Abstract
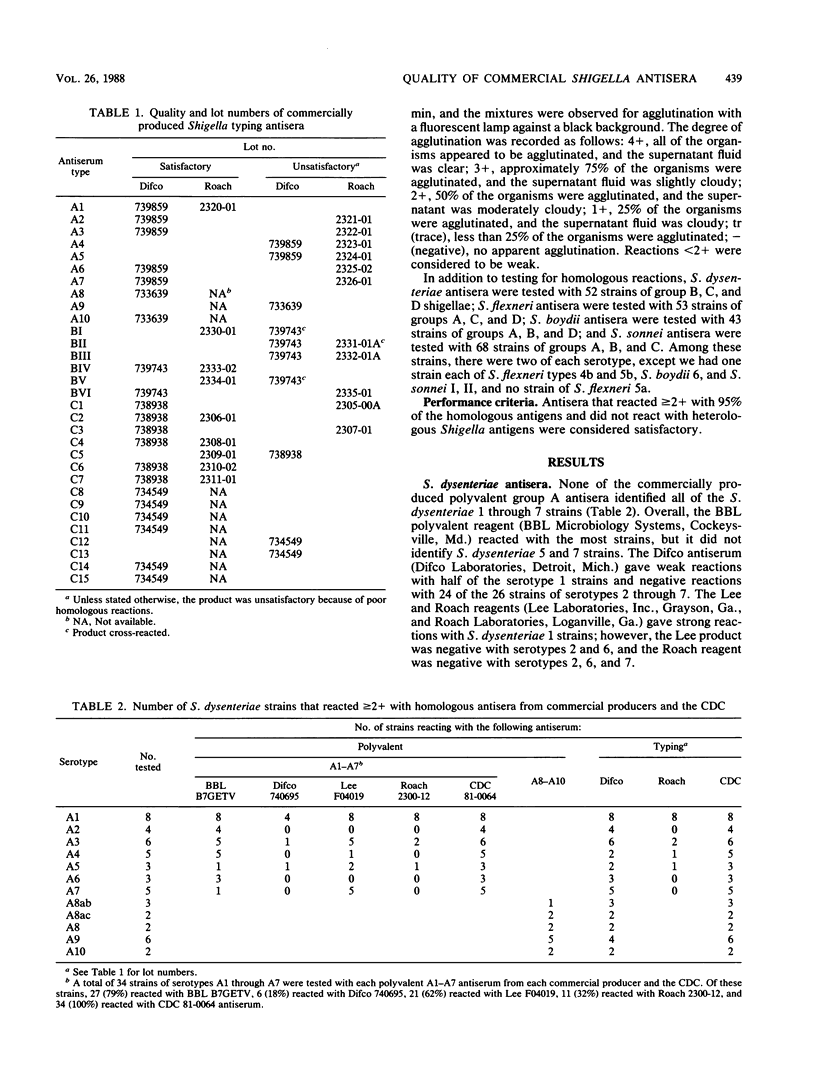
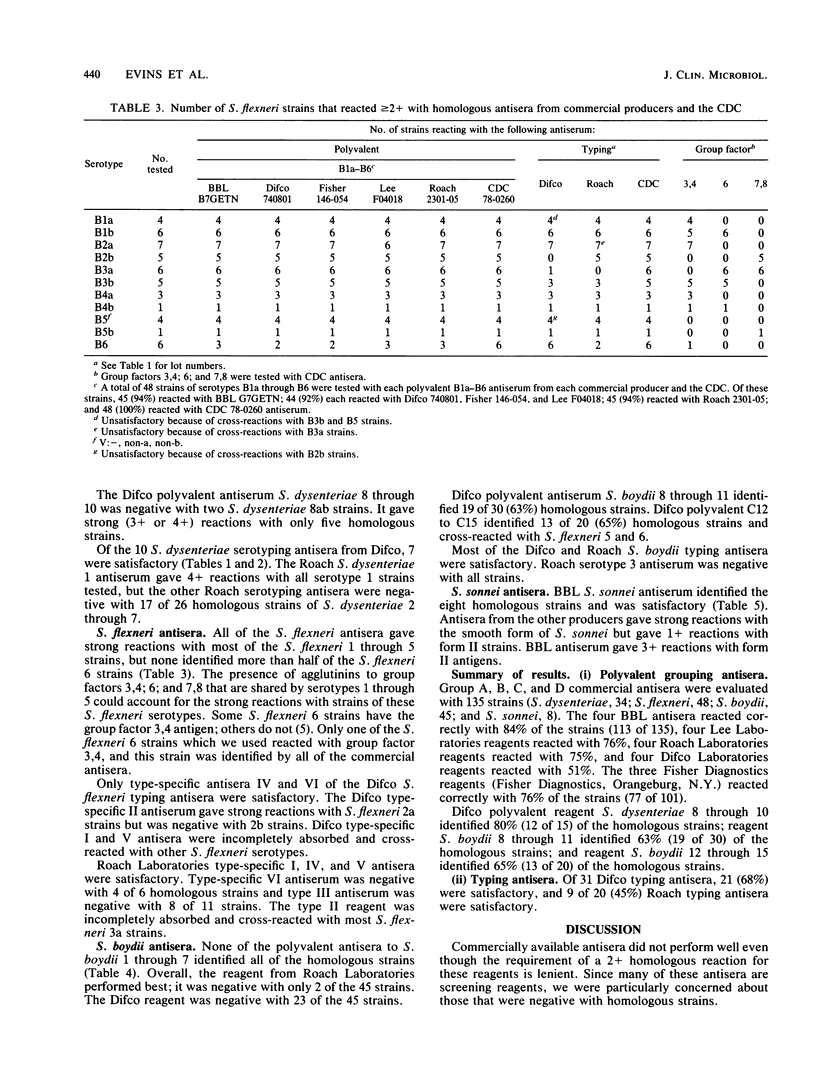
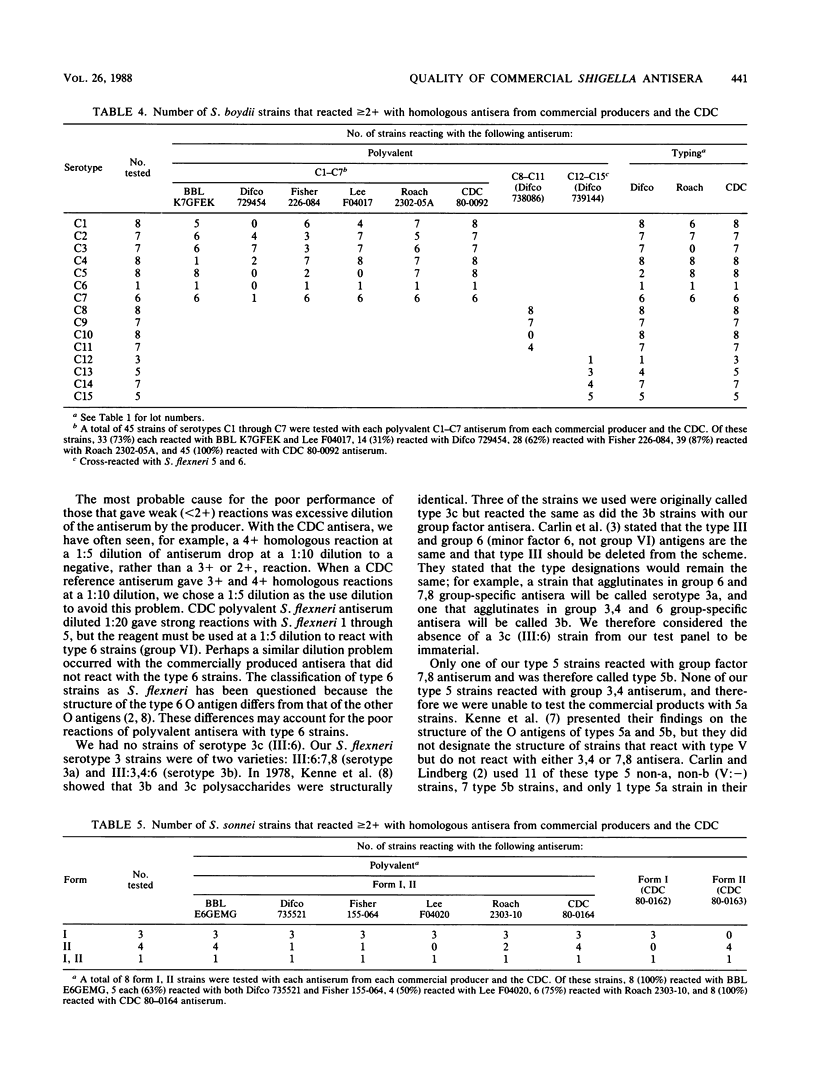
Shigella grouping antisera from five manufacturers and typing antisera from two were purchased and evaluated with homologous and heterologous Shigella strains in the slide agglutination test. Only 31 of 73 (42%) antisera were satisfactory. In many instances, the antisera gave negative, as opposed to weak, reactions when they should have given strong positive reactions. Four reagents cross-reacted with Shigella strains. Of the 19 polyvalent grouping antisera to subgroups Shigella dysenteriae serotypes 1 through 7, S. flexneri serotypes 1 through 6, S. boydii serotypes 1 through 7, and S. sonnei forms I, II, only one S. sonnei reagent and five S. flexneri reagents were satisfactory with greater than or equal to 90% of the homologous strains. The reagent of poorest quality was satisfactory with only 18% of the homologous strains. There were three polyvalent antisera to the higher types of S. dysenteriae and S. boydii, which were available from only one company, that adequately identified 80, 63, and 65% of the homologous strains. Typing antisera were available from only two companies, and 30 of 51 (59%) were satisfactory. Commercially available Shigella antisera are inadequate for the laboratory testing required for planning the development of and evaluating Shigella vaccines.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlin N. I., Lindberg A. A. Monoclonal antibodies specific for O-antigenic polysaccharides of Shigella flexneri: clones binding to II, II:3,4, and 7,8 epitopes. J Clin Microbiol. 1983 Nov;18(5):1183–1189. doi: 10.1128/jcm.18.5.1183-1189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin N. I., Lindberg A. A. Monoclonal antibodies specific for Shigella flexneri lipopolysaccharides: clones binding to type I and type III:6,7,8 antigens, group 6 antigen, and a core epitope. Infect Immun. 1986 Jul;53(1):103–109. doi: 10.1128/iai.53.1.103-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin N. I., Wehler T., Lindberg A. A. Shigella flexneri O-antigen epitopes: chemical and immunochemical analyses reveal that epitopes of type III and group 6 antigens are identical. Infect Immun. 1986 Jul;53(1):110–115. doi: 10.1128/iai.53.1.110-115.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins G. M., Linne L. L., McWhorter A. C. Evaluation of API SerImm Sure strips for screening and grouping Salmonella isolates. Diagn Microbiol Infect Dis. 1985 Jan;3(1):19–23. doi: 10.1016/0732-8893(85)90062-8. [DOI] [PubMed] [Google Scholar]
- Kenne L., Lindberg B., Petersson K., Katzenellenbogen E., Romanowska E. Structural studies of Shigella flexneri O-antigens. Eur J Biochem. 1978 Nov 2;91(1):279–284. doi: 10.1111/j.1432-1033.1978.tb20963.x. [DOI] [PubMed] [Google Scholar]
- Kenne L., Lindberg B., Petersson K., Katzenellenbogen E., Romanowska E. Structural studies of the Shigella flexneri variant X, type 5 a and type 5 b O-antigens. Eur J Biochem. 1977 Jun 15;76(2):327–330. doi: 10.1111/j.1432-1033.1977.tb11599.x. [DOI] [PubMed] [Google Scholar]
- Keren D. F., McDonald R. A., Formal S. B. Secretory immunoglobulin A response following peroral priming and challenge with Shigella flexneri lacking the 140-megadalton virulence plasmid. Infect Immun. 1986 Dec;54(3):920–923. doi: 10.1128/iai.54.3.920-923.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks E. V., Hale T. L., Formal S. B. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986 Jul;53(1):57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Sasakawa C., Makino S., Yoshikawa M. DNA sequence and product analysis of the virF locus responsible for congo red binding and cell invasion in Shigella flexneri 2a. Infect Immun. 1986 Nov;54(2):395–402. doi: 10.1128/iai.54.2.395-402.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun. 1981 Oct;34(1):75–83. doi: 10.1128/iai.34.1.75-83.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Nakamura A. Identification of Shigella sonnei form I plasmid genes necessary for cell invasion and their conservation among Shigella species and enteroinvasive Escherichia coli. Infect Immun. 1986 Aug;53(2):352–358. doi: 10.1128/iai.53.2.352-358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



