Abstract
Bacillus subtilis mutants with resistance against peptide deformylase inhibitors were isolated. All showed a bypass of the pathway through mutations in three genes required for formylation of Met-tRNAfMet, fmt, folD, and glyA. glyA corresponds to a yet uncharacterized locus inducing resistance. The bypass of formylation caused robust fitness reduction but was not accompanied by alterations of the transcription profile. A subtle adaptation of the enzymes of the intermediary metabolism was observed.
In bacteria, all newly synthesized polypeptides transiently carry a formylated N terminus (1, 18, 33). Peptide deformylase (PDF) catalyzes the subsequent removal of the formyl group, and its gene is essential (11, 22, 23). The natural compound actinonin was the first PDF inhibitor (PDFI) characterized (4, 6, 8). Mechanisms causing PDFI resistance involve (i) mutations in the target gene, (ii) bypassing of the formylation pathway, or (iii) efflux of PDFI (4, 9, 10, 15, 17, 19, 20, 25, 31). Bacillus subtilis has two functional PDF genes, def and ykrB (14). The mechanisms of resistance have been studied only in bacteria expressing one active PDF gene. No analysis has approached the implications beyond the fitness cost often associated with PDFI resistance.
To isolate variants resistant to PDFI, 100 μl of an exponential-phase B. subtilis culture was plated onto Mueller-Hinton (MH) agar supplemented with 12 μg/ml of actinonin. The plates were incubated for 2 days at 37°C, after which resistant colonies were restreaked and isolated. Cells were also plated on minimal medium (MM) agar broth (15 mM ammonium sulfate, 80 mM K2HPO4, 45 mM KH2PO4, 3.5 mM sodium citrate, 800 μM MgSO4, 0.5% [wt/vol] glucose) supplemented with tryptophan (50 mg/liter) and glycine (25 mg/liter) where indicated below. To assess the fitness cost, growth rates were measured in different broths at 37°C. Cells (106) were inoculated into 10 ml of MH medium (Fluka) or in MM without glucose and supplemented with 0.5% (wt/vol) of the indicated carbon source, and then the optical density at 600 nm was measured. In order to identify mutations in open reading frames and/or promoters, given gene loci were amplified with specific primers as shown in Table 1, and the sequences were determined.
TABLE 1.
Mutations, resistance levels, and fitness costs of actinonin-resistant strains
| Gene | Identified mutation(s)a | No. of independently isolated mutantsb | MIC of actinonin (μg/ml)c | Fitness costd |
|---|---|---|---|---|
| fmt | Deletion of base pairs 295-639 (codons 99-213; frame conserved); strain Δfmt-114 | 1 | >512 | 0.48 |
| Deletion of base pair 119; frameshift (codon 43) | 3 | 192 | 0.37 | |
| Deletion of base pair 745; frameshift (codon 251) | 9 | 96 | 0.45 | |
| One base pair insertion at position 745; frameshift (codon 251) | 2 | 96 | 0.45 | |
| AGA→TGA; R3Stop | 1 | 192 | 0.44 | |
| CAC→CGC; H108R | 1 | >512 | 0.50 | |
| TCC→CCC; S110P | 1 | >512 | 0.54 | |
| CCG→CTG; P113L | 1 | 96 | 0.36 | |
| CGC→TGC; R116C | 2* | 48 | 0.45 | |
| CGC→CCC; R116P | 1 | >512 | 0.43 | |
| GGT→GAT; G118D | 1 | >512 | 0.46 | |
| CCG→CTG; P120L | 1 | >512 | 0.34 | |
| folD | Deletion of base pairs 199-209 and 219 (codons 67-70 and 73; frame conserved); S71Q, S72Q, and L73P | 1 | >512 | 0.50 |
| Deletion of base pairs 507-518 (codons 172-175; frame conserved) | 1 | >512 | 0.41 | |
| GAA→CAA; E17Q | 2 | 192 | 0.50 | |
| ACG→ATG; T184 M | 1 | 192 | 0.43 | |
| glyA | Deletion of base pairs 661-663 (codon 224; frame conserved) | 1* | >512 | 0.44 |
Nucleotide and amino acid substitutions and their positions are indicated.
An asterisk (*) indicates that one mutant presented mutations in both genes (fmt and glyA).
MICs were determined by the microtiter broth dilution technique (26), using MH liquid broth at 37°C after 17 h. The MIC was the lowest antibiotic concentration which prevented growth (i.e., an optical density at 600 nm of <0.01).
The fitness cost corresponds to the ratio of the doubling time of the WT to that of the resistant derivative.
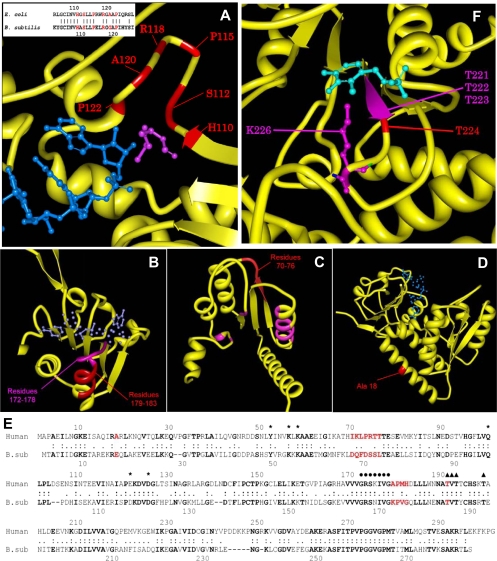
The resistance of B. subtilis 168 to actinonin was challenged on MH agar at four times the MIC. The resistance rate (10−7) was stable, as repeated streaking on drug-free media did not promote loss of resistance. Resistant strains grew at concentrations much higher than four times the MIC (Table 1). Actinonin-resistant mutants did not show any cross-resistance to other antibiotics but were resistant to other classes of PDFI (7). Both open reading frame and promoter regions of genes (def, ykrB, fmt, and folD), the alteration or loss of function of which causes actinonin resistance in other bacteria, were sequenced. No mutation was retrieved in def and ykrB, and 80% were located in fmt (Table 1). Two-thirds of these mutations led to protein sequence alterations, with large deletions due to premature stops, and promoted loss of function of fmt. The Δfmt-114 strain featured a 114-codon deletion. Single changes involved the catalytic mechanism or binding of the substrates (Table 1; Fig. 1A and B).
FIG. 1.
Structural impact of substitutions leading to PDFI resistance in B. subtilis. (A) Structural view of E. coli formylmethionine transferase complexed with formyl methionyl-tRNAfMet (Protein Data Bank code, 2fmt). The enzyme is represented as a yellow ribbon, formylmethionine as pink solid bonds, and tRNAfMet as blue solid bonds. E. coli residues corresponding to mutated B. subtilis residues are in red. The inset shows the sequence alignment of the formylmethionine transferase active sites from E. coli and B. subtilis. Mutated residues are in red. (B through D) Structural view of human methylenetetrahydrofolate dehydrogenase/cyclohydrolase (Protein Data Bank code, 1a4i). The enzyme is represented as a yellow ribbon, the active/binding sites as pink ribbons, and NADP as blue solid bonds. Mutated or deleted residues are in red. (B) NADP binding site and deleted residues downstream. (C) THF binding site and deleted residues. (D) Mutation on Ala18 in the human equivalent of E17Q in B. subtilis. (E) Sequence alignment of methylenetetrahydrofolate dehydrogenase/cyclohydrolase from Homo sapiens and B. subtilis. Mutated or deleted residues are in red. Residues that are conserved between the two sequences are in bold. Residues involved in the THF binding site are marked with an asterisk, residues implied in the NADP binding site are marked with a filled circle, and residues implied in dimer formation are marked with a filled triangle. (F) Structural view of serine hydroxymethyltransferase from Bacillus stearothermophilus in complex with pyridoxal-5′-phosphate (Protein Data Bank code, 1kkj). The enzyme is represented as a yellow ribbon, and pyridoxal-5′-phosphate is represented with blue solid bonds. The pyridoxal-5′-phosphate binding site is in pink. A B. stearothermophilus residue corresponding to a residue deleted in B. subtilis is in red.
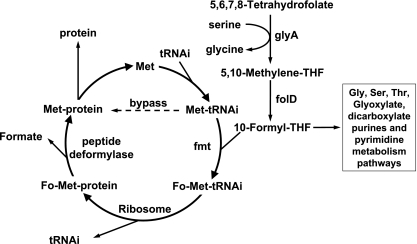
Other mutations were located in folD (Table 1). folD encodes 5,10-methylenetetrahydrofolate dehydrogenase/cyclohydrolase, which produces 10-formyl-tetrahydrofolate (THF), the donor of N-formyl to Met-tRNAfMet. Similar mutations have been described only for Salmonella enterica (24). The loss of function of folD not only bypasses PDF function but also inactivates pathways that use 10-formyl-THF (Fig. 2). When folD is inactivated, the strain cannot grow on MM. None of the resistant strains with a folD alteration could grow on MM, indicating that the mutations induced loss of function. Several mutations corresponded to deletions. The first deletion identified included residues 67 to 70, with modifications of residues 71 to 73. Structural interpretation was based on the three-dimensional model of FolD (29). The THF binding site is composed of residues conserved in both humans (2) and B. subtilis (Fig. 1C to E). The substitutions modify the position of the THF binding site, leading to a dramatic decrease in the reaction efficiency. The second deletion identified (residues 172 to 175) is located next to the NADP binding site 165GRSNIVG171 (172GRSKIVG178 in humans) (Fig. 1B to E).
FIG. 2.
Translation initiation in bacteria, Met-tRNA formylation, ribosome-mediated protein synthesis, and bypass of the pathway in PDFI-resistant bacteria. tRNAi, tRNAfMet.
There was only one strain carrying an fmt mutation that could not grow on MM (Table 1). Given that a nonmutated FolD protein occurred, the origin of the impairment should be due to a mutation upstream in the pathway. One reaction is catalyzed by the glyA product, serine hydroxymethyltransferase (Fig. 2), which produces glycine and 5,10-methylene-THF, the substrate of FolD. As growth of the mutant strain on MM was restored in the presence of glycine, this favored an involvement of glyA. A deletion of codon 224 in glyA was identified. According to the crystallographic model (32), residue 224 lies within the pyridoxal 5′-phosphate (PLP) binding site encompassing residues Thr223 to Lys226 (Fig. 1F). All serine hydroxymethyltransferases have five Thr residues near the active site Lys226, which form the internal glycine-aldimine with PLP (3, 32). Deletion of Thr224 alters PLP binding (Fig. 1F), inactivating the enzyme. The combination of both the glyA and fmt mutations confers a higher resistance than that of the strain carrying only the fmt mutation. The MIC of actinonin for the first strain was increased 10-fold (Table 1), indicating that (i) the Arg116Cys substitution does not completely inactivate fmt and (ii) GlyA modification further completes the bypass of formylation.
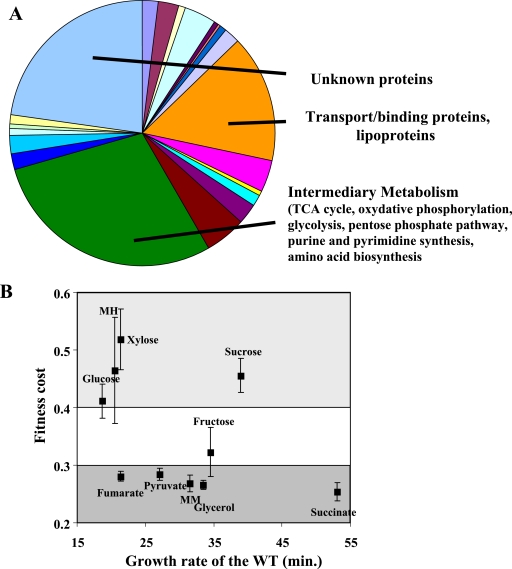
All aforementioned mutations inducing PDFI resistance caused fitness costs with two- to threefold reductions in the growth rate in rich media (Table 1). This decrease was higher in MM. This finding confirms that formylation is needed for rapid growth in bacteria (5, 13, 16, 25, 28). However, the fitness cost was less pronounced than in E. coli (4, 13, 16). Strains with unambiguous inactivation of the fmt gene had distinct doubling times (Table 1). We investigated whether compensatory mechanisms could improve the growth rate by serially passaging several strains in nonselective media through 50 generations. Unlike with PDFI-resistant gram-negative bacteria (12, 21, 25), we were unable to show any improvement in the doubling time. Thus, the fitness burden induced by PDFI resistance is robust. Sequencing of the three B. subtilis tRNAfMet genes revealed no modifications or rearrangement, unlike for various gram-positive bacteria (12, 25, 30). Sequencing of the IF2 gene (infB) did not reveal any mutation, although altered expression contributes to fitness cost reduction in Pseudomonas aeruginosa (30). Two-dimensional gel electrophoresis of protein accumulation in the Δfmt-114 mutant revealed patterns different from those for the wild type (WT), indicative of adaptation mechanisms associated with formylation bypass. Comparison of the transcriptome of the Δfmt-114 strain with that of the WT revealed that the expression of a small number of genes was altered by more than twofold, with 308 upregulated and 25 downregulated (Fig. 3A).
FIG. 3.
Physiological differences between WT and PDFI-resistant strains. (A) Gene categories to which the 333 significantly regulated genes belong. Custom compare analysis of the complete B. subtilis transcriptome of the WT and resistant strains was performed on microarrays at Eurogentech (Herstaing, Belgium). (B) The doubling times of the WT and Δfmt-114 strain were studied in MH medium or MM supplemented with the indicated carbon source. The fitness cost corresponds to the ratio of the doubling time of the WT with that of the resistant derivative. TCA, tricarboxylic acid.
To confirm the expression changes between the WT and the Δfmt-114 strain, quantitative PCR experiments were performed, using rplL for normalization (Table 2). Total RNA was isolated from 5-ml portions of bacterial samples by using an RNeasy minikit (Qiagen) as described by the manufacturer. Genomic DNA was eliminated by RNase-free DNase I treatment during the isolation procedure. cDNAs were prepared, using the SuperScript III First-Strand system. We subjected 5 μg of total RNA to reverse transcription, using random hexamers. The cDNA was then amplified by PCR, using specific oligonucleotides. Real-time PCR was optimized with a LightCycler FastStart DNA Master Sybr green kit (Roche) for each primer pair shown in Table 3 (the amplification efficiency was always >90%), using a standard cDNA. Each cDNA sample was independently quantified three times, with two technical replicates of each. Relative transcript levels were calculated by using the relative expression software tool (REST) (27). Expression of the rplL gene was used as a reference for the determination of induction levels. No evidence for regulation was observed for genes involved in translation. The gene categories showing the highest changes corresponded to genes encoding functions of the intermediary metabolism (Fig. 3A). These categories produce the most-abundant proteins, which suggests that energetic metabolism limits growth and is the root of the fitness cost. Thus, in the tested conditions, (i) there is no global change of gene expression and (ii) the adaptation mechanisms involved are subtle.
TABLE 2.
Absolute gene regulation in the Δfmt-114 mutant
| Gene | Absolute gene regulation valuea | P valueb |
|---|---|---|
| atpH | 1.11 ± 0.05 | 0.001 |
| fhuD | 1.08 ± 0.03 | 0.004 |
| pdhA | 1.08 ± 0.04 | 0.001 |
| katA | 1.13 ± 0.03 | 0.001 |
| gtlB | 1.10 ± 0.03 | 0.001 |
| ahpF | 1.12 ± 0.05 | 0.001 |
| thyA | 1.04 ± 0.03 | 0.012 |
| hisC | 1.12 ± 0.04 | 0.001 |
| srfAC | 0.92 ± 0.03 | 0.031 |
Expressed as the ratio R = EΔCT(WT/Δfmt-114)target gene/EΔCT(WT/Δfmt-114)reference gene, where E is PCR efficiency (10−1/slope) and ΔCT is the cycle threshold.
Pairwise fixed reallocation randomization test results.
TABLE 3.
Nucleotide sequences of the primers used for PCR amplification
| Primera | Sequence (5′ to 3′) |
|---|---|
| DEF/FMT-F1 | ATTTCAATTGATATGAATCCTTATATGATG |
| DEF/FMT-F2 | AAGAGGCATTGCGCCACGGCAT |
| DEF/FMT-R1 | ACCGATTTCAGCAGCAGGTTGCTGTATGCC |
| DEF/FMT-R2 | ATCGGAGCACCGCCGCGCAGTT |
| YKRB-F | TGCATACGCTGTCAAAGAATTGGGCCTTTT |
| YKRB-R | CGGGTCCTGTCCCATGCATCCTGTTAATAT |
| FOLD-F | TATATACTTTGTCAGCGCTGAGACGGTCAC |
| FOLD-R | AGTTTGTAAACGGGGTTCTTTCTAACATTAA |
| GLYA-F1 | TCGTACCTGTATTTTCATTCCGTATATATG |
| GLYA-F2 | CTTGTTACGAAAGGTTTTTCAGGATCATAT |
| GLYA-R1 | AGCCTCGGTTCAGCTCATGTGACATTGGCG |
| GLYA-R2 | ATCTGTTTTGACAAATAAGTACGCAGAAGG |
| ATPH-F | GGAGTCGCTTCACTGAGAATCA |
| ATPH-R | CTTACGCTGCCGTCATAAATCC |
| FHUD-F | CTGCAGGCTACAAAGCTGACAA |
| FHUD-R | GCGAAGTCAGGGAGCTTCTCTA |
| PDHA-F | AGGGAGAAGTCGTGAATGAAGC |
| PDHA-R | GAGATAGAGCGTTGGTCAAGCA |
| KATA-F | GTGAACTTGGCTCTGCTGACAC |
| KATA-R | CCGACGATGTCGTAGTTTCCTT |
| GTLB-F | CAGCTGATGAGCTAAAGGAGCA |
| GTLB-R | AGTCGCGTCCTTCGATTAAGAG |
| AHPF-F | GGCAAGTCTTGAAGAGCATGTG |
| AHPF-R | GCACCTGTTGAAAGGATCACTG |
| THYA-F | CAATCCATCTTCACGCAGACAC |
| THYA-R | CTCAAGGTGGAGTTTCCCATGT |
| HISC-F | GCCGCTCTTAAGCAAGTATTCC |
| HISC-R | AATTCCGTATCCGACTCTGAGC |
| SRFAC-F | CTGATGGCTTGCAGGATGTAAC |
| SRFAC-R | GCATAGCTTGTATGACGGCAAG |
| RPLL-F | CTTTAAGCTCTTCAGCTTCTTCTTTAGC |
| RPLL-R | GTTGTACGTGAAATCACTGGTCTTG |
F, forward; R, reverse.
To understand whether those adaptation mechanisms could be mediated by the growth rate and/or substrate utilization, we tested if fitness could depend on the growth medium. Growth was performed on either MH medium or MM. The growth rate with several carbon sources varied from 18.6 to 53.1 min. There was no correlation between growth rate and fitness cost, and two types of fitness defects could be detected (Fig. 3B). In rich medium or in MM supplemented with a mono- or disaccharide, the doubling time was reduced by twofold. With carboxylic acids such as pyruvate, the growth rate was reduced fourfold. We concluded that sugar utilization in the context of the Δfmt-114 strain is more effective than that of carboxylic acids. This difference reflects the limited efficiency of the tricarboxylic cycle/oxidative phosphorylation and the pentose phosphate pathway in the context of the mutant strain, possibly compensating for the overall reduced translation efficiency of these major proteins induced by the loss of formylation of Met-tRNAfMet.
Acknowledgments
Y.D. was supported by Centre National de la Recherche Scientifique (CNRS, France) and by a postdoctoral grant from the Région Ile-de-France. This work was supported by CNRS and by grant ANR-06-MIME-010 from the Agence Nationale de la Recherche (ANR, France).
Footnotes
Published ahead of print on 26 January 2009.
REFERENCES
- 1.Adams, J. M., and M. Capecchi. 1966. N-Formylmethionine-sRNA as the initiator of protein synthesis. Proc. Natl. Acad. Sci. USA 55:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaire, M., Y. Li, R. E. MacKenzie, and M. Cygler. 1998. The 3-D structure of a folate-dependent dehydrogenase/cyclohydrolase bifunctional enzyme at 1.5 Å resolution. Structure 6:173-182. [DOI] [PubMed] [Google Scholar]
- 3.Angelaccio, S., S. Pascarella, E. Fattori, F. Bossa, W. Strong, and V. Schirch. 1992. Serine hydroxymethyltransferase: origin of substrate specificity. Biochemistry 31:155-162. [DOI] [PubMed] [Google Scholar]
- 4.Apfel, C. M., H. Locher, S. Evers, B. Takács, C. Hubschwerlen, W. Pirson, M. G. P. Page, and W. Keck. 2001. Peptide deformylase as an antibacterial drug target: target validation and resistance development. Antimicrob. Agents Chemother. 45:1058-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold, H. H. 1977. Initiation of protein synthesis in bacillus subtilis in the presence of trimethoprim or aminopterin. Biochim. Biophys. Acta 476:76-87. [DOI] [PubMed] [Google Scholar]
- 6.Boularot, A., C. Giglione, I. Artaud, and T. Meinnel. 2004. Structure-activity relationship and therapeutic potential of peptide deformylase inhibitors. Curr. Opin. Investig. Drugs 5:809-822. [PubMed] [Google Scholar]
- 7.Boularot, A., C. Giglione, S. Petit, Y. Duroc, R. A. Sousa, V. Larue, T. Cresteil, F. Dardel, I. Artaud, and T. Meinnel. 2007. Discovery and refinement of a new structural class of potent peptide deformylase inhibitors. J. Med. Chem. 50:10-20. [DOI] [PubMed] [Google Scholar]
- 8.Chen, D. Z., D. V. Patel, C. J. Hackbarth, W. Wang, G. Dreyer, D. C. Young, P. S. Margolis, C. Wu, Z. J. Ni, J. Trias, R. J. White, and Z. Yuan. 2000. Actinonin, a naturally occurring antibacterial agent, is a potent deformylase inhibitor. Biochemistry 39:1256-1262. [DOI] [PubMed] [Google Scholar]
- 9.Clements, J. M., R. P. Beckett, A. Brown, G. Catlin, M. Lobell, S. Palan, W. Thomas, M. Whittaker, S. Wood, S. Salama, P. J. Baker, H. F. Rodgers, V. Barynin, D. W. Rice, and M. G. Hunter. 2001. Antibiotic activity and characterization of BB-3497, a novel peptide deformylase inhibitor. Antimicrob. Agents Chemother. 45:563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean, C. R., S. Narayan, J. Richards, D. M. Daigle, S. Esterow, J. A. Leeds, H. Kamp, X. Puyang, B. Wiedmann, D. Mueller, H. Voshol, J. van Oostrum, D. Wall, J. Koehn, J. Dzink-Fox, and N. S. Ryder. 2007. Reduced susceptibility of Haemophilus influenzae to the peptide deformylase inhibitor LBM415 can result from target protein overexpression due to amplified chromosomal def gene copy number. Antimicrob. Agents Chemother. 51:1004-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giglione, C., M. Pierre, and T. Meinnel. 2000. Peptide deformylase as a target for new generation, broad spectrum antimicrobial agents. Mol. Microbiol. 36:1197-1205. [DOI] [PubMed] [Google Scholar]
- 12.Guillon, J.-M., S. Heiss, J. Soutourina, Y. Mechulam, S. Laalami, M. Grunberg-Manago, and S. Blanquet. 1996. Interplay of methionine tRNAs with translation elongation factor Tu and translation initiation factor 2 in Escherichia coli. J. Biol. Chem. 271:22321-22325. [DOI] [PubMed] [Google Scholar]
- 13.Guillon, J.-M., Y. Mechulam, J.-M. Schmitter, S. Blanquet, and G. Fayat. 1992. Disruption of the gene for Met-tRNAfMet formyltransferase severely impairs growth of Escherichia coli. J. Bacteriol. 174:4294-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas, M., D. Beyer, R. Gahlmann, and C. Freiberg. 2001. YkrB is the main peptide deformylase in Bacillus subtilis, a eubacterium containing two functional peptide deformylases. Microbiology 147:1783-1791. [DOI] [PubMed] [Google Scholar]
- 15.Hackbarth, C. J., D. Z. Chen, J. G. Lewis, K. Clark, J. B. Mangold, J. A. Cramer, P. S. Margolis, W. Wang, J. Koehn, C. Wu, S. Lopez, I. G. Withers, H. Gu, E. Dunn, R. Kulathila, S. H. Pan, W. L. Porter, J. Jacobs, J. Trias, D. V. Patel, B. Weidmann, R. J. White, and Z. Yuan. 2002. N-Alkyl urea hydroxamic acids as a new class of peptide deformylase inhibitors with antibacterial activity. Antimicrob. Agents Chemother. 46:2752-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey, R. J. 1973. Growth and initiation of protein synthesis in Escherichia coli in the presence of trimethoprim. J. Bacteriol. 114:309-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosowska-Shick, K., K. L. Credito, G. A. Pankuch, B. DeWasse, P. McGhee, and P. C. Appelbaum. 2007. Multistep resistance selection and postantibiotic-effect studies of the antipneumococcal activity of LBM415 compared to other agents. Antimicrob. Agents Chemother. 51:770-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcker, K., and F. Sanger. 1964. N-Formyl-methionyl-S-RNA. J. Mol. Biol. 8:835-840. [DOI] [PubMed] [Google Scholar]
- 19.Margolis, P., C. Hackbarth, S. Lopez, M. Maniar, W. Wang, Z. Yuan, R. White, and J. Trias. 2001. Resistance of Streptococcus pneumoniae to deformylase inhibitors is due to mutations in defB. Antimicrob. Agents Chemother. 45:2432-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margolis, P. S., C. J. Hackbarth, D. C. Young, W. Wang, D. Chen, Z. Yuan, R. White, and J. Trias. 2000. Peptide deformylase in Staphylococcus aureus: resistance to inhibition is mediated by mutations in the formyltransferase gene. Antimicrob. Agents Chemother. 44:1825-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marliere, P., R. Mutzel, and D. Mazel. November 2005. Descendant of bacteria devoid of N-terminal formylation useful for the production of proteins and peptides. U.S. patent 6,962,807 B2.
- 22.Mazel, D., S. Pochet, and P. Marliere. 1994. Genetic characterization of polypeptide deformylase, a distinctive enzyme of eubacterial translation. EMBO J. 13:914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meinnel, T., and S. Blanquet. 1994. Characterization of the Thermus thermophilus locus encoding peptide deformylase and methionyl-tRNAfMet formyltransferase. J. Bacteriol. 176:7387-7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson, A. 2005. Resistance adaptation to novel selection pressure. Ph.D. thesis. Stockholm University, Stockholm, Sweden.
- 25.Nilsson, A. I., A. Zorzet, A. Kanth, S. Dahlstrom, O. G. Berg, and D. I. Andersson. 2006. Reducing the fitness cost of antibiotic resistance by amplification of initiator tRNA genes. Proc. Natl. Acad. Sci. USA 103:6976-6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson, R. D., R. T. Steigbigel, H. T. Davis, and S. W. Chapman. 1980. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob. Agents Chemother. 18:699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaffl, M. W., G. W. Horgan, and L. Dempfle. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuel, C. E., C. L. Murray, and J. C. Rabinowitz. 1972. Methionine transfer ribonucleic acid from folate-sufficient and folate-deficient Streptococcus faecalis R. J. Biol. Chem. 247:6856-6865. [PubMed] [Google Scholar]
- 29.Shen, B. W., D. H. Dyer, J. Y. Huang, L. D'Ari, J. Rabinowitz, and B. L. Stoddard. 1999. The crystal structure of a bacterial, bifunctional 5,10-methylene-tetrahydrofolate dehydrogenase/cyclohydrolase. Protein Sci. 8:1342-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steiner-Mosonyi, M., C. Creuzenet, R. A. Keates, B. R. Strub, and D. Mangroo. 2004. The Pseudomonas aeruginosa initiation factor IF-2 is responsible for formylation-independent protein initiation in P. aeruginosa. J. Biol. Chem. 279:52262-52269. [DOI] [PubMed] [Google Scholar]
- 31.Teo, J. W., P. Thayalan, D. Beer, A. S. Yap, M. Nanjundappa, X. Ngew, J. Duraiswamy, S. Liung, V. Dartois, M. Schreiber, S. Hasan, M. Cynamon, N. S. Ryder, X. Yang, B. Weidmann, K. Bracken, T. Dick, and K. Mukherjee. 2006. Peptide deformylase inhibitors as potent antimycobacterial agents. Antimicrob. Agents Chemother. 50:3665-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trivedi, V., A. Gupta, V. R. Jala, P. Saravanan, G. S. Rao, N. A. Rao, H. S. Savithri, and H. S. Subramanya. 2002. Crystal structure of binary and ternary complexes of serine hydroxymethyltransferase from Bacillus stearothermophilus: insights into the catalytic mechanism. J. Biol. Chem. 277:17161-17169. [DOI] [PubMed] [Google Scholar]
- 33.Webster, R. E., D. L. Engelhardt, and N. D. Zinder. 1966. In vitro protein synthesis: chain initiation. Proc. Natl. Acad. Sci. USA 55:155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]