Abstract
Gallium (Ga) is a semimetallic element that has demonstrated therapeutic and diagnostic-imaging potential in a number of disease settings, including cancer and infectious diseases. Gallium's biological actions stem from its ionic radius being almost the same as that of ferric iron (Fe3+), whereby it can replace iron (Fe) in Fe3+-dependent biological systems, such as bacterial and mammalian Fe transporters and Fe3+-containing enzymes. Unlike Fe3+, ionic gallium (Ga3+) cannot be reduced, and when incorporated, it inactivates Fe3+-dependent reduction and oxidation processes that are necessary for bacterial and mammalian cell proliferation. Most pathogenic bacteria require Fe for growth and function, and the availability of Fe in the host or environment can greatly enhance virulence. We examined whether gallium maltolate (GaM), a novel formulation of Ga, had antibacterial activity in a thermally injured acute infection mouse model. Dose-response studies indicated that a GaM dose as low as 25 mg/kg of body weight delivered subcutaneously was sufficient to provide 100% survival in a lethal P. aeruginosa-infected thermally injured mouse model. Mice treated with 100 mg/kg GaM had undetectable levels of Pseudomonas aeruginosa in their wounds, livers, and spleens, while the wounds of untreated mice were colonized with over 108 P. aeruginosa CFU/g of tissue and their livers and spleens were colonized with over 105 P. aeruginosa CFU/g of tissue. GaM also significantly reduced the colonization of Staphylococcus aureus and Acinetobacter baumannii in the wounds of thermally injured mice. Furthermore, GaM was also therapeutically effective in preventing preestablished P. aeruginosa infections at the site of the injury from spreading systemically. Taken together, our data suggest that GaM is potentially a novel antibacterial agent for the prevention and treatment of wound infections following thermal injury.
The American Burn Association estimates that approximately 500,000 individuals in the United States are treated for thermal injury each year, resulting in over 4,000 deaths (1). Worldwide the numbers are significantly larger, especially in areas of conflict. The World Health Organization reports that over 90% of burns occur in developing or underdeveloped nations, where the mortality for large burns (over 40% total body surface area) approaches 100% (31). As thermal injury removes or impairs the body's natural barrier to microbes, the cause of death in over 75% of these burned individuals is infection (38).
Pseudomonas aeruginosa is one of the most prevalent opportunistic pathogens that infect burn wounds, and the mortality associated with a systemic infection is over 75% (26). The exceptional virulence of this gram-negative bacterium is due to the production of numerous virulence factors, including toxins, lysins and proteases (30). P. aeruginosa proliferates rapidly in burn wounds, and the infection can be divided into two phases (19, 20). In the first phase of infection, P. aeruginosa quickly colonizes the devascularized burnt tissue, which provides a warm, moist, nutrient-rich environment ideally suited for bacterial growth. P. aeruginosa proliferates quickly, forming biofilms in the hypodermis, specifically surrounding blood vessels (36). Once a threshold concentration of P. aeruginosa is reached in the eschar (approximately 109 CFU/g tissue), P. aeruginosa spreads systemically through the bloodstream, causing bacteremia, which is the second stage of infection. P. aeruginosa-induced bacteremia is soon followed by multiple organ failure and eventually death (19, 20). Infections in clinical settings are of growing concern because of the increasing resistance of P. aeruginosa to antibiotics. One-third of P. aeruginosa clinical isolates are resistant to three or more antibiotics, including broad-spectrum cephalosporins and imipenem, which has been the “gold standard” (12, 27). Despite the growing resistance of P. aeruginosa and many other pathogens to current antimicrobials, very few new drugs are in advanced development or clinical trials (29). Therefore, new antimicrobials that target this pathogen are urgently needed.
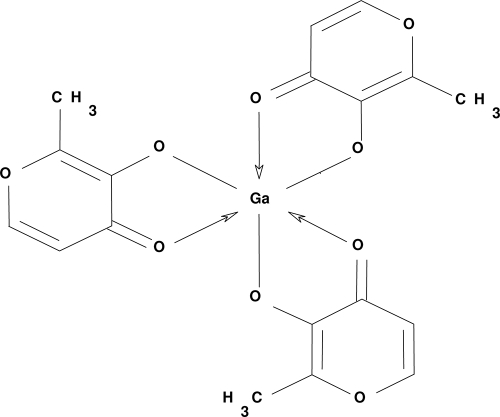
The semimetal gallium (Ga; atomic number 31) was discovered in 1875, and its first therapeutic properties were described in 1971 for the treatment of cancer (15, 16). The antineoplastic effects of Ga are attributed to its inhibitory effects on a cohort of proliferative mechanisms, including DNA and protein synthesis (10), and it is Ga's antitumor activity that has primarily been pursued therapeutically. Ga in the form of citrate-buffered gallium nitrate [Ga(NO3)3] solution (Ganite) is FDA approved for the treatment of hypercalcemia due to malignancy and has also been tested clinically to treat non-Hodgkin's lymphoma and urothelial cancer (10). However, Ga nitrate has to be administered by slow intravenous infusion to avoid the formation of gallate precipitates that can result in nephrotoxicity (10). An alternative formulation, gallium maltolate (GaM), has been developed which consists of Ga bound to three maltol (3-hydroxy-2-methyl-4-pyrone) ligands in a stable and soluble coordination complex with reduced likelihood of forming toxic precipitates (Fig. 1) (4). GaM is cytotoxic to hepatocellular carcinoma cells (8) and Ga nitrate-resistant lymphoma cells in vitro (7, 8) and has been tested to date in over 90 human subjects in dose-escalating safety and pharmacokinetic studies (3, 4). GaM was clinically well tolerated at all dose levels tested, with no serious adverse events, treatment-related withdrawals, or clinically relevant changes to vital signs or parameters (4). Human pharmacokinetic analysis indicated oral bioavailability of 30 to 55% (3), and studies performed with mice and neonatal foals administered GaM resulted in serum gallium concentrations sufficient to suppress growth or kill the facultative intracellular zoonotic pathogen Rhodococcus equi (14, 25).
FIG. 1.
Structure of GaM. GaM is a complex of the trivalent Ga ion surrounded by three deprotonated maltolate ligands.
An important biological attribute of Ga is that it resembles ferric iron (Fe3+). The ionic radii of Ga3+ and Fe3+ are similar and not easily distinguishable by Fe3+-dependent biological systems, including bacterial iron scavengers and transporters. However, Ga3+ cannot be reduced, and when it is incorporated in the place of Fe3+, it inactivates Fe-dependent sequential reduction and oxidation processes. It is well known that most pathogenic bacteria require iron to grow, and the availability of Fe in the host greatly enhances virulence. Recently it was reported that Ga is preferentially taken up over Fe by P. aeruginosa and that Ga nitrate solution could inhibit P. aeruginosa growth and biofilm formation in vitro and in murine lung infection models (22). Here we sought to determine whether Ga nitrate or the novel formulation, GaM, could be used to treat skin infections following thermal injury.
MATERIALS AND METHODS
Bacterial growth and inocula.
P. aeruginosa strain PAO1 (21), S. aureus (American Type Culture Collection #700699), and A. baumannii (American Type Culture Collection #9955) were grown in Luria-Bertani (LB) medium (2). Aliquots (50 μl) of overnight cultures were subcultured in fresh LB broth and grown at 37°C for 4 h to an optical density of approximately 0.9 at 600 nm. A 100-μl aliquot of each culture was then pelleted, washed in phosphate-buffered saline (PBS), and serially diluted (10-fold serial dilutions) in PBS. A 100-μl aliquot of the 10−5 dilution was injected subcutaneously into the burn eschars of each animal. As we have previously determined (18), this dilution represents approximately 2 × 102 to 2 × 103 CFU and this dose of PAO1 results in 82 to 94% mortality in mice by 48 h post-thermal injury infection (17, 35). The exact inoculum of each strain was determined by plating serial dilutions of the inoculum on LB agar plates.
Thermally injured mouse model.
Female Swiss Webster (SW) mice were obtained from Charles River Laboratories (Wilmington, MA). The mice used in these experiments were 6 to 8 weeks old and weighed 17 to 20 g. The virulence of PAO1 was examined by using a modified burned-mouse model of Stieritz and Holder (37). In this modified model, a scald burn is induced. Mice were anesthetized by intraperitoneal injection of 0.4 ml/20 g of body weight of 5% pentobarbital sodium (Nembutal) at 5 mg/ml (Abbott Laboratories, North Chicago, IL), and their backs were shaved. The mice were securely placed into a template with an opening (4.5 by 1.8 cm) exposing their shaved backs. About 15% of the total surface area of the mouse was exposed through the opening on the template. The thermal injury was induced by placing the exposed area of the shaved skin in 90°C water for 10 s. Such an injury is nonlethal but causes a third-degree (full thickness) burn. Fluid replacement therapy consisting of a subcutaneous injection of 0.8 ml lactated Ringer's solution was administered immediately following the burn. Mice were challenged by the subcutaneous inoculation of 100 μl of the bacterial inoculum (see above) directly under the burn. GaM (Titan Pharmaceuticals, Inc., South San Francisco, CA) was prepared as an aqueous solution in sterile phosphate-buffered saline (PBS) at the concentration indicated and filter sterilized. Ga(NO3)3 (Sigma-Aldrich) was buffered in a 100 mM solution of sodium citrate (pH 7) and filter sterilized. Gallium-treated mice were injected sub-eschar (SE) with 100 μl of the indicated dose. Control mice were injected SE with 100 μl of vehicle control (either PBS or 100 mM sodium citrate [pH 7]). Mortality among infected mice was recorded at 24-h intervals, up to 7 days post-thermal injury/infection. Mice were treated humanely and in accordance with the protocol approved by the Animal Care and Use Committee at the Texas Tech University Health Sciences Center (Lubbock, TX).
Quantitation of bacteria within the skin and livers.
At 24 or 48 h postburn/infection, mice were euthanized by intracardial injection of 0.2 ml of Sleepaway (sodium pentobarbital-7.8% isopropyl alcohol euthanasia solution; Fort Dodge Laboratories, Inc., Fort Dodge, IA). Skin sections of approximately 1 by 1 cm were obtained from the burn eschars of mice. Simultaneously, the entire liver and spleen of each animal were obtained. Tissues were weighed, suspended in 2 ml of sterile PBS, and homogenized (Wheaton overhead stirrer; Wheaton Instruments, Millville, NJ). Homogenates were serially diluted and plated on LB agar plates to determine the number of bacterial CFU, which was calculated per gram of tissue.
RESULTS
GaM promotes the survival of P. aeruginosa-infected thermally injured mice.
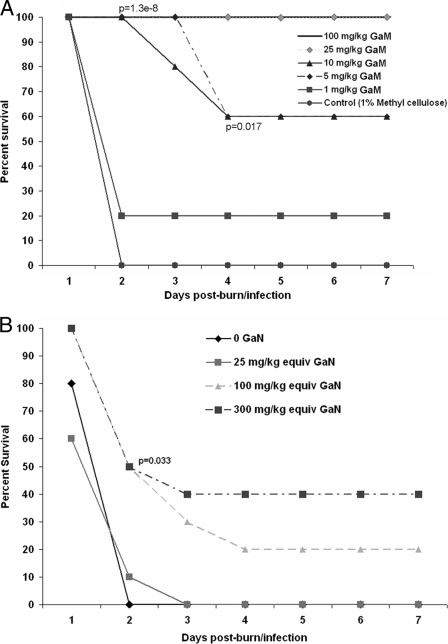
We first sought to examine whether GaM could reduce the mortality associated with P. aeruginosa-infected burn wounds. The thermally injured mouse model closely resembles the burn wound infection sequela typically seen in humans (37). We have previously shown that 90 to 100% of thermally injured mice infected with the P. aeruginosa strain PAO1 die by 48 h postburn/infection (17, 34, 35). Therefore, we compared the mortality rates of thermally injured, P. aeruginosa-infected mice treated with GaM to those of mice treated with vehicle. Groups of mice were given full-thickness scald burns and then immediately infected with PAO1 as described in Materials and Methods. SE injections of 1, 5, 10, 25, or 100 mg/kg GaM, or vehicle (sterile PBS) were given at the time of infection and again at 24 h postinfection. The mice were observed postburn/infection for 7 days, and the percent survival was recorded for each group (Fig. 2A). All of the vehicle-treated mice died within 48 h of the infection (n = 15). In contrast, we observed a dose-dependent increase in the percent survival in mice treated with GaM (n = 10 to 15/experimental group) (Fig. 2A). GaM was completely effective at preventing death when given at doses of 25 and 100 mg/kg. In addition, GaM appeared to be well tolerated at all of the doses delivered, as no gross clinical signs of toxicity, such as gait or postural or feeding difficulties, were observed.
FIG. 2.
Percent survival of P. aeruginosa-infected thermally injured mice treated with increasing doses of GaM. Groups of mice (n = 10 to 15 per group) were thermally injured, inoculated with PAO1, allowed to recover, and observed over 7 days. SE injections of 1, 5, 10, 25, or 100 mg/kg GaM (A), 0.6, 2.6, and 7.7 mM Ga(NO3)3 (B), or vehicle (sterile PBS or 100 mM sodium citrate, respectively) were given at the time of infection and again at 24 h postinfection. Percent survival was assessed at 24-h time points. equiv, equivalents. The data shown represent three independent experiments, Fisher's exact test was used to determine significance between groups.
GaM is more efficacious than Ga(NO3)3 against P. aeruginosa burn wound infections.
Ga in the form of Ga(NO3)3 was recently reported to treat P. aeruginosa infection in a mouse lung model (22). We sought to compare the efficacy of GaM versus Ga(NO3)3 in the treatment of P. aeruginosa-infected burn wounds. Preparations of Ga(NO3)3 containing the equivalent elemental Ga concentrations as 300-, 100-, and 25-mg/kg GaM doses were injected SE in P. aeruginosa-infected thermally injured mice as described above (n = 10 mice/group). In contrast to the GaM-treated mice, a statistically significant difference in percent survival was observed only with the highest dose of GaN tested and only for the 2-day time point (Fig. 2B). Thus, given equivalent doses of elemental Ga, the GaM formulation appears to be significantly more efficacious than the GaN formulation in this mouse burn wound infection model.
GaM prevents P. aeruginosa proliferation in vivo.
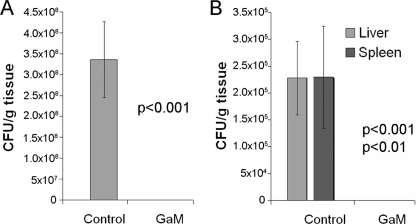
We have previously seen that by 24 h postburn/infection, P. aeruginosa CFU in the burn eschar increase from the infecting dose of 102 CFU/g tissue to approximately 109 CFU/g tissue and that the bacteria can also be isolated from the liver and spleen at this time (33, 35). We examined both the local colonization within the burn eschar and the systemic spread of P. aeruginosa in thermally injured/infected mice. We evaluated the local colonization of bacteria by determining the number of PAO1 CFU in tissue sections extracted from the burn eschars of mice treated with vehicle or GaM.
As in the mortality experiments, mice were thermally injured and inoculated with PAO1. Mice were administered SE injections of vehicle or 100 mg/kg GaM at the time of infection (n = 15/group). At 24 h postburn/infection, the mice were euthanized, burn eschar sections were isolated and homogenized, and the number of PAO1 CFU/g of tissue was determined. While the burn eschars of vehicle-treated mice were colonized with over 108 PAO1 CFU/g of tissue, no detectable bacteria were isolated from the eschars of GaM-treated mice (Fig. 3A). In concurrence with these data, P. aeruginosa was not detected in the livers or spleens of GaM-treated mice, while over 105 PAO1 CFU/g of tissue were detected in both the livers and spleens of mice treated with the vehicle control (Fig. 3B). These data indicate that GaM treatment effectively prevents P. aeruginosa from proliferating in vivo locally, at the burn wound, and systemically.
FIG. 3.
GaM prevents the local proliferation and systemic spread of P. aeruginosa. Mice were anesthetized, thermally injured, and inoculated with PAO1 and the vehicle control or 100 mg/kg GaM. Mice were sacrificed at 24 h postburn/infection. The livers, spleens, and sections of burn eschar were taken from each mouse and homogenized. The samples were diluted and plated on selective media to determine the number of PAO1 microorganisms (CFU)/g tissue in the burn eschar (A) or liver and spleen (B). Values represent the average of 15 mice/group ± standard errors of the mean, and significance was determined using Student's t test. The data shown represent three independent experiments.
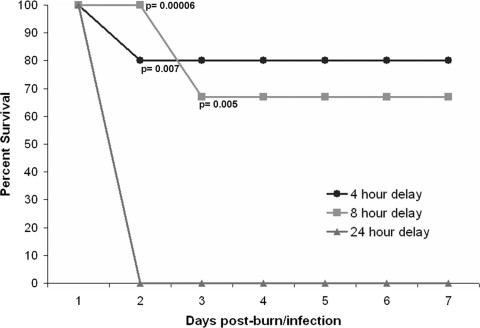
GaM prevents systemic infection in thermally injured mice already colonized with P. aeruginosa.
The above data indicate that GaM treatment prevents P. aeruginosa proliferation in thermally injured mice when the burn wound is dosed with GaM at the time of infection (Fig. 3A). However, human burn wounds are often already colonized by P. aeruginosa, originating from the patient's own flora or the environment, when they seek treatment (9). Therefore, we sought to elucidate the period after infection at which the application of GaM treatment could still significantly improve survival of P. aeruginosa-infected thermally injured mice. Mice were burned and infected with PAO1 as before. At 4, 8, or 24 h postburn/infection, mice were administered an SE injection of 25 mg/kg GaM. The mice were observed over 7 days postburn/infection, and the percent survival was recorded for each group (Fig. 4). GaM treatment delivered 4 and 8 h post-thermal injury/infection, with a second dose given at 24 h post-thermal injury/infection, significantly improved survival to 80% (n = 10; P = 0.007) and 67% (n = 15; P = 0.005), respectively. However, delaying GaM treatment by 24 h resulted in mortality values that were identical to those of untreated thermally injured and infected mice (n = 10). An infecting P. aeruginosa dose of 102 CFU/g tissue proliferates to 106 CFU/g tissue by 8 h in the untreated post-burn wound eschar (35). Therefore, our data indicate that GaM treatment not only prevents P. aeruginosa proliferation but can prevent subsequent systemic infection and death in mice that are already colonized with P. aeruginosa.
FIG. 4.
GaM prevents systemic infection in thermally injured mice with colonized P. aeruginosa. Groups of mice were thermally injured, inoculated with PAO1, allowed to recover, and observed over 7 days. SE injections of 25 mg/kg GaM were given at 4, 8, or 24 h postburn/infection (n = 10, 15, and 5, mice, respectively). The 4- and 8-h-delay groups were given a second 25-mg/kg SE injection of GaM at 24 h postburn/infection. Percent survival was assessed at 24-h time points. The data shown represent three independent experiments. The 4- and 8-h-delay groups displayed significantly higher percent survival by the termination of the experiment (P = 0.007 and 0.005, respectively; Fisher's exact test).
GaM reduces S. aureus and A. baumannii colonization of burn wounds.
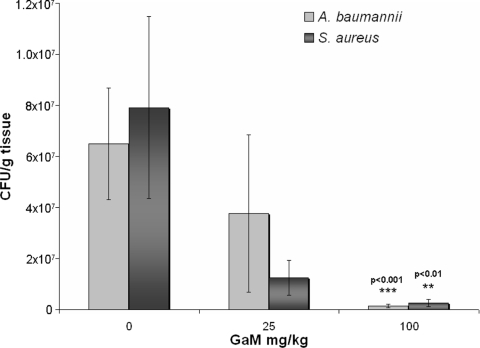
In addition to P. aeruginosa, S. aureus and A. baumannii are two of the most frequent colonizers of burn wounds and causes of nosocomial infections (9, 13). An antibacterial agent effective against all three opportunistic pathogens would, therefore, be highly desirable. A. baumannii in particular has been extremely problematic for wounded military personnel in Afghanistan and Iraq (6). We first tested the virulence of S. aureus and A. baumannii in the thermally injured mouse model. Even at infecting doses up to 107 CFU, we did not observe mortality or systemic infection as evidenced by bacterial growth in the liver or spleen (data not shown). Unlike P. aeruginosa, these strains are nonmotile, which may contribute to their inability to cause bacteremia and fatal infections in the mouse burn model. However, starting at a low infecting dose (102 to 103 CFU) both strains proliferated in the burn wound environment and resulted in the chronic colonization of the burn wound. Therefore, we sought to determine if GaM could prevent or significantly reduce S. aureus or A. baumannii burn wound proliferation and colonization. Mice were burned and infected as described above with S. aureus or A. baumannii, and GaM was administered SE at the time of infection and 24 h postburn/infection. Mice were sacrificed, and the number of CFU/g tissue in the burn eschar was determined at 48 h postburn/infection. Although GaM was not able to completely prevent the growth of these two pathogens as it does with P. aeruginosa, it significantly inhibited the growth of both S. aureus and A. baumannii at a dose of 100 mg/kg (Fig. 5). Therefore, GaM has the potential to treat both gram-negative and gram-positive wound pathogens.
FIG. 5.
GaM inhibition of S. aureus and A. baumannii burn wound colonization. Mice were anesthetized, thermally injured, and inoculated SE with S. aureus or A. baumannii and the vehicle control or 25 or 100 mg/kg GaM. A second dose of GaM was given at 24 h postburn/infection. At 48 h postburn/infection, mice were sacrificed and the number of CFU/g of burn eschar was determined. Values represent the average of 12 to 16 mice/group ± standard error, and significance was determined using the Kruskal-Wallis test (nonparametric analysis of variance), followed by Dunn's multiple comparisons test. The data shown represent at least three independent experiments.
DISCUSSION
P. aeruginosa is an opportunistic pathogen that causes severe mortality and morbidity to individuals with burn wounds. One of the hallmarks of P. aeruginosa infection is its ability to rapidly proliferate in the burn wound, quickly reaching a threshold level of bacteria that then disseminate systemically, causing a bacteremic infection with mortality levels over 75% (26). Therefore, a crucial step in preventing lethal or chronic P. aeruginosa infection is to halt its colonization at the site of the thermal injury. Rapid, topical antimicrobial prophylaxis is currently the primary strategy used for treating burn wounds (28). Antimicrobials that can be applied at the site of injury are chosen specifically to reduce microbial counts and to prevent systemic infection. For example, the metal silver has been used in treating wounds for hundreds of years and is still considered the “gold standard” for early burn injury treatment (23). Silver nitrate and silver sulfadiazine are applied topically and inhibit bacterial and fungal growth by the precipitation of microbial cell wall and cell membrane proteins by silver ions (23, 24). However, the side effects of commonly used silver formulations include adverse local skin reactions, including pain and burning (28). The low eschar-penetrating properties of silver-based topical medications mean that they are most effective early, and their efficacy dramatically decreases once bacteria have spread to underlying tissues.
In our study GaM, was not applied topically but rather SE. This was because P. aeruginosa cells are also injected SE in our thermally injured mouse model. The model design is intended to mimic an acute burn infection, where bacteria have already penetrated the eschar. In most human burn wound infections, bacteria such as P. aeruginosa would initially colonize the skin surface, but may spread to underlying tissues after wound debridement and removal of necrotic tissue infection. Therefore, GaM given locally at the burn wound could potentially protect against local colonization and systemic spread and bacteremia in humans, as in mice. In our study, GaM was 100% effective at preventing P. aeruginosa proliferation in the burn wound and subsequent bacteremia, at doses as low as 25 mg/kg. These results were seen when GaM was given once at the time of infection and again 24 h after infection. Only two GaM doses were given because no bacteria were detected in the burn eschar of mice treated with 25 to 100 mg/kg at 24 h postburn/infection. However, it is possible that the same levels of efficacy could be reached by administering lower doses of GaM for longer durations, such as once daily for 7 to 10 days.
We also demonstrated that GaM not only was effective at preventing initial proliferation but also was efficacious in preventing mortality after P. aeruginosa had colonized the burn wound (Fig. 4). GaM dosed up to 8 h post-thermal injury/infection significantly improved survival in this acute infection model. We have previously shown that by 8 h postinfection, P. aeruginosa had colonized the burn wound, reaching a density of approximately 106 CFU/g tissue (35). Our data also indicated that GaM was not efficacious when dosed 24 h postburn/infection (Fig. 4). This is presumably because the mice already had systemic infections for which the local, topical delivery of GaM would not be very effective. We have previously shown that by 24 h post-thermal injury/infection, P. aeruginosa was present in the liver, spleen, and blood (17, 18, 35). Thus, even the most aggressive topical application-based therapies may not resolve the infection at this point. However, for these experiments, only a single dose of 25 mg/kg GaM was given, and it is possible that higher or more frequent doses could extend the time at which effective therapy could be delivered.
While we have demonstrated that GaM effectively prevents P. aeruginosa proliferation and kills established P. aeruginosa cells in burn wounds, it must also be considered that most human wound infections are not homogenous and may be infected with many different types of microbes. As Ga3+ mimics Fe3+, an element essential for the growth of most microbes, it may demonstrate broad-spectrum antibacterial coverage. In support of this hypothesis, we observed that it was also effective against S. aureus and A. baumannii. Additionally, while GaM is efficacious in treating acute infections in burn wounds, many more wounds are chronic and more difficult to treat. For example, approximately 20 to 25% of diabetic patients will develop foot ulceration during the course of their disease (32). In fact, diabetic foot ulcers are considered the most significant wound care problem in the world, with the cost of care measured in billions of dollars (11). Hospital admissions of patients with foot ulcers are often prolonged by infection, gangrene, and lower extremity amputation and account for more in-hospital days than any other complication of diabetes (5). Several factors contribute to the difficulty of treating these infections, chief among them the presence of polymicrobic infections that resist many current therapeutics. Studies to examine the efficacy of GaM against polymicrobial infections in the thermal injury setting are ongoing.
Our data with GaM are consistent with published observations in murine lung models, where the Ga(NO3)3 formulation was shown to prevent P. aeruginosa proliferation and to kill P. aeruginosa within biofilm (22). However, we demonstrated that GaM was efficacious at significantly lower Ga concentrations than Ga(NO3)3. We hypothesize that the increased lipophilicity of the GaM formulation and its reduced propensity to form insoluble gallate precipitates may account for the observed significantly greater efficacy over the Ga(NO3)3 formulation. As Ga(NO3)3 has been linked to issues of nephrotoxicity in the past, a safe and efficacious Ga-containing formulation to treat a broad spectrum of microbes present in a bacterial biofilm and resistant to conventional antibiotic therapy would be highly desirable. GaM delivered topically, subcutaneously, and/or locally at the site of infection may potentially be beneficial for the treatment of P. aeruginosa and other pathogenic bacterial infections.
Acknowledgments
This work was supported in part by an American Diabetes Association grant to K.P.R.
Footnotes
Published ahead of print on 2 February 2009.
REFERENCES
- 1.American Burn Association. 2007. Burn incidence and treatment in the US: fact sheet. American Burn Association, Chicago, IL.
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1988. Current protocols in molecular biology. Wiley Interscience, New York, NY.
- 3.Bernstein, L. R. 1998. Mechanisms of therapeutic activity for gallium. Pharmacol. Rev. 50:665-682. [PubMed] [Google Scholar]
- 4.Bernstein, L. R., T. Tanner, C. Godfrey, and B. Noll. 2000. Chemistry and pharmacokinetics of gallium maltolate, a compound with high oral gallium bioavailability. Met.-Based Drugs 7:33-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bild, D. E., J. V. Selby, P. Sinnock, W. S. Browner, P. Braveman, and J. A. Showstack. 1989. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 12:24-31. [DOI] [PubMed] [Google Scholar]
- 6.Calhoun, J. H., C. K. Murray, and M. M. Manring. 2008. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin. Orthop. Relat. Res. 466:1356-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chitambar, C. R., D. P. Purpi, J. Woodliff, M. Yang, and J. P. Wereley. 2007. Development of gallium compounds for treatment of lymphoma: gallium maltolate, a novel hydroxypyrone gallium compound, induces apoptosis and circumvents lymphoma cell resistance to gallium nitrate. J. Pharmacol. Exp. Ther. 322:1228-1236. [DOI] [PubMed] [Google Scholar]
- 8.Chua, M. S., L. R. Bernstein, R. Li, and S. K. So. 2006. Gallium maltolate is a promising chemotherapeutic agent for the treatment of hepatocellular carcinoma. Anticancer Res. 26:1739-1743. [PubMed] [Google Scholar]
- 9.Church, D., S. Elsayed, O. Reid, B. Winston, and R. Lindsay. 2006. Burn wound infections. Clin. Microbiol. Rev. 19:403-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collery, P., B. Keppler, C. Madoulet, and B. Desoize. 2002. Gallium in cancer treatment. Crit. Rev. Oncol. Hematol. 42:283-296. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham, A. B. 2006. Biofilms: the hypertextbook. Montana State University, Bozeman, MT.
- 12.Flamm, R. K., M. K. Weaver, C. Thornsberry, M. E. Jones, J. A. Karlowsky, and D. F. Sahm. 2004. Factors associated with relative rates of antibiotic resistance in Pseudomonas aeruginosa isolates tested in clinical laboratories in the United States from 1999 to 2002. Antimicrob. Agents Chemother. 48:2431-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gootz, T. D., and A. Marra. 2008. Acinetobacter baumannii: an emerging multidrug-resistant threat. Expert Rev. Anti-Infect. Ther. 6:309-325. [DOI] [PubMed] [Google Scholar]
- 14.Harrington, J. R., R. J. Martens, N. D. Cohen, and L. R. Bernstein. 2006. Antimicrobial activity of gallium against virulent Rhodococcus equi in vitro and in vivo. J. Vet. Pharmacol. Ther. 29:121-127. [DOI] [PubMed] [Google Scholar]
- 15.Hart, M. M., and R. H. Adamson. 1971. Antitumor activity and toxicity of salts of inorganic group 3a metals: aluminum, gallium, indium, and thallium. Proc. Natl. Acad. Sci. USA 68:1623-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart, M. M., C. F. Smith, S. T. Yancey, and R. H. Adamson. 1971. Toxicity and antitumor activity of gallium nitrate and periodically related metal salts. J. Natl. Cancer Inst. 47:1121-1127. [PubMed] [Google Scholar]
- 17.Haynes, A., III, F. Ruda, J. Oliver, A. N. Hamood, J. A. Griswold, P. W. Park, and K. P. Rumbaugh. 2005. Syndecan 1 shedding contributes to Pseudomonas aeruginosa sepsis. Infect. Immun. 73:7914-7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haynes, A., III, K. P. Rumbaugh, P. W. Park, A. N. Hamood, and J. A. Griswold. 2005. Protamine sulfate reduces the susceptibility of thermally injured mice to Pseudomonas aeruginosa infection. J. Surg. Res. 123:109-117. [DOI] [PubMed] [Google Scholar]
- 19.Holder, I. A. 1993. Pseudomonas aeruginosa burn infections: pathogenesis and treatment, p. 275-295. In M. B. M. Campa and H. Friedman (ed.), Pseudomonas aeruginosa as an opportunistic pathogen. Plenum Press, New York, NY.
- 20.Holder, I. A. 1993. Pseudomonas aeruginosa virulence-associated factors and their role in burn wound infections, p. 235-245. In R. B. Fick (ed.), Pseudomonas aeruginosa: the opportunist. CRC Press, Boca Raton, FL.
- 21.Holloway, B. W., V. Krishnapillai, and A. F. Morgan. 1979. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 43:73-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko, Y., M. Thoendel, O. Olakanmi, B. E. Britigan, and P. K. Singh. 2007. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 117:877-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klasen, H. J. 2000. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 26:117-130. [DOI] [PubMed] [Google Scholar]
- 24.Lansdown, A. B. 2006. Silver in health care: antimicrobial effects and safety in use. Curr. Probl. Dermatol. 33:17-34. [DOI] [PubMed] [Google Scholar]
- 25.Martens, R. J., K. Mealey, N. D. Cohen, J. R. Harrington, M. K. Chaffin, R. J. Taylor, and L. R. Bernstein. 2007. Pharmacokinetics of gallium maltolate after intragastric administration in neonatal foals. Am. J. Vet. Res. 68:1041-1044. [DOI] [PubMed] [Google Scholar]
- 26.McManus, A. T., A. D. Mason, Jr., W. F. McManus, and B. A. Pruitt, Jr. 1985. Twenty-five year review of Pseudomonas aeruginosa bacteremia in a burn center. Eur. J. Clin. Microbiol. 4:219-223. [DOI] [PubMed] [Google Scholar]
- 27.National Nosocomial Infections Surveillance System. 2004. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32:470-485. [DOI] [PubMed] [Google Scholar]
- 28.Noronha, C., and A. Almeida. 2000. Local burn treatment—topical antimicrobial agents. Ann. Burns Fire Disasters 13:216-220. [Google Scholar]
- 29.Norrby, S. R., C. E. Nord, and R. Finch. 2005. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect. Dis. 5:115-119. [DOI] [PubMed] [Google Scholar]
- 30.Pollack, M. 2000. Pseudomonas aeruginosa, p. 2310-2335. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Churchill Livingstone, Philadelphia, PA.
- 31.Potokar, T. S., S. Ali, S. Chamania, S. Prowse, and I. S. Whitaker. 2008. A global overview of burns research highlights the need for forming networks with the developing world. Burns 34:3-5. [DOI] [PubMed] [Google Scholar]
- 32.Reiber, G. E. 1996. The epidemiology of diabetic foot problems. Diabet. Med. 13(Suppl. 1):S6-S11. [PubMed] [Google Scholar]
- 33.Rumbaugh, K. P., J. A. Colmer, J. A. Griswold, and A. N. Hamood. 2001. The effects of infection of thermal injury by Pseudomonas aeruginosa PAO1 on the murine cytokine response. Cytokine 16:160-168. [DOI] [PubMed] [Google Scholar]
- 34.Rumbaugh, K. P., J. A. Griswold, and A. N. Hamood. 1999. Contribution of the regulatory gene lasR to the pathogenesis of Pseudomonas aeruginosa infection of burned mice. J. Burn Care Rehabil. 20:42-49. [DOI] [PubMed] [Google Scholar]
- 35.Rumbaugh, K. P., J. A. Griswold, B. H. Iglewski, and A. N. Hamood. 1999. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect. Immun. 67:5854-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaber, J. A., W. J. Triffo, S. J. Suh, J. W. Oliver, M. C. Hastert, J. A. Griswold, M. Auer, A. N. Hamood, and K. P. Rumbaugh. 2007. Pseudomonas aeruginosa forms biofilms in acute infection independent of cell-to-cell signaling. Infect. Immun. 75:3715-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stieritz, D. D., and I. A. Holder. 1975. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J. Infect. Dis. 131:688-691. [DOI] [PubMed] [Google Scholar]
- 38.Vindenes, H., and R. Bjerknes. 1995. Microbial colonization of large wounds. Burns 21:575-579. [DOI] [PubMed] [Google Scholar]