Abstract
Although information about the efficacy and safety experience with caspofungin at 50 mg/m2 daily is available for children and adolescents, the dosing regimen in infants and toddlers 3 to 24 months of age has yet to be established. We studied the pharmacokinetics and safety of caspofungin at 50 mg/m2 once daily in nine patients 10 to 22 months (median, 13 months) of age with fever and neutropenia who received caspofungin once daily for 2 to 21 (mean, 9.3) days. Plasma caspofungin concentrations were measured by high-performance liquid chromatography assay on days 1 and 4. On day 4, the area under the curve from 0 to 24 h (AUC0-24) was 130.3 μg-h/ml, the peak concentration (C1) was 17.2 μg/ml, and the trough concentration (C24) was 1.6 μg/ml. The day 4 geometric mean ratios (GMRs) and 90% confidence interval (CI) for these parameters in infants/toddlers relative to adults were 1.26 (1.06, 1.50), 1.83 (1.57, 2.14), and 0.81 (0.64, 1.04), respectively. Relative to children (2 to 11 years of age), the day 4 GMRs (and 90% CI) were 1.13 (0.89, 1.44), 1.10 (0.85, 1.42), and 1.12 (0.72, 1.76), respectively. The harmonic mean elimination phase t1/2 in infants/toddlers (8.8 h) was reduced ∼33% relative to adults (13.0 h) but was similar to that in children (8.2 h). Clinical adverse events occurred in seven patients (78%); none were considered drug related. Laboratory adverse events occurred in five patients (56%) and were considered drug related in three (33%). There were no infusion-related events or discontinuations due to toxicity. Caspofungin at 50 mg/m2 daily was well tolerated in infants and toddlers; the AUC and caspofungin C24 were generally comparable to those in adults receiving caspofungin at 50 mg daily.
Incidence rates of Candida and Aspergillus infections are increasing in children with hematological malignancies, congenital immune deficiencies, and other childhood medical conditions that require aggressive immunosuppressive or antibiotic therapy (1, 9, 11). Childhood leukemia and lymphoma and their associated chemotherapy regimens result in prolonged periods of neutropenia that increase the risk of serious fungal infection (21). The use of hematopoietic stem cell transplantation for the treatment of childhood leukemia and lymphoma has also increased in frequency over the last 2 decades; the profound cytopenia and immunosuppression induced by hematopoietic stem cell transplantation place these children at high risk for fungal infections (5, 10). Bloodstream infections with Candida are also occurring at increasing rates in other children with similar hospital exposures and risks, such as central venous catheters, broad-spectrum antibiotics, total parenteral nutrition, and immunosuppressive therapy. In particular, younger children (including neonates) have a high risk of invasive infections with Candida parapsilosis (17).
Caspofungin is a parenteral echinocandin antifungal agent used in adults for the treatment of esophageal candidiasis (20), invasive candidiasis (6, 14), and invasive aspergillosis (12) and as empirical therapy against presumed fungal infections (22). The pharmacokinetics of caspofungin have been evaluated in children and adolescents 2 to 17 years of age (23), and a dosing regimen using a body surface area (BSA) approach of 50 mg/m2 daily (maximum, 70 mg) has been established for this pediatric age group. We conducted a multicenter, open-label study to evaluate the safety, tolerability, and pharmacokinetics of the same dosing regimen of caspofungin in infants and toddlers 3 to 24 months of age.
MATERIALS AND METHODS
This was a multicenter, open-label, noncomparative study conducted from May 2004 through July 2006 at five investigator sites in the United States. The protocol was approved by the institutional review board at each study site, and written informed consent was obtained from the legal guardian of each patient before any study procedures were performed. The protocol is registered on http://www.Clinicaltrials.gov (NCT00292071).
Patients.
Infants and toddlers between the ages of 3 and 24 months with leukemia, lymphoma, or other cancers; bone marrow or peripheral stem cell transplantation; high-dose chemotherapy; or aplastic anemia were eligible for the study if they had an absolute neutrophil count of <500/mm3 (with an anticipated duration of ≥10 days) and at least one recording of fever of >38.0°C within 72 h of screening. Patients were required to have a functioning central venous catheter in place at screening and to start parenteral systemic antibacterial therapy within 72 h prior to screening or within 24 h after screening. Patients were excluded if they were hemodynamically unstable; if they were taking rifampin (rifampicin), cyclosporine A, phenytoin, carbamazepine, phenobarbital, or concomitant antifungal therapy (other than prophylactic fluconazole); or if they had any of the following conditions: proven or probable invasive fungal infection at the time of enrollment, international normalization ratio of >1.6 (or >4.0 if receiving anticoagulants), bilirubin at >3 times the upper limit of normal for their age, aspartate aminotransferase (AST) or alanine aminotransferase (ALT) at >3 times the upper limit of normal for their age, acute hepatitis or cirrhosis due to any cause, or documented human immunodeficiency virus infection of any stage.
Study design.
All patients received caspofungin at 50 mg/m2 once daily as a 1-h intravenous infusion, with a daily maximum dose of 70 mg. Caspofungin was administered daily until the patient recovered from the neutropenic episode (neutropenia resolution for this study was defined as an absolute neutrophil count [ANC] of ≥250/mm3). However, if the patient remained febrile and neutropenic after 4 days of therapy or developed a proven or probable breakthrough invasive fungal infection (according to criteria established in 2002 by the joint committee of the European Organization of Research and Treatment of Cancer and the Mycosis Study Group) (3), caspofungin was to be discontinued and an intravenous formulation of amphotericin B was to be administered. The expected minimum duration of caspofungin study therapy was 4 days, and the maximum duration was 28 days. Patients who received less than 4 days of caspofungin were not counted toward the final enrollment number.
Plasma samples were collected on days 1 and 4 at the following times: immediately predose; upon completion of the 1-h infusion; and at 2, 4, 8, 12, and 24 h after initiation of study drug infusion. A minimum of 1.0 ml of blood was collected at each time point. Trough (C24) samples were also collected 24 h after the start of infusion, just prior to the next day's dose, on days 7, 14, and 28 in patients who were still receiving caspofungin. For patients with a double-lumen central line, pharmacokinetic samples were to be collected from the lumen not used for caspofungin infusion. For patients with a single-lumen central line or peripherally inserted central catheter, all samples were to be drawn from a peripheral intravenous line inserted at the time of enrollment.
Pharmacokinetic methods.
Plasma samples were analyzed for caspofungin concentration by high-performance liquid chromatography with fluorescence detection, as previously described (4). The assay accuracy was within 1.7% of nominal, and the precision was a 5.7% coefficient of variation or better for all standards. The limit of quantitation was 125 ng/ml. Actual sampling times, as recorded by the investigator, were used for calculation of half-lives (t1/2) and area under the curve (AUC). Sampling time criteria were used to define acceptable deviations from the nominal time for end-of-infusion (peak[C1]) and C24 sampling. For C1, a sampling window from 20 min prior to 30 min after the end of infusion was used. For C24, a sampling window from 3 h prior to 3 h after the 24-h-postdose time point was used. Caspofungin pharmacokinetics were characterized using noncompartmental methods, consistent with approaches taken for previously studied older children (23) and adults (2, 8, 19).
AUC0-24 and CL.
The AUC from 0 to 24 h (AUC0-24) was calculated by the linear-log trapezoidal method. For concentration-time profiles with missing values, an AUC was estimated only if the following time points were available: 1 h, 2 h, 4 or 8 h, and 0 or 24 h. For day 1 profiles, a 24-h value was required for determination of the AUC. Estimates of clearance (CL) were determined as the quotients of dose and the day 1 AUC values from 0 h to infinity (AUC0-∞), where the AUC24-∞ was extrapolated as the C24/elimination phase (β) rate constant.
C1 and C24.
The C1 on days 1 and 4 was derived from the observed concentration in plasma immediately following the completion of the first and fourth caspofungin infusions, respectively. The C24 value on day 1 was derived from the observed concentration at 24 h following the first caspofungin infusion (and prior to the caspofungin infusion on day 2). For C24 measurements after day 1, the geometric mean of all values obtained during the time interval from days 3 to 14 was calculated to allow comparison to similar time-averaged parameters from other pharmacokinetic studies.
t1/2.
Although profile data are only available over 24 h in this study, an estimate of the β rate constant was calculated by linear regression of the natural log-transformed plasma concentration data at 8, 12, and 24 h as implemented in WinNonLin software (Pharsight, Mountain View, CA). t1/2 (β-phase) was computed as the quotient of ln(2) and the rate constant. No t1/2 estimates were made for profiles that did not have data at all three points. For t1/2 measurements after day 1, the day 3 to 14 time-averaged t1/2 was calculated as the harmonic mean, and the time-averaged β-rate constant was calculated as the arithmetic mean of all values obtained during the time interval. If only one value was available, that value was used as the mean.
Statistical methods.
The pharmacokinetic parameters (AUC0-24, C1, C24, CL, and t1/2) obtained from the pediatric patients enrolled in this study were compared to similar parameters from adult patients who received caspofungin at 50 or 70 mg daily, corresponding to a BSA dose of ∼28 mg/m2 or ∼40 mg/m2, respectively, for an average-size adult (2, 8, 19), and to those from children (2 to 11 years of age) and adolescents (12 to 17 years of age) who received caspofungin at 50 mg/m2 daily (maximum, 70 mg daily) for new-onset fever and neutropenia (23). The primary pharmacokinetic hypothesis, which compared the day 1 AUC0-24 in infants/toddlers receiving 50 mg/m2 daily to the day 1 AUC0-24 in adults receiving 50 mg daily, was addressed by calculating a 90% confidence interval (CI) for the day 1 AUC0-24 ratio of geometric means in infants/toddlers receiving caspofungin at 50 mg/m2 once daily relative to adults receiving caspofungin at 50 mg once daily. If the 90% CI for the AUC ratio was within the interval (0.7, 1.5), the hypothesis of similarity was supported.
The day 1 AUC0-24 data were natural-log transformed. A 90% CI for the difference in day 1 log AUC0-24 means was calculated using the mean square error (MSE) from the analysis of variance and referencing a t distribution. These limits were exponentiated to obtain the 90% CI for the ratio of day 1 AUC0-24 geometric means. Similar methods were used to compare the day 1 AUC0-24 in infants/toddlers receiving 50 mg/m2 daily to the day 1 AUC0-24 in adults receiving 70 mg daily. Assuming that the variability in day 1 AUCs in these infants/toddlers was similar to that seen in adults, the MSE for log AUC obtained from an analysis of variance having a factor for dose was calculated to be 0.0556 (log) μg·h/ml. Assuming an MSE for log AUC0-24 of 0.0556 (log scale), a two-tailed test, and an α of 0.025, the probability was 97.8% that the 90% CI for the ratio of day 1 AUC0-24 geometric means (infants/toddlers at 50 mg/m2 daily to adults at 50 mg daily [or 70 mg daily]) would lie within the interval (0.7, 1.5) if the true ratio of geometric means was 1.0. Provided that the true GMR was contained in the range of (0.845, 1.184), then the study had at least 80% probability to fall within (0.7, 1.5).
For the analysis of AUC0-24 (and CL and C1) after multiple dosing, the geometric mean of the AUC0-24 (or CL or C1) values on day 4 was calculated for infants/toddlers receiving caspofungin at 50 mg/m2 daily. These data were compared with the time-averaged AUC0-24 (or CL or C1) values in adults, children, and adolescents in a fashion similar to that of the day 1 analysis. The 90% CIs were calculated for each ratio of geometric means.
For C24 comparisons, a time-averaged C24 value for infants/toddlers was calculated as the geometric mean of all C24 values obtained between days 3 and 14. These data were compared with time-averaged C24 values in adults, children, and adolescents by constructing the 90% CIs for each ratio of geometric means (as described above).
RESULTS
Nine patients were enrolled in the study, and all received caspofungin at 50 mg/m2 once daily. Their ages ranged from 10 to 22 months (median, 13 months); four patients were ≤12 months of age, and five were >12 months of age (Table 1). Patient weights ranged from 9.4 to 11.9 kg (median, 11.0 kg), and BSAs ranged from 0.45 to 0.53 m2 (median, 0.49 m2). The most common primary condition was acute myelogenous leukemia (44.4%). Three patients (33.3%) were recipients of cord blood transplants. Most patients (77.8%) entered the study with severe neutropenia (ANC < 100 cells/mm3). The mean duration of caspofungin therapy was 9.6 days (range, 2 to 21 days). Caspofungin was given for more than 7 days to five patients (55.6%), two of whom received caspofungin for more than 14 days.
TABLE 1.
Baseline patient characteristicsa
| Characteristic | n | % |
|---|---|---|
| Gender | ||
| Male | 6 | 66.7 |
| Female | 3 | 33.3 |
| Race | ||
| American Indian or Alaska Native | 3 | 33.3 |
| White | 6 | 66.7 |
| Age (mo) | ||
| 3-6 | 0 | 0.0 |
| 7-12 | 4 | 44.4 |
| 13-18 | 3 | 33.3 |
| 19-24 | 2 | 22.2 |
| Mean (SD) | 15.0 (4.2) | |
| Median (range) | 13.0 (10-22) | |
| Primary condition | ||
| Acute leukemia | ||
| Acute lymphocytic leukemia | 1 | 11.1 |
| Acute myelogenous leukemia | 4 | 44.4 |
| Solid tumor | ||
| Neuroblastoma | 1 | 11.1 |
| Neuroectodermal tumor | 1 | 11.1 |
| Other | ||
| Wiskott-Aldrich syndrome | 1 | 11.1 |
| X-linked chromosomal disorder | 1 | 11.1 |
| Transplant type | ||
| Allogeneic cord blood transplant | 3 | 33.3 |
| ANC (cells/ml) | ||
| < 100 | 7 | 77.8 |
| 100-250 | 2 | 22.2 |
| 251-500 | 0 | 0.0 |
Nine patients were enrolled in the study, and all received caspofungin at 50 mg/m2 once daily.
Pharmacokinetics.
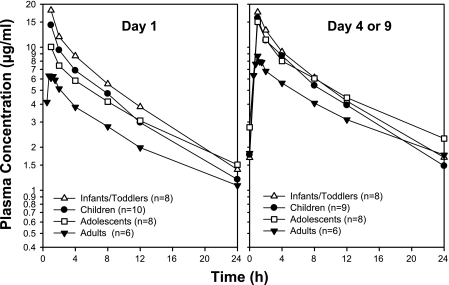
The day 1 AUC0-24 was higher in the infants/toddlers than in adult historical controls who received caspofungin at 50 mg daily (P < 0.001), whereas the day 4 AUC0-24 in infants was similar to the day 3 to 14 AUC0-24 in these adults (P = 0.033) (Table 2). Statistically significant elevations were noted in C1 in infants/toddlers relative to adults on day 1 (128% increase; P < 0.001) and on day 4 (83% increase; P < 0.001 versus days 3 to 14 in adults). However, the difference in C24 between the two populations was not significant on day 1 (P = 0.995) or after multiple doses (P = 0.168). A comparison of the mean plasma profiles in infants/toddlers and adults demonstrates that the rate of decline in the mean plasma concentration-time profiles during the β-phase appeared to be faster in infants/toddlers than in adults (Fig. 1). Consistent with this observation, reductions in β-phase t1/2 were observed in infants/toddlers relative to adults on day 1 (33% decrease) and after multiple doses (32% decrease).
TABLE 2.
Caspofungin pharmacokinetics in infants/toddlers receiving 50 mg/m2 once daily and adults receiving 50 or 70 mg once daily
| Parameter | Infants/toddlers (50 mg/m2)
|
Adults (50 mg daily)
|
GMR (90% CI)b (infants/toddlers: adults [50 mg]) | Adults (70 mg daily)
|
GMR (90% CI)b (infants/toddlers: adults [70 mg] | |||
|---|---|---|---|---|---|---|---|---|
| na | LSM (95% CI)b | n | LSM (95% CI)b | n | LSM (95% CI)b | |||
| Day 1 | ||||||||
| AUC0-24 (μg·h/ml) | 7 | 120.20 (100.93, 143.15) | 32 | 70.60 (65.06, 76.61) | 1.70 (1.45, 2.00) | 29 | 97.13 (89.14, 105.84) | 1.24 (1.05, 1.46) |
| C1 (μg/ml) | 9 | 17.46 (14.75, 20.68) | 38 | 7.67 (7.07, 8.33) | 2.28 (1.95, 2.66) | 37 | 10.04 (9.24, 10.92) | 1.74 (1.49, 2.04) |
| C24 (μg/ml) | 7 | 1.34 (1.03, 1.76) | 33 | 1.35 (1.19, 1.52) | 1.00 (0.78, 1.28) | 29 | 1.95 (1.71, 2.22) | 0.69 (0.54, 0.89) |
| β-Phase t1/2 (h) | 7 | 7.79 (2.31)c | 6 | 11.70 (2.92)c | 6 | 12.25 (2.84)c | ||
| CL (ml/min/m2) | 7 | 6.05 (4.92, 7.45) | 6 | 6.07 (4.85, 7.59) | 1.00 (0.77, 1.29) | 6 | 5.20 (4.16, 6.51) | 1.16 (0.90, 1.50) |
| Day 4 or 3 to 14 time averagedd | ||||||||
| AUC0-24 (μg·h/ml) | 8 | 130.29 (107.46, 157.96) | 38 | 103.38 (94.63, 112.93) | 1.26 (1.06, 1.50) | 35 | 153.65 (140.13, 168.47) | 0.85 (0.71, 1.01) |
| C1 (μg/ml) | 8 | 17.21 (14.56, 20.36) | 38 | 9.39 (8.69, 10.14) | 1.83 (1.57, 2.14) | 35 | 13.32 (12.30, 14.44) | 1.29 (1.11, 1.51) |
| C24 (μg/ml) | 8 | 1.64 (1.24, 2.16) | 60 | 2.01 (1.82, 2.22) | 0.81 (0.64, 1.04) | 57 | 3.33 (3.00, 3.69) | 0.49 (0.38, 0.63) |
| β-Phase t1/2 (h) | 8 | 8.80 (2.10)c | 5 | 13.00 (1.91)c | 5 | 16.46 (7.04)c | ||
n, number of patients included in the analysis.
Least square means (LSM) and GMRs are reported for AUC0-24, C1, and C24.
Harmonic means (jackknife standard deviation) are reported for β-phase t1/2.
Time-averaged parameters were determined as the geometric means of all values obtained between days 3 and 14. The day 4 AUC0-24, C1, and β-phase t1/2 for infants/toddlers were compared to day 3 to 14 time-averaged AUC0-24, C1, and β-phase t1/2 for adult controls. The day 3 to 14 time-averaged C24 for infants/toddlers were compared to the day 3 to 14 time-averaged C24 for adult controls.
FIG. 1.
Mean plasma caspofungin concentrations in infants/toddlers (10 to 24 months), children (2 to 11 years), and adolescents (12 to 17 years) receiving caspofungin at 50 mg/m2 once daily and adults receiving caspofungin at 50 mg once daily.
The day 1 AUC0-24 in infants/toddlers was significantly higher than in adults receiving caspofungin at 70 mg daily (Table 2) for the treatment of esophageal and/or oropharyngeal candidiasis (24% increase; P = 0.033). However, the day 4 AUC0-24 in infants/toddlers was not significantly different from the day 3 to 14 AUC0-24 in these adults (P = 0.128). C1 was significantly higher in the infants/toddlers than in the adults on day 1 (74% increase; P < 0.001) and after multiple doses (29% increase; P < 0.001).
Slightly higher AUC0-24, C1, and C24 values were noted in infants/toddlers than in children 2 to 11 years of age (Table 3), but none of these differences were statistically significant (P > 0.2). The mean plasma profile for caspofungin in infants/toddlers is generally similar to the profile obtained from children (Fig. 1). The shapes of the mean plasma concentration-time profiles in children and infants/toddlers appeared similar, and similar β-phase t1/2 values were found in the two groups. Compared with adolescents (Table 3), infants/toddlers had similar values for AUC0-24, C1, and C24 following multiple doses, but they had significantly higher values for AUC0-24 (55%; P = 0.049) and C1 (95%; P < 0.001) on day 1. This result is consistent with infants and toddlers exhibiting less caspofungin accumulation (Table 2, compare day 1 and day 4 values) than adolescents and adults, which is not unexpected given their shorter t1/2. Reductions in β-phase t1/2 were also observed in infants/toddlers relative to adolescents (26% decrease on day 1 and 21% decrease after multiple doses).
TABLE 3.
Caspofungin Pharmacokinetics in Infants/Toddlers, Children, and Adolescents Receiving 50 mg/m2 Once Daily
| Parameter | Infants/toddlers (ages 3-24 mo)
|
Children (ages 2-11 yr)
|
GMR (90% CI)b (infants/toddlers: children) | Adolescents (ages 12-17 yr)
|
GMR (90% CI)b (infants/toddlers: adolescents) | |||
|---|---|---|---|---|---|---|---|---|
| na | LSM (95% CI)b | n | LSM (95% CI)b | n | LSM (95% CI)b | |||
| Day 1 | ||||||||
| AUC0-24 (μg·h/ml) | 7 | 120.20 (88.39, 163.45) | 9 | 96.40 (73.51, 126.42) | 1.25 (0.89, 1.75) | 7 | 77.58 (57.05, 105.50) | 1.55 (1.08, 2.22) |
| C1 (μg/ml) | 9 | 17.46 (13.90, 21.93) | 10 | 13.99 (11.27, 17.37) | 1.25 (0.96, 1.62) | 8 | 8.95 (7.03, 11.40) | 1.95 (1.48, 2.57) |
| C24 (μg/ml) | 7 | 1.34 (0.87, 2.08) | 9 | 1.09 (0.74, 1.60) | 1.24 (0.76, 2.00) | 7 | 1.26 (0.81, 1.95) | 1.07 (0.64, 1.78) |
| β-Phase t1/2 (h) | 7 | 7.79 (2.31)c | 9 | 7.63 (1.61)c | 7 | 10.51 (2.81)c | ||
| CL (ml/min/m2) | 7 | 6.05 (4.92, 7.45) | 10 | 7.78 (6.54, 9.26) | 0.78 (0.62, 0.97) | 8 | 6.30 (5.19, 7.66) | 0.96 (0.76, 1.22) |
| Day 4 or 3 to 14 time averagedd | ||||||||
| AUC0-24 (μg·h/ml) | 8 | 130.29 (105.26, 161.26) | 9 | 115.23 (94.24, 140.89) | 1.13 (0.89, 1.44) | 8 | 117.19 (94.68, 145.04) | 1.11 (0.87, 1.43) |
| C1 (μg/ml) | 8 | 17.21 (13.76, 21.54) | 9 | 15.61 (12.64, 19.28) | 1.10 (0.85, 1.42) | 8 | 12.90 (10.31, 16.14) | 1.33 (1.03, 1.74) |
| C24 (μg/ml) | 8 | 1.64 (1.10, 2.43) | 9 | 1.46 (1.00, 2.12) | 1.12 (0.72, 1.76) | 8 | 2.15 (1.45, 3.20) | 0.76 (0.48, 1.21) |
| β-Phase t1/2 (h) | 8 | 8.80 (2.10)c | 9 | 8.21 (2.35)‡ | 8 | 11.20 (1.71)c | ||
n, number of patients included in the analysis.
Least square means (LSM) and GMRs are reported for AUC0-24, C1, and C24.
Harmonic means (jackknife standard deviation) are reported for β-phase t1/2.
Time-averaged parameters were determined as the geometric means of all values obtained between days 3 and 14. The day 4 AUC0-24, C1, and β-phase t1/2 for infants/toddlers were compared to day 3 to 14 time-averaged AUC0-24, C1, and β-phase t1/2 for children ages 2 to 11 years. The day 3 to 14 time-averaged C24 for infants/toddlers were compared to the day 3 to 14 time-averaged C24 for children ages 2 to 11 years.
The individual pharmacokinetic-parameter values, including clearance values, demonstrate low variation in caspofungin pharmacokinetics among the infants/toddlers (Table 4). The variation in pharmacokinetics by patient age and weight was also examined in the infants by calculating the slope (β1) of the relationship between the covariate (i.e., age or weight) and the log-transformed pharmacokinetics. No statistically significant variations with age were identified for the day 1 AUC0-24, C1, or C24 or for the day 3 to 14 AUC0-24 or C1. However, the day 3 to 14 C24 of caspofungin showed significant increases with increasing age (P = 0.018). No statistically significant variations with weight were identified for any pharmacokinetic parameters.
TABLE 4.
Individual pharmacokinetics of caspofungin in infants/toddlers
| Patient | Age (mo) | AUC0-24 (μg/ml·h)
|
C1 (μg/ml)
|
C24 (μg/ml)
|
β-Phase t1/2 (h)
|
CL (ml/min/m2)
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 4 | Day 1 | Day 4 | Day 1 | Day 3-14a | Day 1 | Day 4 | Day 1 | ||
| 1 | 12 | 109.4 | 135.0 | 17.53 | 24.79 | 0.65 | 0.94 | 5.02 | 10.51 | 7.30 |
| 2 | 10 | 159.6 | 114.0 | 14.47 | 15.17 | 2.38 | 1.55 | 9.00 | 9.15 | 4.37 |
| 3 | 12 | 119.5 | 122.0 | 18.26 | 15.94 | 1.03 | 1.26 | 6.64 | 6.17 | 6.44 |
| 4 | 13 | 121.4 | 15.75 | 16.87 | 1.48 | 8.35 | ||||
| 5 | 17 | 146.3 | 135.3 | 23.91 | 16.42 | 1.85 | 1.60 | 8.37 | 7.33 | 4.94 |
| 6 | 20 | 97.4 | 122.0 | 13.00 | 12.14 | 1.55 | 2.16 | 11.07 | 11.41 | 6.82 |
| 7 | 17 | 101.7 | 13.90 | 1.15 | 8.59 | 7.19 | ||||
| 8 | 12 | 128.9 | 22.30 | 17.96 | 1.72 | 8.58 | ||||
| 9 | 22 | 119.9 | 171.1 | 21.50 | 21.30 | 1.51 | 3.17 | 8.97 | 12.13 | 5.98 |
| Mean | 121.97 | 131.21 | 17.85 | 17.57 | 1.45 | 1.74 | 7.79b | 8.8b | 6.15 | |
| SD | 23.06 | 17.69 | 3.95 | 3.89 | 0.57 | 0.68 | 2.31b | 2.1b | 1.12 | |
| Geometric mean | 120.2 | 130.28 | 17.46 | 17.21 | 1.34 | 1.64 | 6.05c | |||
Time-averaged parameters determined as the geometric mean of all values obtained between days 3 and 14.
Harmonic means (jackknife standard deviations) are reported for β-phase t1/2.
95% CI for the geometric mean, (4.92, 7.45).
Safety.
All nine patients treated with caspofungin were included in the safety evaluations. Clinical adverse events occurred in seven patients (78%) and were considered serious in two (22%). One patient died 10 days after completion of caspofungin therapy; the cause of death was cytomegalovirus pneumonia. The most commonly reported clinical adverse events were diarrhea, respiratory distress, and tachypnea, which were reported in two patients (22%) each. None of these events were considered by the investigator to be related to caspofungin or resulted in discontinuation of caspofungin therapy.
Laboratory adverse events occurred in five patients (56%) and were considered drug related in three patients (33%): two with elevations in AST and ALT and one with increased blood glucose levels and decreased uric acid levels. Both patients with increased ALT and AST had elevated hepatic transaminases before caspofungin therapy, and these elevations improved even with continuation of caspofungin. None of the laboratory adverse events were serious or resulted in discontinuation of caspofungin. No infusion-related events were reported during the study.
Although efficacy was not specifically assessed in this study, patients were closely evaluated for the development of breakthrough invasive fungal infections. One (11.1%) of the nine patients developed a possible breakthrough infection. The patient was a 17-month-old female (10.2 kg; 80.0 cm) with pre-B acute lymphoblastic leukemia. Caspofungin at 50 mg/m2 (daily dose, 23 mg) was given for 2 days but was discontinued due to possible fungal infection of the lung and spleen (based on computed-tomography findings). Microbiological and histopathological data did not confirm a fungal infection: tissue biopsy of the right lung performed on day 8 showed nonspecific changes and was positive only for Enterococcus faecium. This patient would have been classified as having a possible fungal infection based on the revised European Organization of Research and Treatment of Cancer criteria (7), as well as the original criteria (3). The infection continued through the 14-day posttherapy period. This patient achieved a caspofungin AUC0-24 of 101.7 μg/ml·h and a C24 of 1.15 μg/ml on day 1, which are in the midrange of values observed in this study.
DISCUSSION
This trial represents the first prospective evaluation of caspofungin in pediatric patients between the ages of 3 and 24 months. Patients with known fever (temperature > 38.0°C) and neutropenia (ANC < 500 cells/mm3) received caspofungin within 72 h of the onset of fever (at the same time as antibacterial therapy) for 4 to 28 days. A similar study design was used in a previous pharmacokinetic study of children and adolescents; one notable difference was that the prior study evaluated a weight-based dosing regimen (1 mg/kg of body weight/day) and a BSA regimen (50 mg/m2/day or 70 mg/m2/day) (23). In that study, the AUC0-24 was significantly less (46% after multiple doses) in children receiving 1 mg/kg/day than in adults receiving 50 mg/day, whereas caspofungin at 50 mg/m2/day provided caspofungin plasma exposures comparable to those achieved in adults treated with 50 mg/day. Therefore, caspofungin BSA dosing (50 mg/m2 daily; maximum, 70 mg) was chosen for evaluation in the current study.
The pharmacokinetic results in infants/toddlers who received caspofungin at 50 mg/m2 daily were similar to the historical results from children (2 to 11 years of age) who received the same dosing regimen. The mean concentration-time profiles and the apparent terminal t1/2 for the two groups were similar. Slightly higher AUC0-24, C1, and C24 values were found in infants/toddlers than in children, but none of these differences were statistically significant.
The AUC0-24 values on day 1 were higher in infants/toddlers who received 50 mg/m2 daily than in adults who received caspofungin at 50 mg daily or adolescents who received 50 mg/m2 daily; however, the day 3 to 14 AUC0-24 values were similar between these groups, and the day 1 AUC0-24 values in infants/toddlers were less than those seen at steady state in adults given 70 mg daily. It is unlikely that exceeding the adult exposure for the first few days of therapy prior to reaching steady state would be clinically meaningful. Caspofungin clearance, normalized to BSA, was similar in infants/toddlers and in adults and adolescents (23). Because the apparent terminal t1/2 in infants/toddlers was ∼33% shorter than in adults, it is not possible to identify a dose that would provide comparable values for all three parameters (AUC0-24, C1, and C24). The 50-mg/m2 dosing regimen in infants/toddlers provides AUC and C24 values that are similar to those seen in adults.
In addition, the increased C1 values in infants/toddlers were within the range of caspofungin concentrations measured in adults receiving caspofungin doses higher than 50 mg daily. Peak concentrations in the infants/toddlers were only modestly elevated (29%) relative to those in adults receiving caspofungin at 70 mg daily and were less than those seen in adults receiving caspofungin at 100 mg daily (18). The day 3 to 14 C1 of 17.21 μg/ml in infants/toddlers receiving 50 mg/m2/day was roughly comparable to the values obtained in 35 adults receiving caspofungin at 70 mg/day for the treatment of esophageal and/or oropharyngeal candidiasis (13.32 μg/ml) and in 42 healthy subjects receiving caspofungin at 70 mg/day in several phase I studies (14.89 μg/ml).
The results of this study suggest that steady-state exposure in infants/toddlers receiving caspofungin at 50 mg/m2 daily is similar to that in adults receiving caspofungin at 50 mg daily, that accumulation of caspofungin increases with age, and that the increase in C1 observed in infants/toddlers relative to adults is unlikely to be clinically meaningful. Although there may be less need for a loading dose in infants/toddlers relative to adults, a loading dose is recommended in this age group because the AUC and trough concentrations were lower on day 1 than on day 4 in this study.
The shorter apparent terminal t1/2 of caspofungin in infants/toddlers than in adults may be due to increased plasma clearance, decreased distributional volume, or both in infants/toddlers. A reduction in the terminal t1/2 was also noted in children (aged 2 to 11 years) compared to adults (23). The mechanism(s) for possible changes in plasma clearance with age is unknown. In vitro data suggest that caspofungin tissue distribution may be mediated by uptake transporters; in fact, data suggest that the OATP1B1 transporter may be involved in the hepatic uptake of caspofungin (16). Expression levels of the uptake transporter(s) for which caspofungin is a substrate could change throughout the course of developmental maturity. Differences in physiological factors, such as relative blood flow rates and organ sizes, could also contribute to differences in caspofungin clearance with age. Reduced distributional volume could result from alterations in plasma protein binding in infants relative to adults and adolescents.
Overall, caspofungin was generally well tolerated in this study. Although the majority of patients had one or more clinical adverse events, none were considered drug related or led to discontinuation of caspofungin. In addition, none of the patients in this study had an infusion-related event. Three patients had laboratory adverse events that were considered drug related, but none were serious or required discontinuation of caspofungin. The overall safety profile for caspofungin in this study is consistent with that observed in other pediatric studies (13, 15, 23, 24) and in adult studies. However, only nine infants were enrolled in this study, and none were less than 10 months of age; thus, any conclusions to be drawn regarding the safety of caspofungin in this age group are somewhat limited.
The preliminary results of this study allowed three patients under 24 months of age to be enrolled in a separate study of the efficacy and safety of caspofungin in pediatric patients with documented fungal infections (24). All three patients (ages 6, 12, and 13 months) had a favorable overall response to caspofungin at 50 mg/m2 daily for treatment of candidemia. None of the patients developed a serious drug-related adverse event or had caspofungin therapy discontinued for toxicity reasons. Additional clinical studies are ongoing or in the preparatory stages to further evaluate the 50-mg/m2 once-daily maintenance regimen of caspofungin in older infants and toddlers.
In summary, caspofungin pharmacokinetics observed in infants and toddlers were similar to those observed previously in children 2 to 11 years of age receiving the same dosing regimen (50 mg/m2 daily) and were generally comparable to those in adults receiving caspofungin at 50 mg daily. These results confirm the use of BSA-based dosing as an appropriate method for this age group. The results of this study also suggest that caspofungin is generally well tolerated in infants and toddlers.
Acknowledgments
Funding for this study was provided by Merck & Co., Inc.
Conflicts of interest: M.N., H.S.J., N.S., K.K., and P.C.A. received grant support from Merck to conduct this study; S.K.B., K.M.S., P.S., S.B., M.F.D., J.A.S., and N.A.K. are current or former employees of Merck and may own stock and/or stock options in the company.
Footnotes
Published ahead of print on 29 December 2008.
REFERENCES
- 1.Abelson, J. A., T. Moore, D. Bruckner, J. Deville, and K. Nielsen. 2005. Frequency of fungemia in hospitalized pediatric inpatients over 11 years at a tertiary care institution. Pediatrics 116:61-67. [DOI] [PubMed] [Google Scholar]
- 2.Arathoon, E. G., E. Gotuzzo, L. M. Noriega, R. S. Berman, M. J. DiNubile, and C. A. Sable. 2002. Randomized, double-blind, multicenter study of caspofungin versus amphotericin B for treatment of oropharyngeal and esophageal candidiases. Antimicrob. Agents Chemother. 46:451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 4.Bi, S., M. S. Schwartz, R. B. Desai, A. R. Miller, and B. K. Matuszewski. 2005. A semi-automated procedure for the determination of caspofungin in human plasma using solid-phase extraction and HPLC with fluorescence detection using secondary ionic interactions to obtain a highly purified extract. J. Liq. Chromatogr. Related Technol. 28:2895-2908. [Google Scholar]
- 5.Chanock, S. J., and T. J. Walsh. 1996. Evolving concepts of prevention and treatment of invasive fungal infections in pediatric bone marrow transplant recipients. Bone Marrow Transplant. 18(Suppl. 3):S15-S20. [PubMed] [Google Scholar]
- 6.Cornely, O., M. Lasso, R. Betts, N. Klimko, J. Vazquez, G. Dobb, J. Velez, A. Williams-Diaz, J. Lipka, A. Taylor, C. Sable, and N. Kartsonis. 2007. Caspofungin for the treatment of less common forms of invasive candidiasis. J. Antimicrob. Chemother. 60:363-369. [DOI] [PubMed] [Google Scholar]
- 7.De Pauw, B., T. J. Walsh, J. P. Donnelly, D. A. Stevens, J. E. Edwards, T. Calandra, P. G. Pappas, J. Maertens, O. Lortholary, C. A. Kauffman, D. W. Denning, T. F. Patterson, G. Maschmeyer, J. Bille, W. E. Dismukes, R. Herbrecht, W. W. Hope, C. C. Kibbler, B. J. Kullberg, K. A. Marr, P. Munoz, F. C. Odds, J. R. Perfect, A. Restrepo, M. Ruhnke, B. H. Segal, J. D. Sobel, T. C. Sorrell, C. Viscoli, J. R. Wingard, T. Zaoutis, and J. E. Bennett. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiNubile, M. J., R. J. Lupinacci, R. S. Berman, and C. A. Sable. 2002. Response and relapse rates of candidal esophagitis in HIV-infected patients treated with caspofungin. AIDS Res. Hum. Retrovir. 18:903-908. [DOI] [PubMed] [Google Scholar]
- 9.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 10.Engelhard, D. 1998. Bacterial and fungal infections in children undergoing bone marrow transplantation. Bone Marrow Transplant. 21(Suppl. 2):S78-S80. [PubMed] [Google Scholar]
- 11.Jarvis, W. R. 1995. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin. Infect. Dis. 20:1526-1530. [DOI] [PubMed] [Google Scholar]
- 12.Maertens, J., I. Raad, G. Petrikkos, M. Boogaerts, D. Selleslag, F. B. Petersen, C. A. Sable, N. A. Kartsonis, A. Ngai, A. Taylor, T. F. Patterson, D. W. Denning, and T. J. Walsh. 2004. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin. Infect. Dis. 39:1563-1571. [DOI] [PubMed] [Google Scholar]
- 13.Maertens, J., L. Madero, A. Reilly, T. Lehrnbecher, A. Groll, H. Jafri, M. Green, J. Nania, M. Bourque, B. Wise, A. Taylor, N. Kartsonis, J. Chow, C. Arndt, B. dePauw, and T. Walsh. 2007. A randomized, double-blind, multicenter trial of caspofungin (CAS) versus liposomal amphotericin B (LAMB) for empirical antifungal therapy (EAFRx) of pediatric patients with persistent fever and neutropenia (PFN). Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-621.
- 14.Mora-Duarte, J., R. Betts, C. Rotstein, A. L. Colombo, L. Thompson-Moya, J. Smietana, R. Lupinacci, C. Sable, N. Kartsonis, and J. Perfect. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 15.Saez-Llorens, X., M. Macias, P. Maiya, J. Pineros, H. S. Jafri, A. Chatterjee, G. Ruiz, J. Raghavan, S. K. Bradshaw, N. A. Kartsonis, P. Sun, K. M. Strohmaier, M. Fallon, S. Bi, J. A. Stone, and J. Chow. 2009. Pharmacokinetics and safety of caspofungin in neonates and infants less than 3 months of age. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. 53:869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandhu, P., W. Lee, X. Xu, B. F. Leake, M. Yamazaki, J. A. Stone, J. H. Lin, P. G. Pearson, and R. B. Kim. 2005. Hepatic uptake of the novel antifungal agent caspofungin. Drug Metab. Dispos. 33:676-682. [DOI] [PubMed] [Google Scholar]
- 17.Steinbach, W., and T. J. Walsh. 2006. Mycoses in pediatric patients. Infect. Dis. Clin. N. Am. 20:663-678. [DOI] [PubMed] [Google Scholar]
- 18.Stone, J., E. Migoya, S. Li, P. Deutsch, G. Winchell, and K. Ghosh. 2002. Safety and pharmacokinetics of higher doses of caspofungin. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1389.
- 19.Villaneuva, A., E. G. Arathoon, E. Gotuzzo, R. S. Berman, M. J. DiNubile, and C. A. Sable. 2001. A randomized double-blind study of caspofungin versus amphotericin for the treatment of candidal esophagitis. Clin. Infect. Dis. 33:1529-1535. [DOI] [PubMed] [Google Scholar]
- 20.Villaneuva, A., E. Gotuzzo, E. G. Arathoon, L. M. Noriega, N. A. Kartsonis, R. J. Lupinacci, J. M. Smietana, M. J. DiNubile, and C. A. Sable. 2002. A randomized double-blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasis. Am. J. Med. 113:294-299. [DOI] [PubMed] [Google Scholar]
- 21.Walsh, T. J., C. Gonzalez, C. A. Lyman, S. J. Chanock, and A. Pizzo. 1996. Invasive fungal infections in children: recent advances in diagnosis and treatment. Adv. Pediatr. Infect. Dis. 11:187-290. [PubMed] [Google Scholar]
- 22.Walsh, T. J., H. Teppler, G. R. Donowitz, J. A. Maertens, L. R. Baden, A. Dmoszynska, O. A. Cornely, M. R. Bourque, R. J. Lupinacci, C. A. Sable, and B. E. dePauw. 2004. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 351:1391-1402. [DOI] [PubMed] [Google Scholar]
- 23.Walsh, T. J., P. C. Adamson, N. L. Seibel, P. M. Flynn, M. N. Neely, C. Schwartz, A. Shad, S. L. Kaplan, M. M. Roden, J. A. Stone, A. Miller, S. K. Bradshaw, S. X. Li, C. A. Sable, and N. A. Kartsonis. 2005. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob. Agents Chemother. 49:4536-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaoutis, T. E., H. S. Jafri, L.-M. Huang, F. Locatelli, A. Barzilai, W. Ebell, W. J. Steinbach, J. Bradley, J. M. Lieberman, C.-C. Hsiao, N. Siebel, H. Laws, M. Gamba, M. Petrecz, A. F. Taylor, K. M. Strohmaier, J. W. Chow, N. A. Kartsonis, and A. L. Ngai. A prospective, multicenter study of caspofungin for the treatment of documented Candida or Aspergillus infections in pediatric patients. Pediatrics, in press. [DOI] [PubMed]