Abstract
The aim of this study was to investigate the urinary bactericidal titers (UBTs) and 24-h area under the UBT-versus-time curve (AUBT) of intravenous doripenem (500 mg every 8 h [q8h]), a new carbapenem, versus those of intravenous levofloxacin (250 mg q24h) in patients with complicated urinary tract infections (cUTIs) or pyelonephritis. UBTs and AUBTs are pharmacokinetic/pharmacodynamic parameters able to reflect the activity of an antimicrobial substance in the urine. Doripenem and levofloxacin show comparable urinary excretion of approximately 80% and are therefore registered for the treatment of UTIs. In order to assess and compare the urinary antimicrobial activities of the two substances, UBTs were investigated for 24 patients (10 treated with doripenem and 14 with levofloxacin) for 31 uropathogens and one control strain. Eight strains were tested for all patients and 27 only in the urine of the corresponding patient. Median UBTs (AUBTs) of doripenem for the uropathogens tested ranged between 1.5 and 65,536 (224 and 909,312) and were significantly higher than median UBTs (AUBTs) of levofloxacin, ranging between 0 and 128 (0 and 2,208). Eight microbiological failures were observed, three after doripenem treatment and five after levofloxacin treatment. For levofloxacin, microbiological failures correlated well with low UBTs and AUBTs, whereas for doripenem there was no correlation. From this study, a calculated target attainment rate for levofloxacin predicting therapeutic success in patients with UTIs approximated mean UBTs of 100 over 24 h or AUBTs of 2,240. Doripenem demonstrated excellent urinary bactericidal activity with the dose administered and appears to be a good alternative in the empirical treatment of cUTI.
The gram-negative bacteria Escherichia coli, Klebsiella spp., Enterobacter spp., and Pseudomonas aeruginosa pose particularly significant clinical problems in the treatment of complicated urinary tract infections (cUTIs) due to a clinically important increase in the prevalence of antibiotic resistance (6, 12, 22). Carbapenems, however, still retain their activity against most uropathogens (9). Doripenem is a new 1-β-methyl carbapenem with broad-spectrum activity against gram-negative and gram-positive pathogens (3, 7, 9, 19), including multidrug-resistant members of the Enterobacteriaceae producing extended-spectrum β-lactamases or with AmpC β-lactamases (10) and some P. aeruginosa strains resistant to the other carbapenems (1). In healthy human adults administered a single 500-mg dose of doripenem, approximately 70% of the parent compound and 15% of the ring-opened metabolite, doripenem-M-1, were recovered in the urine within 24 h (http://www.rxlist.com/doribax-drug.htm).
The FDA guidance for developing antimicrobial drugs in the treatment of UTIs defines two broad categories of labeling for anti-infective drugs in the treatment of UTIs (20): (i) uncomplicated UTIs and (ii) cUTIs and pyelonephritis. A cUTI is defined as a clinical syndrome characterized by signs and symptoms of UTI occurring in the presence of a functional or anatomical abnormality of the urinary tract or in the presence of catheterization. Doripenem was compared with levofloxacin in the treatment of cUTIs or pyelonephritis in two phase 3, multinational studies (DORI-05 and DORI-06).
Levofloxacin is one of the most frequently studied fluoroquinolones for UTIs, with a urinary excretion of approximately 80% (21), comparable to that of doripenem.
Pharmacokinetic-pharmacodynamic (PK/PD) probability-of-target-attainment analyses to support phase 3 dosing strategies for doripenem have been described (2). The model, however, was derived from healthy subjects and not from patients for whom the antibiotic is indicated.
MATERIALS AND METHODS
Study design and patient population.
The study was approved by the ethics committee of the Landesärztekammer Bayern, Munich, Germany (no. 03180), and was performed as an investigator-initiated PK/PD substudy within DORI-05 at study center 104 (Straubing, Germany). DORI-05 was a phase 3, multicenter, prospective, randomized, double-blind study of doripenem intravenous (i.v.) infusion versus levofloxacin intravenous infusion in the treatment of cUTI and pyelonephritis.
Criteria for inclusion were ages of ≥18 years, clinical signs and/or symptoms of complicated lower UTI or pyelonephritis, and significant bacteriuria with CFU of ≥105/ml (20).
A physical examination, electrocardiography, acquisition of conventional laboratory data (chemistry panel, blood count, and urinalysis), and urine culture were performed at study entry. Urinalysis and urine culture were performed daily until the final i.v. study drug administration and at each visit thereafter: at the end of therapy, 6 to 9 days after therapy as a test of cure, and 28 to 35 days after therapy as late follow-up. Conventional laboratory data were obtained at day 3 during therapy and with each visit after therapy. Symptoms of UTI and adverse events were recorded daily until the end of therapy and at each visit after therapy. Assessment of clinical and microbiological response was performed at end of therapy, test of cure, and late follow-up.
Drug administration.
The patients were randomly assigned in a 1:1 ratio to receive either doripenem by i.v. infusion, 500 mg every 8 h (q8h) (Doribax, manufactured by Shionogi & Co. Ltd., Osaka, Japan; distributed by Ortho-McNeil Pharmaceuticals, Inc., Raritan, NJ), or levofloxacin by i.v. infusion, 250 mg q24h (Levaquin; Ortho-McNeil Pharmaceuticals, Inc., Raritan, NJ). The study was blinded using either placebo levofloxacin given q24h and active doripenem given q8h or active levofloxacin given q24h and placebo doripenem given q8h. All active drug and placebo doses were administered as 60-min i.v. infusions. After receiving a minimum of 9 doses of i.v. study drug, patients in both study arms were allowed to be switched to oral levofloxacin tablets, 250 mg orally q24h, if the patient was afebrile (<37.8°C) for at least 24 h, had absent or improved signs and/or symptoms of cUTI or pyelonephritis, and had at least two urine cultures with <104 CFU/ml. The total treatment duration of i.v. and oral study drug therapy was 10 days.
The results of the DORI-05 study have been submitted for publication.
The following methods were performed only in this single-center substudy.
Urine collection.
Urine from each of the 24 patients was collected prior to drug administration (antibiotic-free urine) and at day three of i.v. therapy (steady state) at the following time intervals after start of i.v. infusion: 0 to 4, 4 to 8, 8 to 12, 12 to 16, 16 to 20, and 20 to 24 h. All samples were immediately stored at −80°C until investigation. The urine samples were sterile filtered before further processing.
Test strains (Table 1).
TABLE 1.
Strains and MICs of doripenem and levofloxacin used in this studya
| Patient no. or location | Strain | MIC [mg/liter] at baseline (test of cure)
|
Type of UTI | Therapy | Microbiological/ clinical success | Genotypic relationship | |
|---|---|---|---|---|---|---|---|
| Doripenem | Levofloxacin | ||||||
| NAb | P. aeruginosa ATCC 27853 | 0.12-0.5 | 0.25 | NA | NA | NA | NA |
| U.S.b | E. coli 1135121 | ≤0.015 (≤0.015) | 4 (4) | Lower cUTI | Levofloxacin | MFe | Closely related |
| U.S.b | P. mirabilis 1134841 | 0.03 (0.03) | >8 (64) | Lower cUTI | Doripenem | MFe | Identical |
| U.S.b | P. aeruginosa 1134642 | 2 (0.25) | 16 (>8) | Lower cUTI | Doripenem | MFe | Closely related |
| U.S.b | S. aureus 1134684 | 32 (32) | 4 (4) | Lower cUTI | Levofloxacin | MFe | Closely related |
| 07145b,c | E. coli 1949820 B-N | 0.03 (0.03) | 8 (8) | s lower cUTI | Levofloxacin | MF, CF | Indistinguishable |
| 08004b,c | E. faecalis 1780065 B-B | 4 (4) | 1 (1) | as lower cUTI | Levofloxacin | MF | Indistinguishable |
| 07141b,c,d | E. coli 1949821 B-M | ≤0.015 | 0.125 | s lower cUTI | Doripenem | Yes | NA |
| 07141c,d | S. haemolyticus 1949819 B-M | 1 | NA | s lower cUTI | Doripenem | Yes | NA |
| 08014c,d | E. faecalis 2297262-10 B-V | NA | >32 | as lower cUTI | Levofloxacin | Yes | NA |
| 08014c,d | S. epidermidis 2297262-9 B-V | NA | >32 | as lower cUTI | Levofloxacin | Yes | NA |
| 18010c,d | E. faecalis 2215767-10 B-S | NA | >32 | as lower cUTI | Levofloxacin | MF | ND |
| 18010c,d | S. aureus 2215767-9 B-S | NA | >32 | as lower cUTI | Levofloxacin | MF | ND |
| 08015c | E. coli 2297257 B-W | 0.015 | NA | as lower cUTI | Doripenem | Yes | NA |
| 09031c | E. coli 1780064 A-X | NA | 0.047 | Pyelonephritis | Levofloxacin | Yes | NA |
| 07071c | E. coli 1655646 A-Y | NA | 0.047 | s lower cUTI | Levofloxacin | Yes | NA |
| 09034c | E. coli 1780063 B-A | 0.03 | NA | Pyelonephritis | Doripenem | Yes | NA |
| 07090c | E. coli 1821385 B-C | 0.03 | NA | s lower cUTI | Doripenem | Yes | NA |
| 17081c | E. coli 1821386 B-D | NA | <0.03 | s lower cUTI | Levofloxacin | Yes | NA |
| 08007c | E. coli 1889646 B-F | 0.03 (0.03) | >8 (8) | as lower cUTI | Doripenem | MF | Unrelated |
| 07121c | E. coli 1889647 B-H | NA | <0.03 | s lower cUTI | Levofloxacin | Yes | NA |
| 07124c | E. coli 1889645 B-I | 0.03 | NA | s lower cUTI | Doripenem | Yes | NA |
| 07134c | E. coli 1949825 B-K | 0.03 (0.03) | 0.03 (0.03) | s lower cUTI | Doripenem | MF | Closely related |
| 09053c | E. coli 1949826 B-L | 0.03 | NA | Pyelonephritis | Doripenem | Yes | NA |
| 07164c | E. coli 2062717 B-P | ≤0.015 (≤0.015) | 8 (4) | s lower cUTI | Doripenem | MF | Closely related |
| 07180c | E. coli 2062716 B-R | NA | 0.032 | s lower cUTI | Levofloxacin | Yes | NA |
| 08013c | S. marcescens 2297260 B-U | NA | 0.19 | as lower cUTI | Levofloxacin | Yes | NA |
| 08005c | P. aeruginosa 1821383 B-E | NA | 0.25 | as lower cUTI | Levofloxacin | Yes | NA |
| 07168c | P. aeruginosa 2062713 B-Q | 0.5 (0.5) | 0.06 (0.25) | s lower cUTI | Levofloxacin | MF | Indistinguishable |
| 07199c | E. faecalis 2215770 B-T | 0.25 | NA | s lower cUTI | Doripenem | Yes | NA |
| 08003c | S. aureus 1889640 A-Z | NA | 0.06 | as lower cUTI | Levofloxacin | Yes | NA |
| 08008c | S. epidermidis 1949822 B-J | >32 (>32) | 8 (8) | as lower cUTI | Levofloxacin | MF | Unrelated |
NA, not applicable; ND, not done; s, symptomatic; as, asymptomatic; MF, microbiological failure; CF, clinical failure.
Strain was tested for all patients.
Strain was tested in urine sample from individual patient from whom the strain was isolated.
Two strains isolated per patient.
Patients were not part of this study; only strains isolated from these patients were used.
The 32 bacterial strains used in this study represent 31 clinical uropathogens derived from the DORI-05 study and 1 control strain (P. aeruginosa ATCC 27853). Four clinical strains (E. coli 1135121, Proteus mirabilis 1134841, P. aeruginosa 1134642, and Staphylococcus aureus 1134684) were derived from patients of study centers within the United States, all recovered from patients who were counted as microbiological failures at the test-of-cure visit. Twenty-seven clinical strains (15 E. coli strains, 1 Serratia marcescens strain, 2 P. aeruginosa strains, 4 Enterococcus faecalis strains, 1 Staphylococcus haemolyticus strain, 2 Staphylococcus epidermidis strains, and 2 S. aureus strains) were derived from patients of study center 104. For determination of urinary bactericidal titers (UBTs), 7 clinical strains and 1 control strain were tested in the urine of 24 patients (Table 2), and 27 individual patients' strains were tested in the urine of the individual patient only (Table 3).
TABLE 2.
Reciprocal UBTs of doripenem or levofloxacin for seven clinical strains and one control strain tested in urine from 24 patientsa
| Drug or strain | MIC (mg/liter) | UBT for indicated collection period (h)
|
AUBT, 24 h | |||||
|---|---|---|---|---|---|---|---|---|
| 0-4 | 4-8 | 8-12 | 12-16 | 16-20 | 20-24 | |||
| Doripenem | ||||||||
| P. aeruginosa ATCC 27853c | 0.25 | 2,048 (64-65,536)b | 1,024 (32-4,096)b | 3,072 (32-16,384)b | 1,536 (16-4,096)b | 4,096 (16-8,192)b | 512 (4-4,096)b | 69,632 (656-282,624)b |
| E. coli 1135121c | 0.03 | 49,152 (4,096-131,072)b | 24,576 (256-163,084)b | 65,536 (128-131,072)b | 8,192 (64-65,536)b | 32,768 (8,192-131,072)b | 4,096 (256-32,768)b | 909,312 (68,352-1,610,800)b |
| P. mirabilis 1134841c | 0.03 | 2,048 (512-16,384)b | 1,024 (256-8,192)b | 6,144 (256-16,384)b | 1,536 (128-8,192)b | 8,192 (256-16,384)b | 512 (32-16,384)b | 119,040 (5,760-278,528)b |
| P. aeruginosa 1134642c | 2 | 4,096 (64-65,536)b | 1,024 (32-8,192)b | 6,144 (16-65,536)b | 2,048 (16-8,192)b | 4,096 (16-32,768)b | 1,280 (4-4,096)b | 142,848 (592-475,136)b |
| S. aureus 1134684 | 32 | 8 (4-64) | 4 (1-8) | 12 (0-512) | 4 (1-256) | 8 (4-512) | 1.5 (1-16) | 224 (76-5,396) |
| E. coli 1949820 B-Nc | 0.03 | 32,768 (8,192-131,072)b | 12,288 (512-32,768)b | 32,768 (512-131,072)b | 8,192 (256-65,536)b | 32,768 (8,192-131,072)b | 5,120 (512-32,768)b | 708,608 (107,520-1,310,720)b |
| E. faecalis 1780065 B-B | 4 | 128 (16-8,192)b | 32 (8-4,096) | 192 (2-2,048)b | 48 (2-1,024) | 256 (4-4,096)b | 32 (4-8,192)b | 2,880 (192-110,592)b |
| E. coli 1949821 B-Mc | ≤0.015 | 32,768 (4,096-131,072)b | 12,288 (512-32,768)b | 32,768 (512-131,072)b | 8,192 (256-65,536)b | 32,768 (8,192-131,072)b | 5,120 (512-32,768)b | 884,736 (69,632-1,572,864)b |
| Levofloxacin | ||||||||
| P. aeruginosa ATCC 27853c | 0.25 | 16 (4-64)b | 16 (4-64)b | 8 (2-64)b | 8 (2-64)b | 6 (2-64)b | 4 (0-8)b | 256 (56-1,056)b |
| E. coli 1135121c | 4 | 8 (0-16)b | 8 (0-16)b | 3 (0-16)b | 2.5 (0-16)b | 3 (0-16)b | 2 (0-4)b | 128 (0-304)b |
| P. mirabilis 1134841c | 64 | 0 (0-1)b | 0 (0-1)b | 0 (0-0)b | 0 (0-0)b | 0 (0-0)b | 0 (0-0)b | 0 (0-8)b |
| P. aeruginosa 1134642c | 16 | 2 (0-8)b | 2 (1-8)b | 1 (0-4)b | 1 (0-4)b | 1 (0-4)b | 0 (0-1)b | 30 (4-80)b |
| S. aureus 1134684 | 4 | 8 (2-32) | 4 (2-32) | 4 (1-16) | 4 (1-16) | 2 (1-16) | 2 (1-4) | 112 (44-400) |
| E. coli 1949820 B-Nc | 8 | 4 (0-32)b | 2 (0-32)b | 0.5 (0-32)b | 0.5 (0-32)b | 1 (0-32)b | 0 (0-4)b | 48 (0-592)b |
| E. faecalis 1780065 B-B | 1 | 32 (4-128)b | 16 (4-128) | 16 (2-64)b | 16 (2-64) | 8 (2-32)b | 6 (2-32)b | 432 (64-1,664)b |
| E. coli 1949821 B-Mc | 0.125 | 192 (4-2,048)b | 128 (4-2,048)b | 64 (2-2,048)b | 48 (4-2,048)b | 48 (2-1,024)b | 40 (1-256)b | 2,208 (68-29,696)b |
Values are medians (ranges). Ten patients were treated with doripenem and 14 with levofloxacin.
Significantly different (P < 0.05, Mann-Whitney test) results between doripenem and levofloxacin.
Results were significantly different (P < 0.05) using Holm correction for ∑ 0-4, 4-8, 8-12, 12-16, 16-20, and 20-24.
TABLE 3.
Reciprocal UBTs and AUBTs of doripenem or levofloxacin for 27 individual patients' strainsa
| Drug or strain | MIC (mg/liter) | UBT for indicated collection period (h)
|
AUBT, 24 h | Type of UTI | Eradication | Genotype | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0-4 | 4-8 | 8-12 | 12-16 | 16-20 | 20-24 | ||||||
| Doripenem | |||||||||||
| E. coli 1949821 B-Mb | ≤0.015 | 16,384 | 4,096 | 32,768 | 4,096 | 32,768 | 4,096 | 376,832 | s lower cUTI | Yes | NA |
| S. haemolyticus 1949819 B-Mb | 1 | 512 | 128 | 1,024 | 128 | 2,048 | 128 | 15,872 | s lower cUTI | Yes | NA |
| E. coli 2297257 B-W | 0.03 | 4,096 | 2,048 | 16,384 | 4,096 | 32,768 | 4,096 | 253,952 | as lower cUTI | Yes | NA |
| E. coli 1780063 B-A | 0.03 | 4,096 | 1,024 | 4,096 | 1,024 | 4,096 | 1,024 | 61,440 | Pyelonephritis | Yes | NA |
| E. coli 1821385 B-C | 0.03 | 4,096 | 256 | 32,768 | 8,192 | 8,192 | 256 | 215,040 | s lower cUTI | Yes | NA |
| E. coli 1889646 B-F | 0.03 | 65,536 | 32,768 | 32,768 | 8,192 | 65,536 | 65,536 | 1,081,344 | as lower cUTI | MF | Unrelated |
| E. coli 1889645 B-I | 0.03 | 65,536 | 32,768 | 131,072 | 32,768 | 65,536 | 4,096 | 1,327,104 | s lower cUTI | Yes | NA |
| E. coli 1949825 B-K | 0.03 | 16,384 | 4,096 | 8,192 | 2,048 | 4,096 | 256 | 140,288 | s lower cUTI | MF | Closely related |
| E. coli 1949826 B-L | 0.03 | 65,536 | 32,768 | 131,072 | 32,768 | 65,536 | 16,384 | 1,376,256 | Pyelonephritis | Yes | NA |
| E. coli 2062717 B-P | ≤0.015 | 32,768 | 1,024 | 1,024 | 512 | 32,768 | 2,048 | 280,576 | s lower cUTI | MF | Closely related |
| E. faecalis 2215770 B-T | 0.25 | 2,048 | 512 | 1,024 | 512 | 2,048 | 512 | 25,600 | s lower cUTI | Yes | NA |
| Levofloxacin | |||||||||||
| E. faecalis 2297262-10 B-Vb | >32 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | as lower cUTI | Yes | NA |
| S. epidermidis 2297262-9 B-Vb | >32 | 1 | 1 | 4 | 2 | 2 | 2 | 48 | as lower cUTI | Yes | NA |
| E. faecalis 1780065 B-B | 1 | 16 | 8 | 4 | 4 | 4 | 2 | 152 | as lower cUTI | MF | Indistinguishable |
| E. faecalis 2215767-10 B-Sb | >32 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | as lower cUTI | MF | ND |
| S. aureus 2215767-9 B-Sb | >32 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | as lower cUTI | MF | ND |
| E. coli 1780064 A-X | 0.047 | 1,024 | 512 | 256 | 128 | 256 | 128 | 9,216 | Pyelonephritis | Yes | NA |
| E. coli 1655646 A-Y | 0.047 | 2,048 | 2,048 | 2,048 | 1,024 | 1,024 | 256 | 33,792 | s lower cUTI | Yes | NA |
| E. coli 1821386 B-D | <0.03 | 2,048 | 8,192 | 2,048 | 4,096 | 2,048 | 2,048 | 81,920 | s lower cUTI | Yes | NA |
| E. coli 1949820 B-N | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | s lower cUTI | MF, CF | Indistinguishable |
| E. coli 1889647 B-H | <0.03 | 2,048 | 2,048 | 512 | 1,024 | 512 | 256 | 25,600 | s lower cUTI | Yes | NA |
| E. coli 2062716 B-R | 0.032 | 1,024 | 512 | 512 | 256 | 128 | 64 | 9,984 | s lower cUTI | Yes | NA |
| S. marcescens 2297260 B-U | 0.19 | 512 | 256 | 128 | 128 | 32 | 32 | 4,352 | as lower cUTI | Yes | NA |
| P. aeruginosa 1821383 B-E | 0.25 | 32 | 128 | 128 | 128 | 128 | 16 | 2,240 | as lower cUTI | Yes | NA |
| P. aeruginosa 2062713 B-Q | 0.38 | 8 | 4 | 4 | 4 | 4 | 2 | 104 | s lower cUTI | MF | Indistinguishable |
| S. aureus 1889640 A-Z | 0.06 | 256 | 128 | 128 | 128 | 64 | 64 | 3,072 | as lower cUTI | Yes | NA |
| S. epidermidis 1949822 B-J | 8 | 4 | 4 | 2 | 4 | 2 | 1 | 64 | as lower cUTI | MF | Unrelated |
Eleven patients were treated with doripenem and 16 with levofloxacin. MF, microbiological failure; CF, clinical failure; s, symptomatic; as, asymptomatic.
Two strains isolated per patient.
Genotyping procedure.
Strains recovered after treatment failure in the same patient were subjected to genotyping by pulsed-field gel electrophoresis and compared to the genotypes of the initially cultured pathogens (Table 1). Agarose-embedded genomic DNA was prepared using a Chef bacterial genomic DNA plug kit (Bio-Rad, Hercules, CA) as described by the manufacturer. DNA from gram-positive bacteria was digested with SmaI (New England Biolabs, Beverly, MA) and that from gram-negative bacteria with XbaI (New England Biolabs). DNA fragments were separated using a Chef DR III apparatus (Bio-Rad). Fragment patterns were interpreted as suggested by Tenover et al. (18).
Determination of MICs.
MICs of doripenem and levofloxacin were determined according to the CLSI guidelines (4) or using Etest according to the manufacturer's instructions (Etest Doripenem; AB Biodisk, Solna, Sweden).
Determination of UBTs.
A twofold serial dilution (dilution range, 1:0, 1:1 up to 1:131,072) of the urine samples was prepared. The individual patient's antimicrobial agent-free urine collected prior to drug administration was used as a diluent (5, 13, 14, 15). A microdilution test was used to determine UBTs. Each well of the microplates contained 100 μl of the prepared urinary dilution. Bacterial strains were then added to the wells of the microplates using a multipoint inoculator (10 μl). The final inoculum, which was confirmed by actual count, ranged from 1.0 × 106 to 9.5 × 106 CFU/ml. The plates were then incubated at 37°C for 18 h in ambient air. UBTs were determined in a second step after 10 μl of the subcultured urine was transferred to Columbia agar (Merck, Darmstadt, Germany) supplemented with 5% blood. The plates were again incubated for 24 h at 37°C. The number of colonies subsequently grown was used to determine the bactericidal endpoint. Urinary bactericidal activity was defined as a >99.9% (>3-log) reduction of the initially inoculated colony counts. A UBT of 0 was defined as no bactericidal activity, and a UBT of 1 was used when only undiluted urine displayed bactericidal activity. UBTs were transformed into ordinal data and described by using reciprocal numbers.
Determination of AUBT.
The area under the UBT-versus-time curve (AUBT) was calculated as the sum of the products of the reciprocal UBT values and the respective time (h) intervals for each test organism and for each drug. The calculation of AUBT values is an approximation considering the time intervals of 4 h and the nonlinear kinetics in urine.
Statistical analyses.
UBT and AUBT data for the two drugs were compared for each individual by using the Mann-Whitney test. Due to multiple testing in the UBT analyses, a Holm correction was applied. An α value equal to 0.05 was chosen to determine statistical significance. Statistical calculations were performed by using the SPSS 11.0 software program (1989 to 2001; SPSS Inc., Chicago, IL).
RESULTS
The subject population included in this PK/PD study was comprised of 24 consecutive patients (10 female and 14 male patients) from study center 104, with a median age of 74 years (standard deviation, 13.5; range, 20 to 86 years) and a median body mass index of 26.7 kg/m2 (standard deviation, 4.4; range, 21 to 38). Three patients were treated for pyelonephritis and 21 for cUTI. Eight of those patients were assessed as microbiological failures (all with cUTI), three in the doripenem treatment arm and five in the levofloxacin treatment arm. One patient with cUTI in the levofloxacin treatment arm was assessed as both a microbiological and clinical failure. There were no serious adverse events noted among the 24 patients.
The MICs of strains are shown in Table 1. Twenty-seven clinical strains were initially cultured from 24 patients participating in the DORI-05 PK/PD substudy. Three patients had mixed UTIs with two strains each. For the seven selected clinical strains tested in the urine of all 24 patients, both doripenem and levofloxacin showed lower MICs for five strains and higher MICs for two strains (methicillin-resistant S. aureus and E. faecalis) for doripenem than for levofloxacin. For the control strain, the MICs of the two antimicrobials were about the same. For the remaining strains, only the MIC of the antimicrobial with which the patient was treated during the PK/PD study phase was determined. The MICs of the strains causing microbiological or clinical failures at baseline and test of cure are also presented in Table 1. The MICs at baseline of levofloxacin and doripenem for strains causing failures were 0.38, 1, 4, 4, 8, 8, >32, and >32 mg/liter for levofloxacin and ≤0.015, 0.03, 0.03, 0.03, and 2 g/ml for doripenem, respectively. Increases in MICs of levofloxacin from baseline (0.06 g/ml) to test-of-cure (0.25 g/ml) isolates were seen for one P. aeruginosa strain (P. aeruginosa 2062713 B-Q) after levofloxacin treatment. The genotyping results obtained with pulsed-field gel electrophoresis analysis of the strains causing microbiological or clinical failure are shown in Table 1.
The median values (ranges) of UBTs and AUBTs tested for all patients against the seven selected clinical strains and the control strain are shown in Table 2. Median UBTs of doripenem ranged between 1.5 and 65,536; individual ranges were between 1 and 131,072. Median 24-h AUBTs of doripenem ranged between 224 and 909,312, and the interindividual ranges were between 76 and 1,610,800.
Median UBTs of levofloxacin ranged between 0 and 128; the individual ranges were between 0 and 2,048. Median 24-h AUBTs of levofloxacin ranged between 0 and 2,208, and the interindividual ranges were between 0 and 29,696.
UBTs and AUBTs of doripenem were significantly higher than those of levofloxacin against uropathogens investigated, with the exception of S. aureus 1134684.
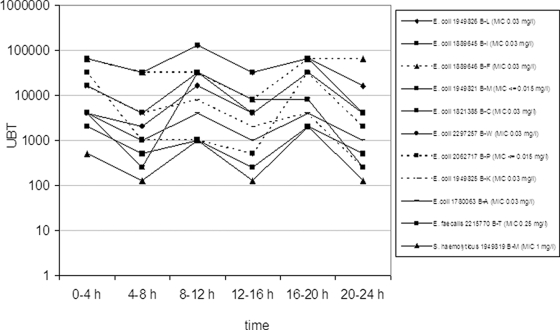
UBTs and AUBTs for each individual patient's strain are shown in Table 3 and UBTs in Fig. 1 and 2. The UBTs of doripenem investigated for 10 patients (1 patient with mixed infections) ranged between 128 and 131,072. The 24-h AUBTs of doripenem ranged between 15,872 and 1,327,104.
FIG. 1.
Reciprocal UBTs of doripenem (500 mg q8h) for 11 individual patients' strains (dotted lines show microbiological failures).
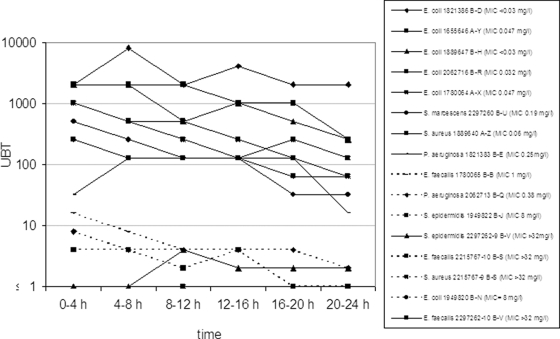
FIG. 2.
Reciprocal UBTs of levofloxacin (250 mg q24h) for 16 individual patients' strains (dotted lines show microbiological failures).
The UBTs of levofloxacin investigated for 14 patients (two patients with mixed infections) ranged between 0 and 4,096. The 24-h AUBTs of levofloxacin ranged between 0 and 81,920.
AUBTs of doripenem against seven E. coli strains exhibiting similar MICs (0.03 mg/liter) varied between 61,440 and 1,376,256 (22-fold) for different patients, and AUBTs of levofloxacin against two E. coli strains exhibiting similar MICs (0.047 mg/liter) varied between 9,216 and 33,792 (fourfold) for different patients.
DISCUSSION
In many infections, antimicrobial susceptibility levels are often gauged relative to what minimal inhibitory antibiotic concentration is achievable in the blood. In UTI, however, a significant portion of bacteria reside in the urine filling the hollow urinary tract. Therefore, the urinary antimicrobial activity may be consulted to evaluate the activity of an antibiotic substance in the treatment of UTI. The investigation of UBTs and AUBTs can be considered a PK/PD assessment of an antibiotic substance in the urine. In urine, bactericidal concentrations are preferred over inhibitory concentrations, because urine is a rather turbid medium where the inhibition of bacterial growth is difficult to visualize. PK/PD studies are often not carried out for those individuals in whom the antibiotic substance is determined to be used. In this substudy, patients with cUTIs or pyelonephritis were included for such PK/PD investigation.
Both antibiotics investigated show a favorable urinary excretion rate of approximately 80%, and they are comparable in that respect (21). One of the differences between the two antibiotics is the different PK/PD parameters correlating best with their antibacterial effects. For doripenem, a target time during which the antibiotic concentration is above the MIC of the target pathogen (T>MIC) of 35% or higher has been determined in target attainment analyses (2). For levofloxacin, an area under the concentration-time curve/MIC target of 125 or higher has been calculated, at least for gram-negative bacteria (17). These PK/PD parameters, however, have not been evaluated for the treatment of cUTIs or pyelonephritis. In the present study, UBTs and AUBTs for the eight bacterial strains (seven uropathogens from this study and one control strain) tested for all 24 patients were significantly higher for patients treated with doripenem than for those treated with levofloxacin, except for one methicillin-resistant S. aureus strain. These differences were generally marked (1,000- to 10,000-fold). However, this must be considered in the context of dosing and MICs of the two antimicrobials. Assuming a similar urinary excretion rate, a factor of six could be explained by the difference in daily dosages. In addition, the generally lower MICs of doripenem would also explain higher reciprocal UBTs. Taking all these factors into account, the urinary bactericidal activity of doripenem is more potent than that of levofloxacin. This is best illustrated when comparing the UBTs for the control P. aeruginosa strain, which exhibits comparable MICs for the two antimicrobials. The median reciprocal UBTs for doripenem were 272-fold higher than for levofloxacin (Table 2). Considering a factor of six, explained by different daily dosing, the bactericidal activity of doripenem still remained 45-fold higher than that of levofloxacin.
Interindividual variation of AUBT values for patients with strains exhibiting similar MIC levels was extensive (Table 3). There was an approximately 22-fold variation of AUBT values investigated for seven different patients with cUTIs caused by E. coli (MIC, 0.03 mg/liter) and treated with doripenem and an approximately fourfold variation for two different patients with cUTIs caused by E. coli (MIC, 0.047 mg/liter) and treated with levofloxacin.
There were three patients with microbiological failures (E. coli 1889646 B-F, E. coli 1949825 B-K, and E. coli 2062717 B-P) but no clinical failure in patients treated with doripenem (Table 3). The three microbiological failures in the doripenem arm did not correlate with the MICs of the strains and the UBT and AUBT values. One strain (E. coli 1889646 B-F) was genotypically different from the strain at the follow-up visit. The female patient harboring these two strains carried out intermittent self-catheterization because of residual urine on the basis of atonic bladder function. The second strain (E. coli 1949825 B-K) was genotypically closely related to the strain at the follow-up visit. The male patient infected with this strain was included in the study on the basis of a lower cUTI and abscess-forming epididymitis leading to a consecutive ablatio testis, without correction of the complicating factor (enlargement of the prostate) responsible for the development of the symptomatic cUTI and epididymitis. The third strain (E. coli 2062717 B-P) was genotypically closely related to the strain at the follow-up visit. The female patient infected with this strain had ureteral stenosis with an indwelling ureteral stent. The ureteral catheter was removed during the course of antibiotic treatment, but the ureteral stenosis was not surgically corrected during this time period. Considering the results of genotyping, true microbiological failures were observed in two patients and the possible cause of this failure was the persistent underlying complicating factors within the urinary tract. Thus, the microbiological failures in patients treated with doripenem in spite of high UBT values were most probably related to the insufficient treatment of the urological complicating factor. Additionally, this shows that achieving higher drug concentrations with β-lactam antibiotics does not result in increased bacterial killing.
There were five patients with microbiological failure in the levofloxacin treatment arm, including one patient who was also a clinical failure (Table 3). Of the eight strains (six patients) with AUBTs of 152 or less, six strains (five patients) were associated with microbiological failure; no patient with a strain with an AUBT of 2,240 or greater was reported as a microbiological failure (Fig. 1). Only one patient with a mixed cUTI with E. faecalis and S. epidermidis exhibiting low AUBTs (0 and 48, respectively) and high MICs (>32 mg/liter) showed a disappearance of the original strains at test of cure and late follow-up. Spontaneous clearance in this patient might be a possible explanation.
In general, failures with doripenem were exclusively due to gram-negative organisms, whereas failures with levofloxacin comprised gram-negative and -positive bacteria.
The emergence of resistance during therapy is difficult to assess with a rather low number of patients investigated. For one patient infected with a P. aeruginosa strain and treated with levofloxacin, however, an increase in the MIC of levofloxacin from baseline (0.06 mg/liter) to test of cure (0.25 mg/liter) was noted, and the isolate was genotypically indistinguishable. In all other cases, no significant increase of MICs was demonstrable (Table 1).
There is still no validated target attainment rate predicting therapeutic success for patients with cUTI or pyelonephritis. From the present study, a target attainment rate of an AUBT of 2,240 can be calculated for levofloxacin, which approximates mean UBTs of 100 over 24 h (Fig. 1 and 2). Because there were no data available between AUBTs of 152 and 2,240, a narrower breakpoint between success and failure cannot be determined. These results are also in agreement with an in vitro catheter-associated urinary infection model, in which a minimum of 32-fold the MIC of levofloxacin was needed to suppress the growth of a P. aeruginosa strain to a nondetectable limit, corresponding to an AUBT of 768 (32 × 24) (8). Therefore, in order to achieve higher UBTs for levofloxacin, higher doses, e.g., 750 mg levofloxacin per day, should be applied. Such a dose was recently studied and has already been approved for use in the United States and Canada (16). For doripenem, no such target attainment rate can be concluded from this study, due to several reasons. (i) Interpretation of AUBTs might be suitable only for concentration-dependent and not for time-dependent antimicrobials. (ii) The excessively high UBTs of doripenem most likely did not result in higher killing than lower UBTs, because with β-lactams the bactericidal effect is related to the T>MIC and not the ratio of antibiotic concentration over the MIC. This has been shown in an in vivo pharmacodynamic profiling study of doripenem against P. aeruginosa in which the exposure of ≥40% T>MIC was critical for a bactericidal effect whereas exposure of ≈20% T>MIC resulted in a bacteriostatic effect (11).
Conclusion.
In this PK/PD study with patients with cUTI or pyelonephritis to whom doripenem was administered at 500 mg i.v. q8h or levofloxacin was administered at 250 mg i.v. q24h, the investigated UBTs and AUBTs were significantly higher for patients treated with doripenem than for those treated with levofloxacin. The urinary bactericidal activity of doripenem remained overproportionally higher than that of levofloxacin, even after the different daily dosages and MICs of the two antimicrobials were considered. In the levofloxacin group, microbiological failures were observed only in patients with AUBT levels of 152 or lower. In the doripenem group, no correlation of treatment failure and AUBT level was found, and the persistence of underlying complicating factors that were not removed during therapy was considered the most likely cause. Doripenem exhibited excellent urinary bactericidal activity with the dose administered and appears to be a good alternative in the empirical treatment of cUTI and pyelonephritis.
Acknowledgments
This study was supported by an unrestricted grant from Johnson & Johnson.
We thank the following employees of Johnson & Johnson Pharmaceutical PRD (Raritan, NJ) for their assistance with the manuscript: Todd Davies for providing genotyping data, Koné Kaniga and Robert Flamm for providing microbiological outcome and MIC data, and Shin-Yir Tong and Iolanda Cirillo for providing clinical data.
Florian M. E. Wagenlehner, Christine Wagenlehner, and Kurt G. Naber were taking part in doripenem licensing clinical studies.
Footnotes
Published ahead of print on 2 February 2009.
REFERENCES
- 1.Anderson, D. L. 2006. Doripenem. Drugs Today (Barcelona) 42:399-404. [DOI] [PubMed] [Google Scholar]
- 2.Bhavnani, S. M., J. P. Hammel, B. B. Cirincione, M. A. Wikler, and P. G. Ambrose. 2005. Use of pharmacokinetic-pharmacodynamic target attainment analyses to support phase 2 and 3 dosing strategies for doripenem. Antimicrob. Agents Chemother. 49:3944-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, S. D., and M. M. Traczewski. 2005. Comparative in vitro antimicrobial activity of a new carbapenem, doripenem: tentative disc diffusion criteria and quality control. J. Antimicrob. Chemother. 55:944-949. [DOI] [PubMed] [Google Scholar]
- 4.CLSI. 2007. Performance standards for antimicrobial susceptibility testing (M100-S17). CLSI/NCCLS, Wayne, PA.
- 5.Edberg, S. 1986. The measurement of antibiotics in human body fluids: techniques and significance, p. 466-467. In V. Lorian (ed.), Antibiotics in laboratory medicine. The Williams & Wilkins Co., Baltimore, MD.
- 6.Ena, J., F. Arjona, C. Martinez-Peinado, M. del Mar Lopez-Perezagua, and C. Amador. 2006. Epidemiology of urinary tract infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Urology 68:1169-1174. [DOI] [PubMed] [Google Scholar]
- 7.Fritsche, T. R., M. G. Stilwell, and R. N. Jones. 2005. Antimicrobial activity of doripenem (S-4661): a global surveillance report (2003). Clin. Microbiol. Infect. 11:974-984. [DOI] [PubMed] [Google Scholar]
- 8.Goto, T., Y. Nakame, M. Nishida, and Y. Ohi. 1999. Bacterial biofilms and catheters in experimental urinary tract infection. Int. J. Antimicrob. Agents 11:227-231, 237-239. [DOI] [PubMed] [Google Scholar]
- 9.Jones, R. N., H. K. Huynh, and D. J. Biedenbach. 2004. Activities of doripenem (S-4661) against drug-resistant clinical pathogens. Antimicrob. Agents Chemother. 48:3136-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, R. N., H. S. Sader, and T. R. Fritsche. 2005. Comparative activity of doripenem and three other carbapenems tested against Gram-negative bacilli with various beta-lactamase resistance mechanisms. Diagn. Microbiol. Infect. Dis. 52:71-74. [DOI] [PubMed] [Google Scholar]
- 11.Kim, A., M. A. Banevicius, and D. P. Nicolau. 2008. In vivo pharmacodynamic profiling of doripenem against Pseudomonas aeruginosa by simulating human exposures. Antimicrob. Agents Chemother. 52:2497-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livermore, D. M., and N. Woodford. 2006. The beta-lactamase threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol. 14:413-420. [DOI] [PubMed] [Google Scholar]
- 13.Naber, C. K., M. Hammer, M. Kinzig-Schippers, C. Sauber, F. Sorgel, E. A. Bygate, A. J. Fairless, K. Machka, and K. G. Naber. 2001. Urinary excretion and bactericidal activities of gemifloxacin and ofloxacin after a single oral dose in healthy volunteers. Antimicrob. Agents Chemother. 45:3524-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naber, K. G., U. Theuretzbacher, M. Kinzig, O. Savov, and F. Sorgel. 1998. Urinary excretion and bactericidal activities of a single oral dose of 400 milligrams of fleroxacin versus a single oral dose of 800 milligrams of pefloxacin in healthy volunteers. Antimicrob. Agents Chemother. 42:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NCCLS. 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline M26-A. NCCLS, Wayne, PA.
- 16.Peterson, J., S. Kaul, M. Khashab, A. C. Fisher, and J. B. Kahn. 2008. A double-blind, randomized comparison of levofloxacin 750 mg once-daily for five days with ciprofloxacin 400/500 mg twice-daily for 10 days for the treatment of complicated urinary tract infections and acute pyelonephritis. Urology 71:17-22. [DOI] [PubMed] [Google Scholar]
- 17.Schentag, J. J., K. K. Gilliland, and J. A. Paladino. 2001. What have we learned from pharmacokinetic and pharmacodynamic theories? Clin. Infect. Dis. 32(Suppl. 1):S39-S46. [DOI] [PubMed] [Google Scholar]
- 18.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuji, M., Y. Ishii, A. Ohno, S. Miyazaki, and K. Yamaguchi. 1998. In vitro and in vivo antibacterial activities of S-4661, a new carbapenem. Antimicrob. Agents Chemother. 42:94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. FDA. 1998. Complicated urinary tract infections and pyelonephritis—developing antimicrobial drugs for treatment. FDA, Rockville, MD. http://www.fda.gov/cder/guidance/2567dft.pdf.
- 21.Wagenlehner, F. M., M. Kinzig-Schippers, U. Tischmeyer, C. Wagenlehner, F. Sorgel, A. Dalhoff, and K. G. Naber. 2006. Pharmacokinetics of ciprofloxacin XR (1000 mg) versus levofloxacin (500 mg) in plasma and urine of male and female healthy volunteers receiving a single oral dose. Int. J. Antimicrob. Agents 27:7-14. [DOI] [PubMed] [Google Scholar]
- 22.Wagenlehner, F. M. E., A. H. Niemetz, W. Weidner, and K. G. Naber. 2008. Spectrum and antibiotic resistance of uropathogens from hospitalised patients with urinary tract infections: 1994-2005. Int. J. Antimicrob. Agents 31S:S25-S34. [DOI] [PubMed] [Google Scholar]