either 0.5- or 3-h infusions achieved nearly identical PTAs at higher MICs as those achieved by the max dose (2 g every 8 h, as 3-h infusion) in patients with normal CLCR. This suggests that the benefit of the prolonged infusion lessens as a patient's renal function declines; we currently advocate the administration of a 2-g dose every 12 h (0.5-h infusion) empirically for VAP patients with a CLCR of 30 to 49 ml/min at our hospital to target nonsusceptible organisms, reserving the prolonged infusion for those with normal renal function only. For patients with a CLCR range of 10 to 29 ml/min, all of the simulated dosage regimens achieved similar PTAs, but none were able to maintain high probabilities of achieving 50% fT>MIC at 16 μg/ml (∼66%) or 32 μg/ml (∼33%). We currently utilize a regimen of 1 g every 12 h (0.5-h infusion) for these patients, but further study is required to determine a dose that achieves higher PTAs at 50% fT>MIC at 16 or 32 μg/ml while retaining a low likelihood for toxicity.
In summary, this is the first cefepime population pharmacokinetic model developed and validated for critically ill patients with VAP treated with a high-dose, prolonged-infused regimen. These data demonstrate that cefepime dosed 2 g every 8 h as a 3-h prolonged infusion will improve the likelihood of pharmacodynamic target attainment over that of standard 30-min infusions. This report also provides suggestions for doses that are able to maintain these PTAs in patients with decreased renal function. Lastly, this covariate model will be useful in predicting cefepime exposures in VAP patients who do not have concentration data available.
Acknowledgments
We thank Christina Sutherland for her assistance with the analytical determination of cefepime and Somvadee Laohaveleeson for her assistance with the collection of patient samples.
Footnotes
Published ahead of print on 2 February 2009.
REFERENCES
- 1.Barradell, L. B., and H. M. Bryson. 1994. Cefepime: a review of its antimicrobial activity, pharmacokinetic properties and therapeutic use. Drugs 47:471-505. [DOI] [PubMed] [Google Scholar]
- 2.Bhat, S. V., A. Y. Peleg, T. P. Lodise, Jr., K. A. Shutt, B. Capitano, B. A. Potoski, and D. L. Paterson. 2007. Failure of current cefepime breakpoints to predict clinical outcomes of bacteremia caused by gram-negative organisms. Antimicrob. Agents Chemother. 51:4390-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boselli, E., D. Breilh, F. Duflo, M. C. Saux, R. Debon, D. Chassard, and B. Allaouchiche. 2003. Steady-state plasma and intrapulmonary concentrations of cefepime administered in continuous infusion in critically ill patients with severe nosocomial pneumonia. Crit. Care Med. 31:2102-2106. [DOI] [PubMed] [Google Scholar]
- 4.Burgess, D. S., R. W. Hastings, and T. C. Hardin. 2000. Pharmacokinetics and pharmacodynamics of cefepime administration by intermittent and continuous infusion. Clin. Ther. 22:66-75. [DOI] [PubMed] [Google Scholar]
- 5.Bustad, A., D. Terziivanov, R. Leary, R. Port, A. Schumitzky, and R. Jelliffe. 2006. Parametric and nonparametric populations methods. Their comparative performance in analyzing a clinical dataset and two Monte Carlo simulation studies. Clin. Pharmacokinet. 45:365-383. [DOI] [PubMed] [Google Scholar]
- 6.Chong, Y., K. Lee, and O. H. Kwon. 1993. In vitro activity of cefepime against Enterobacter cloacae, Serratia marcescens, Pseudomonas aeruginosa and other aerobic gram-negative bacilli. J. Antimicrob. Chemother. 32(Suppl. B):21-30. [DOI] [PubMed] [Google Scholar]
- 7.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 8.Georges, B., J. M. Contil, T. Seguin, E. Dieye, P. Cougot, J. F. Decun, M. Lavit, K. Samil, G. Houin, and S. Saivin. 2008. Cefepime in intensive care unit patients: validation of a population pharmacokinetic approach and influence of covariables. Int. J. Clin. Pharmacol. Ther. 46:157-164. [DOI] [PubMed] [Google Scholar]
- 9.Jaruratanasirikul, S., S. Sriwiriyajan, and J. Punyo. 2005. Comparison of the pharmacodynamics of meropenem in patients with ventilator-associated pneumonia following administration by 3-hour infusion or bolus injection. Antimicrob. Agents Chemother. 49:1337-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, R. N., J. T. Kirby, and P. R. Rhomberg. 2008. Comparative activity of meropenem in US medical centers (2007): initiating the 2nd decade of MYSTIC program surveillance. Diagn. Microbiol. Infect. 61:203-213. [DOI] [PubMed] [Google Scholar]
- 11.Kim, A., C. A. Sutherland, J. L. Kuti, and D. P. Nicolau. 2007. Optimal dosing of piperacillin-tazobactam for the treatment of Pseudomonas aeruginosa infections: prolonged or continuous infusion? Pharmacotherapy 27:1490-1497. [DOI] [PubMed] [Google Scholar]
- 12.Knaus, W. A., E. A. Draper, D. P. Wagner, and J. E. Zimmerman. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818-829. [PubMed] [Google Scholar]
- 13.Kuti, J. L., K. M. Moss, D. P. Nicolau, and R. F. Knauft. 2004. Empiric treatment of multidrug-resistant Burkholderia cepacia lung exacerbation in a patient with cystic fibrosis: application of pharmacodynamic concepts to meropenem therapy. Pharmacotherapy 24:1641-1645. [DOI] [PubMed] [Google Scholar]
- 14.Kuti, J. L., A. M. Nicasio, E. Shore, M. Palter, J. Pepe, and D. P. Nicolau. 2007. Outcomes of an empiric antibiotic algorithm (EAA) for ventilator-associated pneumonia (VAP) that considers local MIC distributions and pharmacodynamics (PD), abstr. P-1093, p. 249. Abstr. 45th Annu. Meet. Infect. Dis. Soc. Am. Infect. Dis. Soc. Am., San Diego, CA.
- 15.Leary, R., R. Jelliffe, A. Schumitzky, and M. van Guilder. 2002. A unified parametric/nonparametric approach to population PK/PD modeling, abstr. 302, p.11. Abstr. 11th Annu. Meet. Popul. Appr. Group Eur. Paris, France.
- 16.Lima-Rogel, V., E. L. Medina-Rojas, R. Del Carmen Milán-Segovia, D. E. Noyola, K. Nieto-Aguirre, A. López-Delarosa, and S. Romano-Moreno. 2008. Population pharmacokinetics of cefepime in neonates with severe nosocomial infections. J. Clin. Pharm. Ther. 33:295-306. [DOI] [PubMed] [Google Scholar]
- 17.Lipman, J., S. C. Wallis, and C. Rickard. 1999. Low plasma cefepime levels in critically ill septic patients: pharmacokinetic modeling indicates improved troughs with revised dosing. Antimicrob. Agents Chemother. 43:2559-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lodise, T. P., Jr., B. Lomaestro, and G. L. Drusano. 2007. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin. Infect. Dis. 44:357-363. [DOI] [PubMed] [Google Scholar]
- 19.Maglio, D., C. Ong, M. A. Banevicius, Q. Geng, C. H. Nightingale, and D. P. Nicolau. 2004. Determination of the in vivo pharmacodynamic profile of cefepime against extended-spectrum-beta-lactamase-producing Escherichia coli at various inocula. Antimicrob. Agents Chemother. 48:1941-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicasio, A. M., J. L. Kuti, and D. P. Nicolau. 2008. The current state of multidrug-resistant gram-negative bacilli in North America. Pharmacotherapy 28:235-249. [DOI] [PubMed] [Google Scholar]
- 21.Rafati, M. R., M. R. Rouini, M. Mojtahedzadeh, A. Najafi, H. Tavakoli, K. Gholami, and M. R. Fazeli. 2006. Clinical efficacy of continuous infusion of piperacillin compared with intermittent dosing in septic critically ill patients. Int. J. Antimicrob. Agents 28:122-127. [DOI] [PubMed] [Google Scholar]
- 22.Roos, J. F., J. Bulitta, J. Lipman, and C. M. Kirkpatrick. 2006. Pharmacokinetic-pharmacodynamic rationale for cefepime dosing regimens in intensive care units. J. Antimicrob. Chemother. 58:987-993. [DOI] [PubMed] [Google Scholar]
- 23.Tam, V. H., P. S. McKinnon, R. L. Akins, G. L. Drusano, and M. J. Rybak. 2003. Pharmacokinetics and pharmacodynamics of cefepime in patients with various degrees of renal function. Antimicrob. Agents Chemother. 47:1853-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tam, V. H., A. Louie, B. M. Lomaestro, and G. L. Drusano. 2003. Integration of population pharmacokinetics, a pharmacodynamic target, and microbiological surveillance data to generate a rational empiric dosing strategy for cefepime against Pseudomonas aeruginosa. Pharmacotherapy 23:291-295. [DOI] [PubMed] [Google Scholar]
- 25.Yamaoka, K., T. Nakagawa, and T. Uno. 1978. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 6:165-175. [DOI] [PubMed] [Google Scholar]