Abstract
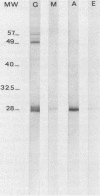
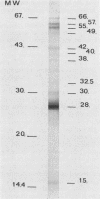
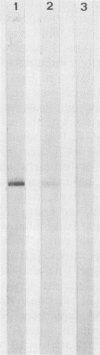
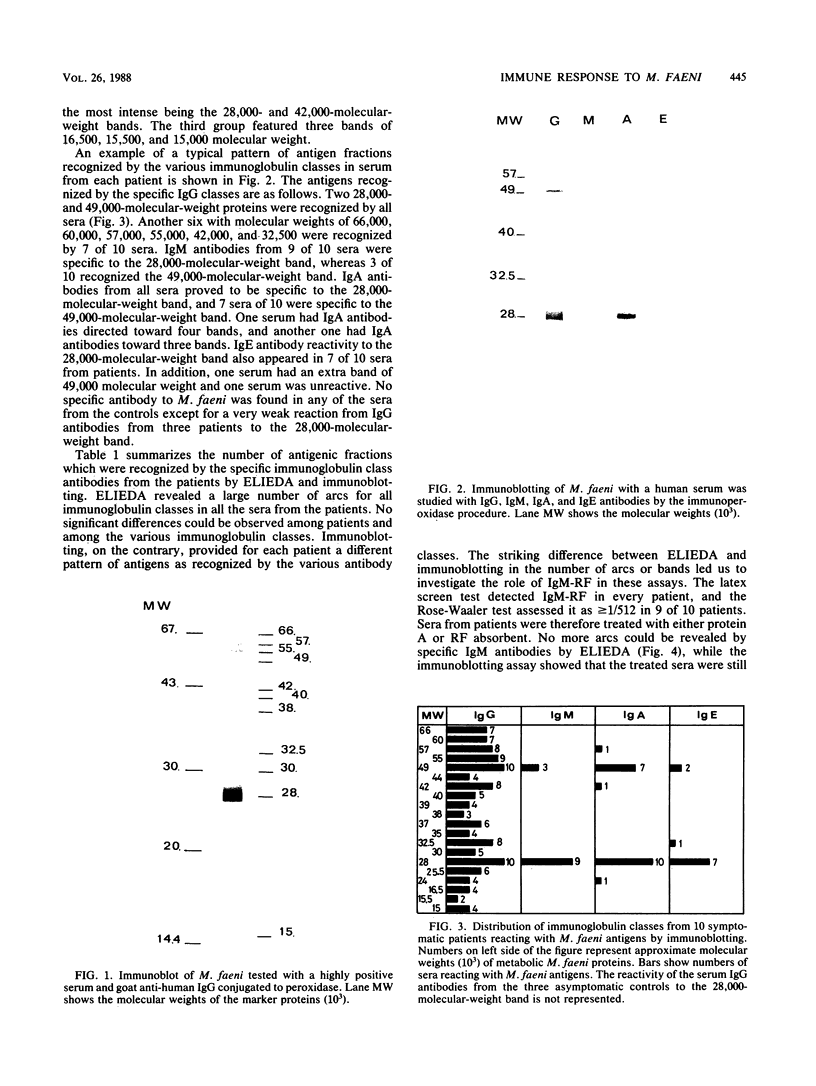
Immune response to Micropolyspora faeni was analyzed in 10 patients suffering from farmer's lung by two techniques: enzyme-linked immunoelectrodiffusion assay (ELIEDA) and immunoblotting. ELIEDA revealed the presence in all patients of various specific immunoglobulin G (IgG), IgM, IgA, and IgE antibodies, with the number of arcs ranging from 4 to 19. M. faeni proteins were isolated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and immunoblotted with human sera and specific immunoglobulin-peroxidase conjugates. In immunoblotting, the predominant immunoglobulin class was IgG for all patients. At least 20 bands ranging from 15,000 to 60,000 in molecular weight were observed in a highly positive serum, whereas IgM- and IgA-specific reactivity was directed mainly to the 28,000- and 49,000-molecular-weight bands; M. faeni-specific IgE antibodies appeared less often. The rheumatoid factor (IgM-RF), which also had high titers in these patients (greater than 1/512), interfered with ELIEDA, while only slightly interfering with the immunoblotting detection of specific IgM. This latter technique provided a better characterization of immune response in patients with farmer's lung than ELIEDA did and should also permit discrimination of recently exposed individuals from chronic patients. Moreover, this technique should make it possible to determine whether the response of one particular immunoglobulin class to an antigen fraction can be associated with a specific state of the disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banaszak E. F., Thiede W. H., Fink J. N. Hypersensitivity pneumonitis due to contamination of an air conditioner. N Engl J Med. 1970 Aug 6;283(6):271–276. doi: 10.1056/NEJM197008062830601. [DOI] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Gordon M. A., Almy R. E., Greene C. H., Fenton J. W., 2nd Diagnostic mycoserology by immunoelectroosmophoresis: a general, rapid, and sensitive microtechnic. Am J Clin Pathol. 1971 Oct;56(4):471–474. doi: 10.1093/ajcp/56.4.471. [DOI] [PubMed] [Google Scholar]
- Jameson J. E. Rapid and sensitive precipitin test for the diagnosis of farmer's lung using immunoosmophoresis. J Clin Pathol. 1968 May;21(3):376–382. doi: 10.1136/jcp.21.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBAYASHI M., STAHMANN M. A., RANKIN J., DICKIE H. A. Antigens in moldy hay as the cause of farmer's lung. Proc Soc Exp Biol Med. 1963 Jun;113:472–476. doi: 10.3181/00379727-113-28400. [DOI] [PubMed] [Google Scholar]
- Kurup V. P., John K. V., Ting E. Y., Somasundaram K., Resnick A., Marx J. J., Jr Immunochemical studies of a purified antigen from Micropolyspora faeni. Mol Immunol. 1984 Mar;21(3):215–221. doi: 10.1016/0161-5890(84)90076-2. [DOI] [PubMed] [Google Scholar]
- Kurup V. P., Ting E. Y., Fink J. N., Calvanico N. J. Characterization of Micropolyspora faeni antigens. Infect Immun. 1981 Nov;34(2):508–512. doi: 10.1128/iai.34.2.508-512.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landini M. P., Re M. C., Mirolo G., Baldassarri B., La Placa M. Human immune response to cytomegalovirus structural polypeptides studied by immunoblotting. J Med Virol. 1985 Dec;17(4):303–311. doi: 10.1002/jmv.1890170403. [DOI] [PubMed] [Google Scholar]
- Lind I., Harboe M., Folling I. Protein A reactivity of two distinct groups of human monoclonal IgM. Scand J Immunol. 1975;4(8):843–848. doi: 10.1111/j.1365-3083.1975.tb03726.x. [DOI] [PubMed] [Google Scholar]
- Marx J. J., Jr, Gray R. L. Comparison of the enzyme-linked immunosorbent assay and double immunodiffusion test for the detection and quantitation of antibodies in farmer's lung disease. J Allergy Clin Immunol. 1982 Aug;70(2):109–113. doi: 10.1016/0091-6749(82)90237-8. [DOI] [PubMed] [Google Scholar]
- Partanen P., Seppänen H., Suni J., Vaheri A. Selective reactivity of antibodies to human immunoglobulins G, M, and A with rubella virus proteins. J Clin Microbiol. 1985 May;21(5):800–802. doi: 10.1128/jcm.21.5.800-802.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon J. M., Dropsy G. ELIEDA (enzyme-linked-immuno-electro-diffusion-assay): application of a combined immunoelectrodiffusion and immunoenzyme method to the study of immune response in parasitic infections. J Immunol Methods. 1977;16(1):15–22. doi: 10.1016/0022-1759(77)90035-7. [DOI] [PubMed] [Google Scholar]
- Terho E. O., Lindström P., Mäntyjärvi R., Tukiainen H., Wager O. Circulating immune complexes and rheumatoid factors in patients with farmer's lung. Allergy. 1983 Jul;38(5):347–352. doi: 10.1111/j.1398-9995.1983.tb04129.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walbaum S., Biguet J., Tran-Van-Ky P. Structure antigénique de Thermopolyspora polyspora. Répercussions pratiques sur le diagnostic du "poumon du fermier". Ann Inst Pasteur (Paris) 1969 Nov;117(5):673–693. [PubMed] [Google Scholar]