Abstract
The bactericidal activity of amoxicillin was investigated against Enterococcus faecalis JH2-2 and against an isogenic mutant deficient in the production of the N-acetylglucosaminidase AtlA. Comparison of the two strains indicated that this autolysin contributes to killing by amoxicillin both in vitro and in a rabbit model of experimental endocarditis.
Enterococci have long been recognized as a frequent cause of community-acquired infections, including urinary tract and intra-abdominal infections and endocarditis (15). Since the mid-1980s, enterococci have also become significant nosocomial pathogens (21). A presumed major reason for their spread in the hospital environment is their ability to resist most of the available antibiotics (18). Enterococci are intrinsically more resistant to penicillin than streptococci, due to the production of a penicillin-binding protein, PBP5, that displays decreased affinity for β-lactams (22). In addition, several studies revealed that large proportions of clinical isolates are tolerant to all antibiotics that inhibit cell wall synthesis, including β-lactams, since the minimal bactericidal concentrations (MBCs) of the antibiotics are ≥32-fold higher than the MICs (5, 7, 11). Recommendations for the treatment of severe enterococcal infections, such as endocarditis, include the combination of a cell-wall-active agent with an aminoglycoside (2). The combination is synergistic (1, 9) and may improve the outcome of endocarditis (13).
Although the targets of antibiotics have been thoroughly characterized at both the molecular and physiological levels, the exact sequence of events that leads to cell death remains poorly understood. A recent study concluded that the three major classes of bactericidal drugs, β-lactams, aminoglycosides, and quinolones, utilize a common mechanism of killing involving the production of lethal doses of hydroxyl radicals (10). In Streptococcus pneumoniae, inhibition of peptidoglycan synthesis by β-lactams triggers cells lysis that contributes to the bactericidal activity of the antibiotics (20). β-Lactam-induced bacteriolysis of the pneumococcus requires production of specific enzymes, autolysins, that digest the peptidoglycan network and disrupt its essential osmo-protective function. We have recently characterized the major autolysin of Enterococcus faecalis JH2-2, AtlA, as a glucosaminidase which cleaves the β-1,4 bond between N-acetylglucosamine and N-acetylmuramic acid (4). Here, we investigate the contribution of AtlA to the bactericidal activity of amoxicillin against E. faecalis JH2-2 (8) both in vitro and in vivo, using a rabbit model of infective endocarditis.
E. faecalis JH2-2 ΔatlA is a stable mutant resulting from an in-frame deletion of 2,052 bp within the atlA open reading frame (737 codons) (14). In order to prevent any polar effect, the deletion was in frame and the resulting locus encoded a 58-amino-acid peptide, comprising the signal peptide of AtlA followed by four C-terminal residues of this protein. The MICs and MBCs of amoxicillin (GlaxoSmithKline Laboratories, Marly-le-Roi, France) were determined by the macrodilution method, according to CLSI (3). Briefly, glass tubes containing twofold dilutions of antibiotic (0.25 to 1,024 μg/ml) in 2 ml of brain heart infusion (BHI) broth were incubated with 0.1 ml of exponentially growing cells (ca. 108 CFU/ml). After 24 h of incubation at 37°C, surviving bacteria were enumerated on BHI agar supplemented with 0.015 U/ml of Bacillus cereus penicillase (Sigma, St. Louis, MO). The MIC was defined as the lowest drug concentration that inhibited the visible bacterial growth. The MBC was defined as the lowest drug concentration that killed ≥99.9% of the original inoculum (16). Using the standard procedure, the MICs of amoxicillin were 0.5 μg/ml for E. faecalis JH2-2 and 1 μg/ml for E. faecalis JH2-2 ΔatlA. The MBC of amoxicillin was 2 μg/ml for E. faecalis JH2-2, with an MBC/MIC ratio of 4. Although the decrease in the CFU was close to the threshold of ≥99.9% at 2 μg/ml, none of the tested concentration of amoxicillin was bactericidal against JH2-2 ΔatlA (MBC, >1,024 μg/ml). By definition (6), the mutant was tolerant to amoxicillin since the MBC/MIC ratio was ≥32. These results suggested that the autolysin AtlA contributed to the bactericidal activity of amoxicillin in E. faecalis JH2-2. In agreement, inactivation of atlA in E. faecalis strain OG1RF was previously shown to significantly reduce bacterial killing by penicillin (17). However, the impact of the deletion was more limited, suggesting that the contribution of AtlA to the killing by β-lactams may vary between strains.
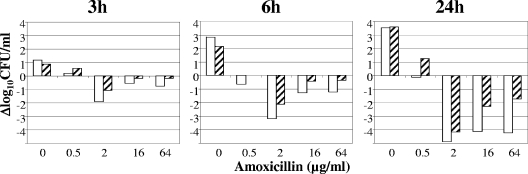
To gain insight into the kinetics of bacterial killing, 107 CFU/ml of exponentially growing E. faecalis were incubated with 0, 0.5, 2, 16, and 64 μg/ml of amoxicillin and surviving bacteria were enumerated after 0, 3, 6, and 24 h of incubation. Each experiment was performed at least four times with similar results. Figure 1 shows the result of a representative experiment. The most efficient concentration of amoxicillin for killing of E. faecalis JH2-2 and JH2-2 ΔatlA was 2 μg/ml (Fig. 1). The reduced killing observed for higher drug concentrations (Fig. 1) has been previously reported for β-lactams, a paradoxical response referred to as the “Eagle effect” (19). Table 1 shows the mean (± standard deviation) decreases in cell counts obtained after 3, 6, and 24 h of incubation in the presence of the concentration of amoxicillin that produced maximum bacterial killing (2 μg/ml). Comparison of E. faecalis JH2-2 and JH2-2 ΔatlA revealed that deletion of atlA decreased the observed bacterial killing at 3, 6, and 24 h for all drug concentrations (Fig. 1 and Table 1). However, bacterial killing occurred independently from AtlA and the difference observed between E. faecalis JH2-2 and JH2-2 ΔatlA did not remain statistically significant after 24 h of exposure to 2 μg/ml of amoxicillin (Table 1). Thus, our analysis suggests that AltA-mediated autolysis contributes to bacterial killing, although this is not the only pathway to cell death.
FIG. 1.
Impact of the deletion of atlA on the bactericidal activity of amoxicillin. E. faecalis JH2-2 (open bars) and E. faecalis JH2-2 ΔatlA (hatched bars) were incubated with various concentrations of amoxicillin for 3, 6, and 24 h. The variations in the number of CFU are expressed as Δlog10 CFU/ml.
TABLE 1.
Impact of atlA deletion on the in vitro activity of 2 μg/ml amoxicillin
| Incubation time | Decrease in cell counts (log10 CFU/ml) after incubation with 2 μg/ml amoxicillina
|
P valueb | |
|---|---|---|---|
| JH2-2 | JH2-2 ΔatlA | ||
| 3 h | 2.57 ± 0.44 | 1.50 ± 0.15 | 0.0002 |
| 6 h | 5.59 ± 0.62 | 3.93 ± 0.52 | 0.0005 |
| 24 h | 7.82 ± 1.42 | 6.46 ± 1.69 | 0.26 |
Each experiment was performed at least four times. Data are expressed as the mean ± standard deviation difference between log10CFU/ml obtained with and without amoxicillin after 3, 6, or 24 h of incubation.
Student's t test.
To study the impact of AtlA on the in vivo activity of amoxicillin, aortic endocarditis was induced in 28 female New Zealand White rabbits by insertion of a polyethylene catheter through the right carotid artery into the left ventricle (12). Twenty-four hours after catheter insertion, each rabbit was inoculated by the ear vein with E. faecalis JH2-2 (109 CFU) or JH2-2 ΔatlA (5 × 108 CFU) in 1 ml of 0.9% NaCl. The catheter was left in place throughout the experiment. Forty-eight hours after inoculation, animals were treated intramuscularly with amoxicillin (50 mg/kg of body weight three times a day for 3 days). Control animals were left untreated and were sacrificed 48 h after inoculation. Animals were killed 8 h after the last antibiotic injection, and the vegetations from each rabbit were excised, rinsed in saline, pooled, and weighed. The vegetations were homogenized in 1 ml of sterile saline, and surviving bacteria were enumerated on BHI agar for each rabbit. Colony counts were expressed as log10 CFU per gram of vegetation. As shown in Table 2, bacterial counts in the vegetations of control rabbits infected with either of the two strains were not different (P = 0.37). Thus, deletion of atlA had no impact on the capacity of E. faecalis JH2-2 to colonize the aortic vegetations. The levels of virulence of E. faecalis OG1RF and its atlA mutant were also found to be similar in the mouse peritonitis model (17). The amoxicillin regimen decreased bacterial counts in the vegetations of rabbits infected by E. faecalis JH2-2 (P = 0.012), as previously observed (1). In contrast, bacterial counts were not reduced by amoxicillin in rabbits infected by E. faecalis JH2-2 ΔatlA (P = 0.746).
TABLE 2.
Activity of amoxicillin in experimental endocarditis due to E. faecalis JH2-2 and JH2-2 ΔatlA
| Strain | Bacterial counts (log10 CFU/g vegetation) for treatment regimena
|
P value for treated vs controlsd | |
|---|---|---|---|
| Noneb | Amoxicillinc | ||
| JH2-2 | 9.40 ± 0.62 (4) | 7.69 ± 1.32 (8) | 0.012 |
| JH2-2 ΔatlA | 8.34 ± 1.66 (8) | 8.55 ± 0.68 (8) | 0.746 |
Data are expressed as means ± standard deviations. Numbers of rabbits are shown in parentheses.
Controls at the start of therapy.
Intramuscular administration of 50 mg/kg/day for 3 days.
Student's t test.
In conclusion, AtlA contributes to bactericidal activity of amoxicillin both in vitro and in vivo. Further studies are mandatory to investigate the contribution of other enterococcal autolysins to the bactericidal activity of antibiotics.
Acknowledgments
We thank Jean-Luc Mainardi for critical reading of the manuscript.
Footnotes
Published ahead of print on 2 February 2009.
REFERENCES
- 1.Aslangul, E., R. Ruimy, F. Chau, L. Garry, A. Andremont, and B. Fantin. 2005. Relationship between the level of acquired resistance to gentamicin and synergism with amoxicillin in Enterococcus faecalis. Antimicrob. Agents Chemother. 49:4144-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonow, R. O., B. A. Carabello, C. Kanu, A. C. de Leon, Jr., D. P. Faxon, M. D. Freed, W. H. Gaasch, B. W. Lytle, R. A. Nishimura, P. T. O'Gara, R. A. O'Rourke, C. M. Otto, P. M. Shah, J. S. Shanewise, S. C. Smith, Jr., A. K. Jacobs, C. D. Adams, J. L. Anderson, E. M. Antman, V. Fuster, J. L. Halperin, L. F. Hiratzka, S. A. Hunt, R. Nishimura, R. L. Page, and B. Riegel. 2006. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 114:e84-231. [DOI] [PubMed] [Google Scholar]
- 3.CLSI. 2008. Performance standards for antimicrobial susceptibility testing. Eighteenth informational supplement. M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Eckert, C., M. Lecerf, L. Dubost, M. Arthur, and S. Mesnage. 2006. Functional analysis of AtlA, the major N-acetylglucosaminidase of Enterococcus faecalis. J. Bacteriol. 188:8513-8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontana, R., A. Grossato, M. Ligozzi, and E. A. Tonin. 1990. In vitro response to bactericidal activity of cell wall-active antibiotics does not support the general opinion that enterococci are naturally tolerant to these antibiotics. Antimicrob. Agents Chemother. 34:1518-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Handwerger, S., and A. Tomasz. 1985. Antibiotic tolerance among clinical isolates of bacteria. Rev. Infect. Dis. 7:368-386. [DOI] [PubMed] [Google Scholar]
- 7.Hodges, T. L., S. Zighelboim-Daum, G. M. Eliopoulos, C. Wennersten, and R. C. Moellering, Jr. 1992. Antimicrobial susceptibility changes in Enterococcus faecalis following various penicillin exposure regimens. Antimicrob. Agents Chemother. 36:121-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jawetz, E., J. B. Gunnison, and V. R. Coleman. 1950. The combined action of penicillin with streptomycin or chloromycetin on enterococci in vitro. Science 111:254-256. [DOI] [PubMed] [Google Scholar]
- 10.Kohanski, M. A., D. J. Dwyer, B. Hayete, C. A. Lawrence, and J. J. Collins. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797-810. [DOI] [PubMed] [Google Scholar]
- 11.Krogstad, D. J., and A. R. Parquette. 1980. Defective killing of enterococci: a common property of antimicrobial agents acting on the cell wall. Antimicrob. Agents Chemother. 17:965-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefort, A., and B. Fantin. 1999. Rabbit model of bacterial endocarditis, p. 611-617. In O. Zak and M. Sande (ed.), Handbook of animal models of infection. Academic Press, London, United Kingdom.
- 13.Mandell, G. L., D. Kaye, M. E. Levison, and E. W. Hook. 1970. Enterococcal endocarditis. An analysis of 38 patients observed at the New York Hospital-Cornell Medical Center. Arch. Intern. Med. 125:258-264. [DOI] [PubMed] [Google Scholar]
- 14.Mesnage, S., F. Chau, L. Dubost, and M. Arthur. 2008. Role of N-acetylglucosaminidase and N-acetylmuramidase activities in Enterococcus faecalis peptidoglycan metabolism. J. Biol. Chem. 283:19845-19853. [DOI] [PubMed] [Google Scholar]
- 15.Murray, B. E. 1990. The life and times of the enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson, R. D., R. T. Steigbigel, H. T. Davis, and S. W. Chapman. 1980. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob. Agents Chemother. 18:699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin, X., K. V. Singh, Y. Xu, G. M. Weinstock, and B. E. Murray. 1998. Effect of disruption of a gene encoding an autolysin of Enterococcus faecalis OG1RF. Antimicrob. Agents Chemother. 42:2883-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaberg, D. R., D. H. Culver, and R. P. Gaynes. 1991. Major trends in the microbial etiology of nosocomial infection. Am. J. Med. 91:72S-75S. [DOI] [PubMed] [Google Scholar]
- 19.Shah, P. M. 1982. Paradoxical effect of antibiotics. I. The ‘Eagle effect.’ J. Antimicrob. Chemother. 10:259-260. [DOI] [PubMed] [Google Scholar]
- 20.Tomasz, A., and S. Waks. 1975. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc. Natl. Acad. Sci. USA 72:4162-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincent, J. L. 2003. Nosocomial infections in adult intensive-care units. Lancet 361:2068-2077. [DOI] [PubMed] [Google Scholar]
- 22.Williamson, R., C. le Bouguenec, L. Gutmann, and T. Horaud. 1985. One or two low affinity penicillin-binding proteins may be responsible for the range of susceptibility of Enterococcus faecium to benzylpenicillin. J. Gen. Microbiol. 131:1933-1940. [DOI] [PubMed] [Google Scholar]