Abstract
Miltefosine (hexadecylphosphocholine, MLF) is the first oral drug with recognized efficacy against both visceral and cutaneous leishmaniasis. However, some clinical studies have suggested that MLF shows significantly less efficiency against the cutaneous leishmaniasis caused by Leishmania braziliensis. In this work, we have determined the cellular and molecular basis for the natural MLF resistance observed in L. braziliensis. Four independent L. braziliensis clinical isolates showed a marked decrease in MLF sensitivity that was due to their inability to internalize the drug. MLF internalization in the highly sensitive L. donovani species requires at least two proteins in the plasma membrane, LdMT, a P-type ATPase involved in phospholipid translocation, and its β subunit, LdRos3. Strikingly, L. braziliensis parasites showed highly reduced levels of this MLF translocation machinery at the plasma membrane, mainly because of the low expression levels of the β subunit, LbRos3. Overexpression of LbRos3 induces increased MLF sensitivity not only in L. braziliensis promastigotes but also in intracellular amastigotes. These results further highlight the importance of the MLF translocation machinery in determining MLF potency and point toward the development of protocols to routinely monitor MLF susceptibility in geographic areas where L. braziliensis might be prevalent.
Pentavalent antimonials have been the first-line treatment for both visceral leishmaniasis (VL) and cutaneous leishmaniasis (CL); however, the drugs' toxicity and the emergence of resistance in VL strains in India limit their use. Lipid amphotericin B formulations are used as the second line of treatment of VL (4). Miltefosine (hexadecylphosphocholine, MLF), registered as Impavido, has become the first oral drug with recognized efficacy for the treatment of VL and CL (16, 20, 21), although sensitivity to MLF and other alkyl-lysophospholipids is known to vary between Leishmania species (3, 5, 22). Among the different clinically relevant species studied so far, Leishmania donovani and Leishmania braziliensis seem to be the most sensitive and one of the less sensitive, respectively, at least in in vitro studies. This intrinsic MLF resistance observed in L. braziliensis has also been demonstrated in a number of clinical studies (8, 16-18). While MLF induced a rapid clinical and parasitological cure in 94% of the VL cases caused by L. donovani (2), its efficacy against CL caused by L. braziliensis was only 33% in Guatemala (16) and 58 to 88% in Bolivia (18, 19).
The mechanisms of action of MLF are not properly understood, but a clear correlation between the accumulation of the drug within the parasite and its toxic effects has already been described (13). Consequently, the variation in the abilities of different Leishmania species to internalize the drug seems to correlate with MLF susceptibility, as observed in different eukaryotic cells (9, 15, 23). MLF is primarily taken up by specific protein translocation machinery present at the plasma membrane (PM) in Leishmania parasites (10). This translocation machinery is composed of at least two proteins, LdMT, a member of the P4 subfamily of P-type ATPases involved in phospholipid translocation, and its β subunit, LdRos3, a member of the Lem3/CDC50 family (11, 12).
In this study, we have determined the molecular basis for the decreased MLF sensitivity of L. braziliensis. Different L. braziliensis strains showed an extreme reduction of the ability to internalize the drug from the extracellular medium, mainly due to the low expression levels of the MLF translocation machinery at the parasite PM. Overexpression of the LbRos3 β subunit in the L. braziliensis promastigote and intracellular amastigote stages restored MLF uptake and sensitivity to levels closer to those of L. donovani. We suggest a careful assessment of the prevalent species and their sensitivity to MLF before its widespread use in areas where leishmaniasis is endemic to prolong the useful therapeutic life span of this oral drug.
MATERIALS AND METHODS
Chemical compounds.
MLF was from Æterna Zentaris (Frankfurt, Germany). Hexadecylphospho[1,2-ethylene-14C]choline ([14C]MLF; 1.33 MBq/mmol) was synthesized by Amersham Pharmacia Biotech (Buckinghamshire, United Kingdom). All other chemicals were of the highest quality available.
Strains and culture conditions.
Promastigote forms of L. braziliensis MHOM/BR/75/M-2904 (Brazilian isolate; WHO reference strain); L. braziliensis Peruvian isolates MHOM/PE/03/LH-2419, MHOM/PE/02/LH-2210, and MHOM/PE/03/LH-2224; and derivative lines were maintained at 22°C in RPMI medium (Invitrogen, Carlsbad, CA) supplemented with 20% heat-inactivated fetal bovine serum (IFBS; Invitrogen). Promastigotes of parental L. donovani MHOM/ET/67/HU3 (WHO reference strain) and derivative lines LdMT knockout (LdMT−/−), LdRos3 knockout (LdRos3−/−), and LdMT−/− overexpressing LdMT-green fluorescent protein (GFP) (12) were maintained at 28°C in M-199 medium (Invitrogen) supplemented with 40 mM HEPES (Sigma-Aldrich, St. Louis, MO), 100 μM adenosine (Sigma-Aldrich), 0.2% hemin (Sigma-Aldrich), and 10% IFBS.
Isolation of LbMT and LbRos3 and DNA constructs.
The orthologue of LdMT (GenBank accession no. AY321297) from L. braziliensis, LbMT (GenBank accession no. XM_001563228), was isolated from genomic DNA by PCR using primers MTB1 (5′-ATCCCGGGATGTCCGGCCAAG) and MTB2 (5′-GGATCCTCAGATATCCCGCATGCCGC). The orthologue of LdRos3 (GenBank accession no. DQ205096) from L. braziliensis, LbRos3 (GenBank accession no. XM_001567366), was isolated from genomic DNA by PCR using primers ROB1 (5′-CCCGGGATGGTGGATCTA) and ROB2 (5′-GGATCCCTAGATATCCTTTGTATATC). Restriction sites were added (underlined in the sequences) for further cloning. Nucleotide sequences were determined automatically as described previously (7).
To generate GFP fusions at the carboxyl terminus of LbMT (LbMT-GFP), the open reading frame was amplified without the stop codon and subcloned into the pXG-′GFP+ expression vector (6) as previously described for LdMT-GFP (12).
LdRos3-GFP (12) and LbRos3 were subcloned into the integrating stable expression vector pIR1SAT to generate the LdRos3-GFP-pIR1SAT and LbRos3-pIR1SAT constructs (provided by S. Beverley, Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO; unpublished data).
Cell transfection.
Parasites were transfected as previously described (11). Briefly, 3 ×107 promastigotes were transfected by electroporation (450 V, 500 μF) and then maintained in culture medium with the corresponding selected antibiotic. Parasites transfected with the LbMT-GFP or LdMT-GFP construct were selected with G418 at 200 μg/ml. Parasites transfected with the LdRos3-GFP-pIR1SAT or LbRos3-pIR1SAT construct were selected with nourseothricin at 50 μg/ml.
MLF internalization, efflux, and drug susceptibility assay.
The internalization and efflux of [14C]MLF were measured as described previously (10). Briefly, for determination of MLF internalization, 2 × 107 promastigotes in culture medium were incubated with 0.09 μCi/ml [14C]MLF (2.5 μM) for 60 min at 28°C. After washing with phosphate-buffered saline (PBS; 1.2 mM KH2PO4, 8.1 mM Na2HPO4, 130 mM NaCl, 2.6 mM KCl, pH 7) containing 10 mg/ml bovine serum albumin (BSA) at 2°C to allow for the removal of the drug fraction bound to the outer leaflet of the PM, followed by a second PBS wash, both protein concentration and counts per minute were determined. Additionally, the initial ratio of [14C]MLF internalization was determined as described previously for other phospholipid analogues (1). Briefly, 1 ×108 promastigotes in culture medium were incubated with 0.09 μCi/ml [14C]MLF (2.5 μM) for 5 min at 2°C. After washing two times with PBS at 2°C, an aliquot was taken and both protein concentration and counts per minute were determined, representing the total MLF incorporated. The remaining washed parasites were incubated at 28°C, aliquots were taken at different times and washed with PBS plus BSA as described above, and the internalized amount of [14C]MLF was measured. MLF efflux measurements were carried out by loading 2 × 107 promastigotes per ml at 28°C with 0.25 and 2.5 μM [14C]MLF for L. donovani and L. braziliensis, respectively, for 1 h in culture medium. After washing with PBS plus BSA, efflux was initiated at 28°C and the retained radioactivity was measured at different time points (0, 10, 20, 40, and 60 min) as described above.
To determine parasite sensitivity to MLF, 2 × 106 promastigotes per ml were incubated for 72 h at different drug concentrations before determining cell proliferation by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) colorimetric assay as previously described (11). The 50% effective concentration (EC50) was defined as the drug concentration required for half-maximal inhibition of the cellular growth rate. The EC50 for each line was calculated by nonlinear regression analysis using SigmaPlot 2000 for Windows (SPSS Inc.).
Generation of LdMT and LdRos3 antibodies.
DNA fragments of LdMT (corresponding to amino acid residues 371 to 868, the hydrophilic loops between transmembrane domains 4 and 5) and LdRos3 (amino acid residues 56 to 333, the putative extracytosolic loop) were isolated from genomic DNA of L. donovani by PCR using primer pair MT1 (5′-CATATGGAAGTGTGCAAAGTG) and MT2 (5′-AAGCTTCTTGTGGAAGCTAACC) and primer pair ROB3 (5′-CATATGACGACACGGCTTGATTTTCGC) and ROB4 (5′-AAGCTTATGGCTTCTACCCCCGATCCA), respectively. Both fragments were cloned into the pET-21b expression vector (Novagen, Merck KGaA, Darmstadt, Germany) to get six-His-tagged recombinant polypeptides that were expressed in Escherichia coli BL21(DE3). Polypeptides were purified by Ni2+-nitrilotriacetic acid affinity chromatography (Qiagen, Merck KGaA, Darmstadt, Germany). Polyclonal anti-LdMT and anti-LdRos3 antibodies were obtained after several subcutaneous injections of New Zealand White rabbits with 100 μg of purified recombinant polypeptides. In order to determine the level of LbRos3 recognition by polyclonal anti-LdRos3, the recombinant LbRos3 polypeptide was generated as described above. DNA fragments of LbRos3 (amino acid residues 51 to 332) were isolated from genomic DNA of L. braziliensis by PCR using primers ROB5 (5′-CATATGGTGAGCGGGGATAG) and ROB6 (5′-AAGCTTGCCGAGATGGTGGTTC). The resulting fragment was cloned into the pET-21b expression vector to get six-His-tagged recombinant polypeptides.
Preparation of crude membranes.
Leishmania promastigotes (3 × 108 per ml) in the late log phase of growth were harvested by centrifugation and washed three times in cold PBS. Parasites were suspended in hypotonic buffer (10 mM Tris-HCl, pH 7.4) plus a protease inhibitor cocktail (Sigma-Aldrich) and disrupted by N2 cavitation at 104 kPa for 30 min at 4°C. Unbroken cells and large cell debris were removed by centrifugation at 1,000 × g for 10 min at 4°C. The supernatant was centrifuged at 100,000 × g for 1 h at 4°C. The resulting pellet (total membranes) was solubilized in lysis buffer (10 mM Na2HPO4, 5 M urea, 1% 2-mercaptoethanol, 1% sodium dodecyl sulfate [SDS]). Insoluble material was removed by centrifugation at 21,000 × g for 10 min at 4°C. The clarified lysate was mixed with 2× Laemmli buffer and resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE).
Cell surface labeling.
Promastigotes (1 × 108) were washed with PBS and incubated on ice with 1 mM EZ-Link Sulfo-NHS-SS-biotin (Pierce, Thermo Fisher, Rockford, IL) in 1 ml PBS for 2 h at 4°C. The biotinylation reaction was quenched by washing the cells three times with 50 mM Tris-HCl, pH 7.4. Cells were incubated on ice for 30 min in 100 μl lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Nonidet P-40) plus a protease inhibitor cocktail (Sigma-Aldrich). The cell lysate was clarified by centrifugation at 21,000 × g for 10 min at 4°C, and the supernatant was incubated for 1 h at room temperature with 100 μl of packed streptavidin-agarose (Pierce) prewashed in lysis buffer. Agarose beads were washed two times with 1 ml of PBS, and the biotinylated proteins were eluted in 30 μl of 2× Laemmli buffer.
Immunoblotting.
Protein samples were fractionated by SDS-PAGE under standard conditions and electrotransferred onto Immobilon P membranes (Millipore, Bedford, MA). Immunodetection was performed with 1:200 polyclonal anti-LdMT and 1:3,000 polyclonal anti-LdRos3 in buffer A (PBS containing 0.01% Tween 20 and 0.1% BSA). After washing, membranes were incubated with horseradish peroxidase-conjugated secondary goat anti-rabbit (1:5,000) immunoglobulin G (Dako, Barcelona, Spain) in buffer A. Immunodetection of both LdMT and LbMT fused with GFP was performed with 1:5,000 polyclonal anti-GFP (Invitrogen). Six-His-tagged recombinant polypeptides of LdRos3 and LbRos3 were detected using 1:5,000 HisProbe-HRP (Pierce), a nickel (Ni2+)-activated derivative of horseradish peroxidase (HRP). These approaches were carried out to normalize the signal intensities of immunodetection with anti-LdMT and anti-LdRos3 and the respective L. braziliensis orthologues. Immunodetection of α-tubulin was performed with monoclonal anti-α-tubulin antibody (Sigma-Aldrich) at 1:5,000. Additionally, α-tubulin may be used as a loading control for crude membrane fractions considering that Leishmania parasites possess a subpellicular microtubule cytoskeleton attached to the PM. Signals were detected by the ECL chemiluminescent substrate (Pierce). Quantification of protein expression was performed by densitometry using the Quantity One software (Bio-Rad, Hercules, CA).
Deglycosylation reaction.
We used peptide N-glycosidase F (PNGase F; New England BioLabs, Ipswich, MA) by following the instructions of the manufacturer. Briefly, 5 × 107 promastigotes were washed with PBS and then solubilized in 100 μl lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Nonidet P-40) plus a protease inhibitor cocktail (Sigma-Aldrich). Insoluble material was removed by centrifugation at 21,000 × g for 10 min at 4°C. The supernatant was mixed with 10× N-glycosidase buffer and incubated with PNGase F (1,000 U) at 37°C for 1 h. Sample was mixed with 2× Laemmli buffer and separated by SDS-PAGE.
In vitro Leishmania amastigote sensitivity.
Late-stage promastigotes were used to infect isolated peritoneal macrophages from BALB/c mice (Charles River Ltd.) at a macrophage-to-parasite ratio of 1:10 as previously described (14). After infection, cultures were maintained at 37°C with 5% CO2 with different MLF concentrations in RPMI 1640 medium plus 10% IFBS. After 72 h, samples were fixed for 20 min at 4°C with 2% (wt/vol) paraformaldehyde in PBS, followed by permeabilization with 0.1% Triton X-100 in PBS for 10 min. Intracellular parasites were detected by nuclear staining (Prolong-Gold antifade reagent with DAPI [4′,6′-diamidino-2-phenylindole]; Invitrogen). Two hundred macrophages per well were evaluated microscopically.
RESULTS
Reduced drug internalization accounts for the intrinsic low sensitivity of L. braziliensis to MLF.
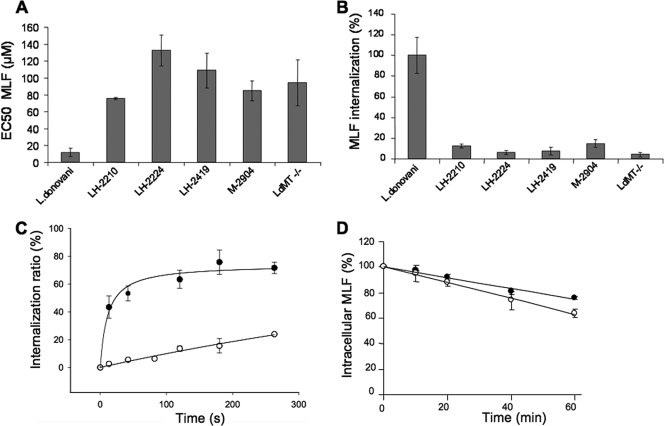
In order to study the molecular basis of the refractory response of L. braziliensis to MLF, we decided to compare four different L. braziliensis strains with our well-characterized, highly sensitive L. donovani strain, for which a number of resistance controls have already been generated and characterized (11, 12). Drug sensitivity experiments demonstrated a 6- to 10-fold decreased MLF sensitivity for the promastigote stages of the different L. braziliensis strains with respect to the L. donovani wild-type line (Fig. 1A). The previously characterized LdMT−/− L. donovani line (12) showed a resistance index of 10-fold. We then measured the accumulation of radiolabeled MLF by the different L. donovani and L. braziliensis strains. In all cases, L. braziliensis showed levels of intracellular MLF of around 10% of those of L. donovani wild-type parasites (Fig. 1B), suggesting, once again, a direct correlation between intracellular drug levels and MLF sensitivity in different Leishmania spp. Because of the low, albeit significant, residual MLF internalization activity observed for L. braziliensis, we tried to determine the kinetic parameters of MLF internalization by Michaelis-Menten analysis. MLF internalization was measured over a range of [14C]MLF concentrations (2 to 50 μM) that were insufficient to obtain substrate saturation curves, and higher drug concentrations were toxic for the parasites under these assay conditions (data not shown). Consequently, we decided to study the initial ratio of internalization of radiolabeled MLF. Parasites were incubated with a fixed concentration of [14C]MLF on ice to promote binding of the drug to the surface of the cells, the excess drug was eliminated with a PBS wash, and incubations were started by increasing the temperature to 28°C. For every time point analyzed, labeled parasites were washed with PBS containing BSA to remove the surface-bound drug that had not been internalized. Measurements were expressed as percentages of the initial amount of drug bound to the parasite surface (1). After 4 min of incubation, the ratios of MLF internalization (ratio of radiolabeled MLF internalized to radiolabeled MLF bound to the outer leaflet of the PM) were 75 and 20% for the parental L. donovani and L. braziliensis LH-2419 strains, respectively (Fig. 1C). The amount of MLF in the outer leaflet of the PM at the beginning of the incubation was 232% for L. braziliensis parasites, compared to wild-type L. donovani, discarding a reduced level of MLF incorporation at the parasite PM as the cause for the low internalization rate. Finally, to study whether the reduced MLF accumulation in L. braziliensis was due to increased efflux of the drug, L. braziliensis and L. donovani parasites were loaded under conditions that yielded similar intracellular MLF levels and then maintained in drug-free medium. Time-dependent MLF efflux was similar in both species (Fig. 1D), leading to the conclusion that the reduced MLF accumulation observed in L. braziliensis is due to a lower rate of internalization.
FIG. 1.
L. braziliensis strains are less sensitive to MLF and have less drug internalization than L. donovani. (A) MLF sensitivity of promastigotes of the L. donovani wild-type and LdMT−/− lines and L. braziliensis strains LH-2210, LH-2224, LH-2419, and M-2904. Shown are EC50s after 72 h of culture. The results shown are the mean ± standard deviation of three independent experiments. (B) [14C]MLF internalization into promastigotes of the L. donovani wild-type and LdMT−/− lines and L. braziliensis strains LH-2210, LH-2224, LH-2419, and M-2904. Internalization was measured after 60 min of incubation at 28°C and expressed as a percentage of the internalization by wild-type L. donovani. Bars represent the mean ± standard deviation of three independent experiments. (C) Initial rate of [14C]MLF internalization in promastigotes of the L. donovani wild-type (black circles) and L. braziliensis LH-2419 (open circles) strains. The data are expressed as the percentage of [14C]MLF inside the cells with respect to the drug bound to the outer leaflet of the PM at the indicated time points. Each point represents the mean ± standard deviation of three independent experiments. (D) [14C]MLF efflux. Promastigotes of the L. donovani wild-type (black circles) and L. braziliensis LH-2419 (white circles) strains were preincubated with [14C]MLF at 28°C, and the decay in radioactivity was monitored at different times (10, 20, 40, and 60 min). The data are expressed as the percentage of the initial amount of [14C]MLF incorporated. Each point represents the mean ± standard deviation of three independent experiments.
Quantitative analysis of the expression levels of the MLF transport machinery in different Leishmania strains.
MLF internalization into L. donovani parasites requires at least two proteins located at the PM, LdMT, a P-type ATPase involved in phospholipid translocation, and its β subunit, LdRos3 (11, 12). We hypothesized that the reduced MLF internalization observed in L. braziliensis lines might be related to a lower efficiency of the MLF transport machinery in these parasites. At least three different scenarios which are not necessarily exclusive could account for this reduced activity, i.e., (i) lower steady-state levels of the MLF transporter and/or its specific β subunit, LbRos3; (ii) reduced trafficking of both proteins to the PM; and (iii) lower activity of the MLF transporter.
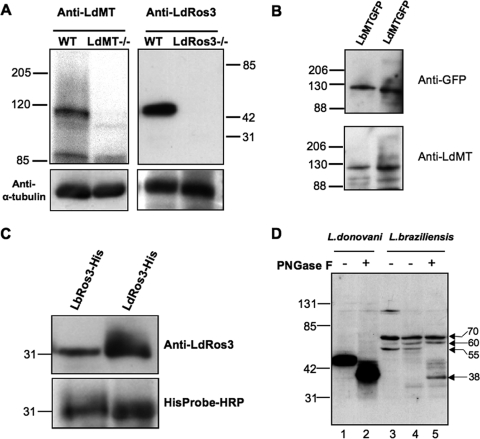
To compare the total expression levels of both proteins in L. donovani and L. braziliensis parasites, we generated rabbit polyclonal antibodies against recombinant polypeptides for LdMT and LdRos3. Polyclonal anti-LdMT antibodies recognized a 110-kDa protein by immunoblotting of a wild-type L. donovani crude membrane fraction (Fig. 2A), which is in agreement with the predicted molecular mass of LdMT. The 100-kDa protein was not detected in membranes of LdMT−/− parasites. Thus, this protein is equivalent to LdMT. The LdMT polypeptide used to generate antibodies has 84.2% identity to the L. braziliensis orthologue LbMT. In order to determine the level of recognition toward LbMT, we transfected LdMT−/− parasites with plasmids containing the LdMT-GFP and LbMT-GFP chimeras. Total cell lysates from both transfectant lines were immunoblotted using anti-LdMT, and the intensities of the bands were normalized by developing the blots against anti-GFP antibodies (Fig. 2B). Cleavage products of the chimeric transporters were not detected below 88 kDa (data not shown). The level of LbMT recognition by anti-LdMT antibodies was around 85%. Thus, anti-LdMT antibodies cross-react with the L. braziliensis LbMT orthologue, allowing for the quantitative analysis of LbMT expression levels in L. braziliensis.
FIG. 2.
Antibodies to LdMT and LdRos3 cross-react with their L. braziliensis orthologues. (A) Specificity of the anti-LdMT and anti-LdRos3 antibodies. Crude membrane fractions from promastigotes of wild-type (WT) L. donovani and the LdMT−/− and LdRos3−/− lines were subjected to SDS-PAGE and immunoblotted with rabbit polyclonal antibodies against recombinant polypeptides of LdMT and LdRos3. An anti-α-tubulin monoclonal antibody was used as a probe for a protein loading control. The positions of molecular mass markers (kilodaltons) are indicated. (B) Level of recognition of LbMT by anti-LdMT antibodies. Aliquots of crude membrane fractions of L. donovani LdMT−/− promastigotes transfected with LbMT-GFP or LdMT-GFP were subjected to SDS-PAGE and immunoblotted with anti-LdMT and anti-GFP as a normalization control. A Western blot representative of at least three independent experiments is shown. The positions of molecular mass markers (kilodaltons) are indicated on the left. (C) Level of recognition of LbRos3 by anti-LdRos3 antibodies. Aliquots of six-His-tagged LdRos3 and LbRos3 recombinant polypeptides (LdRos3-His, LbRos3-His) were subjected to SDS-PAGE and immunoblotted for anti-LdRos3. HisProbe-HRP was used as a normalization control. A Western blot representative of at least three independent experiments is shown. The positions of molecular mass markers (kilodaltons) are indicated on the left. (D) LdRos3 and LbRos3 are glycosylated proteins. Crude membrane fractions of L. donovani and L. braziliensis LH-2419 wild-type strain promastigotes were treated with PNGase F at 37°C for 1 h and analyzed by Western blotting with anti-LdRos3 antibodies. Lanes 1 and 3, untreated protein samples; lanes 2 and 5, PNGase F-treated samples; lane 4, sample incubated with the N-glycosidase buffer. The positions of molecular mass markers (kilodaltons) are indicated on the left. The L. braziliensis 38- to 70-kDa proteins recognized by anti-LdRos3 are indicated on the right.
Polyclonal anti-LdRos3 antibodies recognized a 44-kDa protein by immunoblotting analysis of the crude membrane fraction from L. donovani parasites (Fig. 2A) whose molecular mass is slightly greater than the predicted 40 kDa. This protein band was absent from membranes of LdRos3−/− parasites, demonstrating the specificity of the antibodies to LdRos3. LdRos3 has three putative N-linked glycosylation sites, and the treatment with PNGase F decreased the apparent mobility of the protein from 44 kDa (Fig. 2D, lane 1) to 40 kDa (Fig. 2D, lane 2). The LdRos3 polypeptide used to generate polyclonal antibodies shows 72.8% identity to the L. braziliensis orthologue LbRos3. Both six-His-tagged proteins were expressed in bacteria to determine the LbRos3 recognition of anti-LdRos3 by immunoblotting analysis. The level of LbRos3 recognition by cross-reactivity with anti-LdRos3 antibodies was around 60% (Fig. 2C). Immunoblotting experiments using a crude membrane fraction from L. braziliensis with anti-LdRos3 antibodies detected proteins of 55 to 70 kDa (Fig. 2D, lane 3). LbRos3 has six putative N-glycosylation sites. To determine whether these proteins corresponded to different rates of N glycosylation, protein samples from L. braziliensis were subjected to PNGase F treatment. The apparent mobility of the 55-kDa protein decreased to 38 kDa (Fig. 2D, compare lanes 4 and 5), consistent with the predicted molecular weight of LbRos3. Furthermore, the 60- and 70-kDa proteins were resistant to PNGase F treatment, suggesting that the polyclonal antibody unspecifically recognizes both proteins. The 120-kDa protein (Fig. 2D, lane 3) could be aggregated proteins because this band disappeared after sample incubation in buffered 1% NP-40 for 1 h at 37°C (Fig. 2, lanes 4 and 5) or boiling (data not shown). Therefore, the anti-LdRos3 antibody also cross-recognized the LbRos3 orthologue as a 55-kDa protein that represents a glycosylated form of LbRos3.
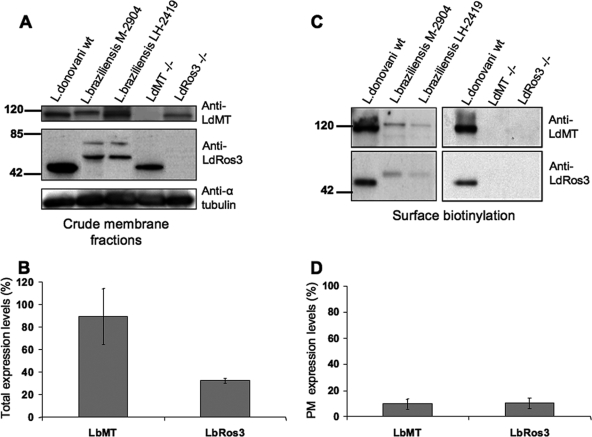
Different crude membrane preparations from different L. braziliensis strains showed a mean LbMT expression level of around 90% of that of LdMT in wild-type L. donovani (Fig. 3A and B). These data indicate that the lower intrinsic activity of MLF against L. braziliensis cannot be accounted for by reduced expression of LbMT. However, different L. braziliensis strains showed a lower LbRos3 expression level of around 32% of that of LdRos3 in wild-type L. donovani (Fig. 3A and B). This reduced expression could explain the low level of MLF internalization by L. braziliensis.
FIG. 3.
L. braziliensis expresses low amounts of the MLF translocation machinery at the parasite PM. (A) Comparative expression analysis of the total levels of LMT and LRos3 in crude membrane fractions of promastigotes of the L. donovani wild-type (wt), LdMT−/−, and LdRos3−/− and L. braziliensis M-2904 and LH-2419 strains by immunoblotting with anti-LdMT and anti-LdRos3 antibodies. An anti-α-tubulin monoclonal antibody was used as a probe for a protein loading control. Western blot assays representative of at least six independent experiments are shown. The positions of molecular mass markers (kilodaltons) are indicated on the left. (B) Quantitative analysis of total LbMT and LbRos3 expression levels in two different L. braziliensis strains (M-2904 and LH-2419). Bars represent the means of six independent experiments ± the standard deviations, and the results are expressed as percentages of the wild-type L. donovani protein level. (C) Biotinylated proteins from the promastigote surface were analyzed by immunoblotting with anti-LdMT and anti-LdRos3 antibodies as described in Materials and Methods. Western blot assays representative of at least four independent experiments are shown. The positions of molecular mass markers (kilodaltons) are indicated on the left. (D) Quantitative analysis of LbMT and LbRos3 expression levels at the PM of two different L. braziliensis strains (M2904 and LH2419). Bars represent the means of four independent experiments ± the standard deviations, and the results are expressed as in panel B.
Reduced levels of the MLF transport machinery at the PM of L. braziliensis.
MLF internalization occurs at the parasite PM, from where it is specifically translocated by the LdMT-LdRos3-dependent machinery. We have previously shown that both proteins are mutually dependent for their delivery to the PM, as assessed by fluorescence microscopy analysis using GFP chimeras (12). We quantified the expression levels of both components of the MLF transport system at the parasite PM by surface biotinylation. LdMT and LdRos3 present at the PM of wild-type L. donovani parasites were detected in the fraction eluted from streptavidin beads by immunoblotting (Fig. 3C). As negative controls, we used LdRos3−/− and LdMT−/− parasites, which should retain all of the LdMT and LdRos3 synthesized, respectively, at the endoplasmic reticulum level (12). In both cases, no protein could be detected at the PM, even though their total levels in the lysates were clearly detected, demonstrating the ability of the biotinylation procedure to isolate the PM pool of both proteins specifically. Furthermore, no immunoreactive bands were detected after immunoblotting of biotinylated proteins against anti-α-tubulin (data not shown). This control confirms that labeling reagent did not penetrate the PM. A similar procedure undertaken with L. braziliensis promastigotes showed very weak signal bands corresponding to both members of the translocation machinery (Fig. 3C). Of all of the bands recognized by anti-LdRos3 antibody in total lysates of L. braziliensis, only the 55-kDa protein appeared in the PM fractions of L. braziliensis (Fig. 3C). Therefore, this 55-kDa band represents the mature LbRos3 orthologue. We can compare the expression levels of the orthologues of the MLF transport complex of L. braziliensis and L. donovani because the lysine residues putatively exposed to the cells surface, which are necessary to the biotinylation reaction, are conserved. Quantitative analysis indicates that L. braziliensis strains express around 10% of both members of the MLF transporter machinery at the PM compared to L. donovani wild-type parasites (Fig. 3D). These results are in agreement with the rates of MLF accumulation (Fig. 1B). Thus, the inability of L. braziliensis strains to accumulate normal MLF levels comes from a reduced expression of the translocation machinery at the parasite PM.
The LbMT orthologue is partially able to functionally rescue LdMT−/− parasites.
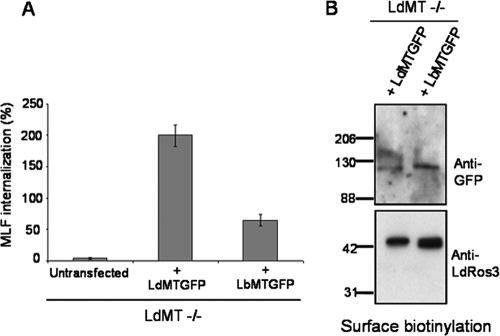
In order to study the functionality of LbMT, we transfected LdMT−/− parasites with LbMT-GFP. Both the reduced MLF accumulation and the resistance phenotype could be partially rescued, but only to levels fivefold lower than those achieved upon transfection with LdMT-GFP (Fig. 4A), even though both transfected parasites showed similar levels of MLF transporter-GFP chimeras at the PM, as determined by the biotinylation assay (Fig. 4B). Thus, there is a correlation with MLF sensitivity assays in both transfected parasite lines, showing that LbMT-GFP parasites have threefold lower MLF sensitivity than LdMT-GFP parasites. The EC50s were 94.4 ± 22.9, 26.1 ± 1.1, and 9.5 ± 1.1 μM for the LdMT−/−-, LbMT-GFP-, and LdMT-GFP-transfected lines, respectively. This result suggests a lower intrinsic activity of LbMT than of LdMT, even though a reduced affinity for other associated proteins involved in MLF transport (including LdRos3) cannot be ruled out.
FIG. 4.
LbMT-GFP partially rescues MLF uptake in LdMT−/− parasites. L. donovani LdMT−/− promastigotes were transfected with LdMT-GFP or LbMT-GFP. (A) [14C]MLF internalization was measured after 60 min of incubation at 28°C and is expressed as a percentage of the internalization by wild-type L. donovani. Results are shown as the mean ± standard deviation of three independent experiments. (B) Affinity-purified biotinylated proteins from the surface of live parasites were analyzed by immunoblotting with anti-GFP and anti-LdRos3 antibodies. Western blot assays representative of at least three independent experiments are shown. The positions of molecular mass markers (kilodaltons) are indicated on the left.
Overexpression of LbRos3 increases MLF internalization and sensitivity in L. braziliensis.
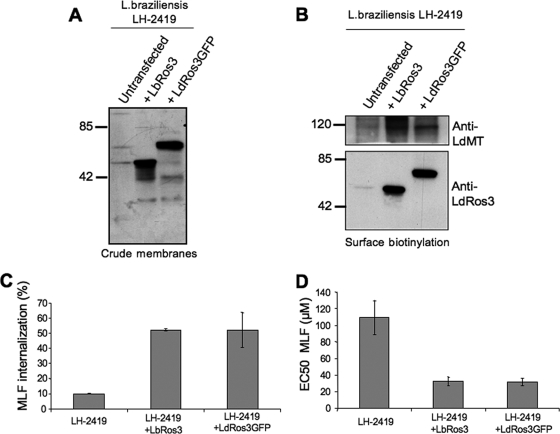
The previous results suggested that the lower levels of the MLF transporter machinery at the PM of L. braziliensis parasites are due to decreased expression of the β subunit LbRos3, which would behave as the rate limiting factor of the MLF transport complex. To test this hypothesis, L. braziliensis parasites (MHOM/PE/03/LH-2419) were transfected with constructs containing LbRos3 and LdRos3-GFP, a functional chimera previously described (12) (Fig. 5A). Biotinylation experiments showed a notable increase in LbMT levels at the PM in parasites overexpressing LdRos3-GFP and LbRos3 with respect to the parental line (Fig. 5B). MLF internalization was similarly increased around fivefold in each transfected line (Fig. 5C). Furthermore, the sensitivity to MLF was also increased three- to fourfold (Fig. 5D). Parasites transfected with the empty vector showed MLF internalization and sensitivity values similar to those of the wild-type line (data not shown).
FIG. 5.
Overexpression of LbRos3 increases LbMT trafficking to the PM and MLF uptake and sensitivity in L. braziliensis promastigotes. (A) Overexpression of LbRos3 and LdRos3-GFP in crude membrane fractions of L. braziliensis LH-2419 promastigotes was detected by immunoblotting with anti-LdRos3 antibodies. The positions of molecular mass markers (kilodaltons) are indicated on the left. (B) L. braziliensis LH-2419 promastigotes transfected with the LbRos3 or LdRos3-GFP construct expressed increased levels of LbMT and LbRos3 or LdRos3-GFP, at the PM. Western blot assays representative of at least three independent experiments are shown. The positions of molecular mass markers (kilodaltons) are indicated on the left. (C) [14C]MLF internalization after 60 min in L. braziliensis LH-2419 promastigotes overexpressing LbRos3 and LdRos3-GFP. Bars shown are the mean ± standard deviation of three independent experiments, and results are expressed as a percentage of the internalization by wild-type L. donovani. (D) MLF sensitivity after 72 h of culture in L. braziliensis LH-2419 parasites overexpressing LbRos3 and LdRos3-GFP. The EC50s shown are the mean ± standard deviation of three independent experiments.
L. braziliensis intracellular amastigotes maintain the decreased susceptibility to MLF in an LbRos3-dependent manner.
We have previously reported that MLF resistance is maintained from promastigote to intracellular amastigote forms in in vitro models (14). Following a similar approach, late-stage promastigotes of both the L. braziliensis LH-2419 parental and LbRos3-overexpressing lines were used to infect primary isolated mouse peritoneal macrophages. Infected cultures were maintained with different MLF concentrations for 72 h. Results showed that the ratio of EC50s of MLF for parental and LbRos3-transfected L. braziliensis persisted in both the promastigote and amastigote life cycle stages (ratio = 3.3) (Table 1). Furthermore, both the promastigote and amastigote stages of L. donovani are more sensitive to MLF, with EC50s of 9.36 and 3.02 μM, respectively (14). Transfected parasites remained infective and maintained virulence, as determined by the percentage of infected macrophages and the average number of amastigotes per infected macrophage (Table 1).
TABLE 1.
MLF sensitivity profiles of promastigotes and intracellular amastigotes of L. braziliensis linesa
| Line | EC50 (μM)b (RI)c
|
% Infectiond | No. of amastigotes/celle | |
|---|---|---|---|---|
| Promastigotes | Amastigotes | |||
| LH-2419 | 109.0 ± 20.1 (3.3) | 17.9 ± 5.4 (3.3) | 50 ± 6 | 4.6 ± 1.4 |
| LH-2419 + LbRos3 | 32.4 ± 5.1 | 5.4 ± 0.5 | 45 ± 16 | 3.6 ± 0.9 |
L. braziliensis (L-H2419) parasites were grown for 72 h in the presence of increasing concentrations of MLF as described in Materials and Methods. Subsequently, promastigote and amastigote viability was determined by using an MTT-based assay and DAPI staining, respectively. Data are the means ± the standard deviations of three independent experiments.
Results are expressed as the MLF concentration necessary to inhibit parasite growth by 50% (EC50).
The resistance index (RI) was calculated by dividing the EC50 for LH-2419 by that for parasites overexpressing LbRos3 (LH-2419 + LbRos3).
Percent infection indicates the percentage of macrophages infected in the absence of drug at the endpoint of the assay for Leishmania amastigotes.
The number of amastigotes per cell is the average number of intracellular amastigotes per infected macrophage in the absence of drug after 72 h of infection.
DISCUSSION
In this work, we have determined the cellular and molecular basis for the natural resistance to MLF observed in L. braziliensis isolates. These parasites accumulate much less MLF because of reduced drug internalization. The lower capacity to internalize MLF can be explained, in part, by a lower expression level of LbRos3 than that of the L. donovani orthologue LdRos3, which leads to a very low expression levels of the MLF transport complex components LbRos3 and LbMT at the parasite PM. Furthermore, the intrinsic activity of LbMT seems lower than that of LdMT. Differences between the two transporters could reflect differences in Vmax, Km, or both.
These findings point once again to MLF internalization as the main determinant of drug sensitivity and, more precisely, to the levels of the MLF translocation complex present at the PM. For the first time, and thanks to the development of new specific polyclonal antibodies, we are able to quantitatively measure the amounts of LMT and LRos3 at the PM for L. donovani and L. braziliensis. LdMT had been described as the limiting molar factor of the translocation machinery in L. donovani because wild-type parasites overexpressing LdMT were hypersensitive to the drug and internalize greater amounts of MLF (11). Furthermore, overexpression of LdMT in Leishmania tarentolae, a lizard parasite also naturally resistant to MLF, increased MLF accumulation and sensitivity to levels similar to those of L. donovani (11). Consistent with this idea, when LdRos3 was overexpressed in L. donovani, no further change in MLF accumulation or sensitivity was observed (12). The situation is reversed in L. braziliensis. LbRos3 is only poorly expressed in wild-type strains (Fig. 3), and its overexpression can rescue MLF accumulation and sensitivity (Fig. 5C and D) to levels much closer to those of L. donovani. Furthermore, LbRos3 overexpression led to a concomitant increase in the amount of LbMT present at the parasite PM (Fig. 5B), indicating that LMT and LRos3 traffic together along the secretory pathway from the endoplasmic reticulum to the PM. Therefore, either LMT or LRos3 can be the rate-limiting factor in MLF internalization, and their levels at the parasite PM ultimately determine MLF potency.
The clinical relevance of these findings is further supported by the correlation of the MLF sensitivities of amastigotes and promastigotes of the different L. braziliensis lines. This correlation is also in agreement with previous findings on L. donovani (14). Therefore, we propose that the lower efficacy of MLF observed in some CL clinical trials (16) is most likely due to the low intrinsic sensitivity of certain L. braziliensis lines to MLF. All of the L. braziliensis strains tested in this study (Brazilian and Peruvian isolates) showed the same trend regarding MLF accumulation and sensitivity. Nevertheless, the situation in the field seems far more complicated. Indeed, the CL cure rates of MLF have varied not only with the species responsible for the infection but with the geographic area as well. In Guatemala, the efficacy of MLF against CL caused by L. braziliensis was only 33%, a value similar to that of the placebo control (38%) (16). In the same study, the cure rate in Colombia, where L. panamensis was prevalent, was 91%. Recently, a cure rate of 88% after MLF treatment of CL has been reported in Bolivia (19), even though the etiological agent was L. braziliensis. Therefore, closer monitoring of the sensitivity of clinical isolates to MLF is strongly recommended for different geographic areas before the widespread use of MLF as the first-line treatment for New World CL, especially because of the taxonomic complexity of the Leishmania subgenus Viannia. L. (Viannia) braziliensis, L. (V.) guyanensis, and L. (V.) lainsoni CL isolates from Peru present notable differences in intrinsic sensitivity to MLF (22). Because promastigote sensitivity to MLF clearly and completely correlates with that of intracellular amastigotes (14; this work), MLF sensitivity could be monitored in clinical isolates grown as the easier-to-handle promastigote forms. In this manner, basic clinical laboratories could perform sensitivity tests on a large percentage of their clinical isolates. Similarly, for clinical isolates refractory to MLF—or geographic areas where those species and subspecies are prevalent—we suggest a combinatorial therapy including MLF, since the residual MLF activity observed in these naturally resistant strains could still be significant to promote parasite clearance.
We conclude that the levels of the MLF transport machinery at the parasite PM are the most important factor determining Leishmania susceptibility to the drug. It is therefore important to develop new reagents able to measure in more simple and quantitative ways the relative levels of LMT/LRos3 at the PM of the different Leishmania spp.
Acknowledgments
This work was supported by Spanish grants SAF2005-01639 (to S.C.) and ISCIII-Red de Investigación Cooperativa en Enfermedades Tropicales (RICET) RD06/0021/0002 (to F.G.) and by the Plan Andaluz de Investigación (Cod. BIO130). M.P.S.-C. is the recipient of an F.P.U. fellowship from the Ministerio de Innovación y Ciencia.
We also thank Æterna Zentaris (Frankfurt, Germany) for providing the MLF used in this study and S. Beverley (Washington University, St. Louis, MO) for the pXG, pXG-′GFP+, and pIR1SAT Leishmania expression vectors.
Footnotes
Published ahead of print on 2 February 2009.
REFERENCES
- 1.Araújo-Santos, J. M., F. Gamarro, S. Castanys, A. Herrmann, and T. Pomorski. 2003. Rapid transport of phospholipids across the plasma membrane of Leishmania infantum. Biochem. Biophys. Res. Commun. 306:250-255. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya, S. K., P. K. Sinha, S. Sundar, C. P. Thakur, T. K. Jha, K. Pandey, V. R. Das, N. Kumar, C. Lal, N. Verma, V. P. Singh, A. Ranjan, R. B. Verma, G. Anders, H. Sindermann, and N. K. Ganguly. 2007. Phase 4 trial of miltefosine for the treatment of Indian visceral leishmaniasis. J. Infect. Dis. 196:591-598. [DOI] [PubMed] [Google Scholar]
- 3.Cabrera-Serra, M. G., J. Lorenzo-Morales, M. Romero, B. Valladares, and J. E. Piñero. 2007. In vitro activity of perifosine: a novel alkylphospholipid against the promastigote stage of Leishmania species. Parasitol. Res. 100:1155-1157. [DOI] [PubMed] [Google Scholar]
- 4.Chappuis, F., S. Sundar, A. Hailu, H. Ghalib, S. Rijal, R. W. Peeling, J. Alvar, and M. Boelaert. 2007. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 5:873-882. [DOI] [PubMed] [Google Scholar]
- 5.Escobar, P., S. Matu, C. Marques, and S. L. Croft. 2002. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Trop. 81:151-157. [DOI] [PubMed] [Google Scholar]
- 6.Ha, D. S., J. K. Schwarz, S. J. Turco, and S. M. Beverley. 1996. Use of the green fluorescent protein as a marker in transfected Leishmania. Mol. Biochem. Parasitol. 77:57-64. [DOI] [PubMed] [Google Scholar]
- 7.Lario, A., A. González, and G. Dorado. 1997. Automated laser-induced fluorescence DNA sequencing: equalizing signal-to-noise ratios significantly enhances overall performance. Anal. Biochem. 247:30-33. [DOI] [PubMed] [Google Scholar]
- 8.Minodier, P., and P. Parola. 2007. Cutaneous leishmaniasis treatment. Travel Med. Infect. Dis. 5:150-158. [DOI] [PubMed] [Google Scholar]
- 9.Mollinedo, F., J. L. Fernández-Luna, C. Gajate, B. Martín-Martín, A. Benito, R. Martínez-Dalmau, and M. Modolell. 1997. Selective induction of apoptosis in cancer cells by the ether lipid ET-18-OCH3 (edelfosine): molecular structure requirements, cellular uptake, and protection by Bcl-2 and Bcl-XL. Cancer Res. 57:1320-1328. [PubMed] [Google Scholar]
- 10.Pérez-Victoria, F. J., S. Castanys, and F. Gamarro. 2003. Leishmania donovani resistance to miltefosine involves a defective inward translocation of the drug. Antimicrob. Agents Chemother. 47:2397-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez-Victoria, F. J., F. Gamarro, M. Ouellette, and S. Castanys. 2003. Functional cloning of the miltefosine transporter. A novel P-type phospholipid translocase from Leishmania involved in drug resistance. J. Biol. Chem. 278:49965-49971. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Victoria, F. J., M. P. Sánchez-Cañete, S. Castanys, and F. Gamarro. 2006. Phospholipid translocation and miltefosine potency require both L. donovani miltefosine transporter and the new protein LdRos3 in Leishmania parasites. J. Biol. Chem. 281:23766-23775. [DOI] [PubMed] [Google Scholar]
- 13.Pérez-Victoria, F. J., M. P. Sánchez-Cañete, K. Seifert, S. L. Croft, S. Sundar, S. Castanys, and F. Gamarro. 2006. Mechanisms of experimental resistance of Leishmania to miltefosine: implications for clinical use. Drug Resist. Updat. 9:26-39. [DOI] [PubMed] [Google Scholar]
- 14.Seifert, K., F. J. Pérez-Victoria, M. Stettler, M. P. Sánchez-Cañete, S. Castanys, F. Gamarro, and S. L. Croft. 2007. Inactivation of the miltefosine transporter, LdMT, causes miltefosine resistance that is conferred to the amastigote stage of Leishmania donovani and persists in vivo. Int. J. Antimicrob. Agents 30:229-235. [DOI] [PubMed] [Google Scholar]
- 15.Small, G. W., J. C. Strum, and L. W. Daniel. 1997. Characterization of an HL-60 cell variant resistant to the antineoplastic ether lipid 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine. Lipids 32:715-723. [DOI] [PubMed] [Google Scholar]
- 16.Soto, J., B. A. Arana, J. Toledo, N. Rizzo, J. C. Vega, A. Díaz, M. Luz, P. Gutiérrez, M. Arboleda, J. D. Berman, K. Junge, J. Engel, and H. Sindermann. 2004. Miltefosine for New World cutaneous leishmaniasis. Clin. Infect. Dis. 38:1266-1272. [DOI] [PubMed] [Google Scholar]
- 17.Soto, J., and J. Berman. 2006. Treatment of New World cutaneous leishmaniasis with miltefosine. Trans. R. Soc. Trop. Med. Hyg. 100(Suppl. 1):S34-S40. [DOI] [PubMed] [Google Scholar]
- 18.Soto, J., J. Toledo, L. Valda, M. Balderrama, I. Rea, R. Parra, J. Ardiles, P. Soto, A. Gómez, F. Molleda, C. Fuentelsaz, G. Anders, H. Sindermann, J. Engel, and J. Berman. 2007. Treatment of Bolivian mucosal leishmaniasis with miltefosine. Clin. Infect. Dis. 44:350-356. [DOI] [PubMed] [Google Scholar]
- 19.Soto, J., J. Rea, M. Balderrama, J. Toledo, P. Soto, L. Valda, and J. D. Berman. 2008. Efficacy of miltefosine for Bolivian cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 78:210-211. [PubMed] [Google Scholar]
- 20.Sundar, S., A. Makharia, D. K. More, G. Agrawal, A. Voss, C. Fischer, P. Bachmann, and H. W. Murray. 2000. Short-course of oral miltefosine for treatment of visceral leishmaniasis. Clin. Infect. Dis. 31:1110-1113. [DOI] [PubMed] [Google Scholar]
- 21.Sundar, S., T. K. Jha, C. P. Thakur, S. K. Bhattacharya, and M. Rai. 2006. Oral miltefosine for the treatment of Indian visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 100(Suppl. 1):S26-S33. [DOI] [PubMed] [Google Scholar]
- 22.Yardley, V., S. L. Croft, S. De Doncker, J. C. Dujardin, S. Koirala, S. Rijal, C. Miranda, A. Llanos-Cuentas, and F. Chappuis. 2005. The sensitivity of clinical isolates of Leishmania from Peru and Nepal to miltefosine. Am. J. Trop. Med. Hyg. 73:272-275. [PubMed] [Google Scholar]
- 23.Zoeller, R. A., M. D. Layne, and E. J. Modest. 1995. Animal cell mutants unable to take up biologically active glycerophospholipids. J. Lipid Res. 36:1866-1875. [PubMed] [Google Scholar]