Abstract
The role of combination antibiotic therapy with a beta-lactam and a fluoroquinolone for bacteremia caused by gram-negative bacilli, to our knowledge, has not been previously described. Much of the previous study of combination therapy has included beta-lactams and aminoglycosides. We conducted a large retrospective cohort study to evaluate 28-day all-cause mortality in patients with monomicrobial bacteremia due to aerobic gram-negative bacilli who received either a combination of beta-lactams and fluoroquinolones or beta-lactam monotherapy. We enrolled adult patients admitted to Mayo Clinic hospitals from 1 January 2001 to 31 October 2006 in the study. After stratification of patients by Pitt bacteremia scores, we used Cox regression models to estimate the hazard ratios (HR) for 28-day all-cause mortality after adjusting for the propensity to receive combination therapy. We identified 398 and 304 unique patients with bacteremia caused by gram-negative bacilli who received single and combination antibiotic therapy, respectively. In less severely ill patients with Pitt bacteremia scores of <4, combination therapy was associated with lower 28-day mortality than single therapy (4.2% [9 of 214] versus 8.8% [28 of 319]; adjusted HR, 0.44; 95% confidence interval [CI], 0.20 to 0.98; P = 0.044). In critically ill patients with Pitt bacteremia scores of ≥4, there was no difference in 28-day mortality between combination and single therapy (25.6% [23 of 90] versus 27.8% [22 of 79]; adjusted HR, 0.87; 95% CI, 0.47 to 1.62; P = 0.660). These findings were consistent for 14-day all-cause mortality. In this large cohort, we found for the first time that combination therapy with beta-lactams and fluoroquinolones was associated with a reduction in 28-day all-cause mortality among less severely ill patients with bacteremia caused by gram-negative bacilli.
The efficacy of combination antimicrobial therapy in patients with bacteremia caused by gram-negative bacilli has been previously examined. The majority of studies have included beta-lactam and aminoglycoside antibiotic combinations. Overall, there was no significant reduction in mortality with beta-lactam and aminoglycoside combinations compared to beta-lactam monotherapy in patients with bacteremia caused by gram-negative bacilli (16, 18, 25). However, decreased mortality was demonstrated in subgroups of patients with Pseudomonas aeruginosa bacteremia (4, 14, 25) and critically ill patients with Klebsiella species bacteremia (17). A clinical benefit with combination therapy was also seen in neutropenic patients with bacteremia caused by gram-negative bacilli (10, 18). As a result, beta-lactam and aminoglycoside combination therapy has been frequently used in patients with P. aeruginosa bacteremia, but not in patients with bacteremia due to other gram-negative bacilli (6). This approach is particularly useful in patients with risk factors for P. aeruginosa bacteremia for whom combination therapy is started empirically; otherwise, there is little benefit if the decision to start combination therapy is delayed until a gram-negative organism in blood cultures is identified (4, 14, 15). In addition, due to the potential drug-related adverse events associated with aminoglycosides in critically ill patients, particularly nephrotoxicity, empirical use of these drugs has been limited (21, 26).
With the increase in antibiotic resistance among gram-negative bacilli, use of combination antibiotic therapy for bacteremia caused by gram-negative bacilli has reemerged. Studies have shown that inappropriate antimicrobial treatment can be reduced with empirical administration of combination therapy (19). Despite the increasing use of fluoroquinolones due to their relatively broad spectrum of antimicrobial activity and an acceptable safety profile, the inclusion of fluoroquinolones in combination antimicrobial regimens for bacteremia caused by gram-negative bacilli, to our knowledge, has not been exclusively studied. A meta-analysis showed comparable mortality, favorable clinical outcomes, and less nephrotoxicity with ciprofloxacin and beta-lactam combinations than with aminoglycoside and beta-lactam combinations in patients with febrile neutropenia (3). Recent studies also showed that the combination of a fluoroquinolone with a beta-lactam had in vitro and in vivo synergy against extended-spectrum beta-lactamase-producing Escherichia coli and P. aeruginosa isolates, respectively (8, 22). This combination contributed less frequently than did monotherapy to in vitro selection for resistance in three different gram-negative bacilli (8, 9).
We hypothesized that the empirical use of fluoroquinolones in combination with beta-lactam antibiotics, prior to identification of the gram-negative bacilli and availability of susceptibility results, decreases mortality in patients with bacteremia caused by gram-negative bacilli compared to single-beta-lactam treatment. The primary aim of this cohort study was to compare the 28-day all-cause mortality in adult patients with bacteremia caused by gram-negative bacilli who received a combination of beta-lactam and fluoroquinolone antibiotics to that in patients who received single-beta-lactam treatment after stratification by acute severity of illness.
(A poster of this study was presented at the 108th General Meeting of the American Society for Microbiology, 4 June 2008, Boston, MA.)
MATERIALS AND METHODS
Setting.
The study was conducted at two Mayo Clinic hospitals: Saint Mary's Hospital and Rochester Methodist Hospital, located in Rochester, MN. Both are large tertiary-care hospitals that have a combined total of over 1,950 licensed beds and provide care for local residents, as well as referral patients, in a wide variety of medical and surgical subspecialties. The study was approved by the institutional review board at the Mayo Clinic, Rochester, MN.
Case definition.
Bacteremia caused by gram-negative bacilli in this study was defined as the growth of any aerobic gram-negative bacillus in a blood culture. Monomicrobial bacteremia caused by gram-negative bacilli was defined as the growth of only one gram-negative bacillus in a blood culture, and polymicrobial bacteremia was defined as the growth of more than one organism in a blood culture, excluding coagulase-negative staphylococci, Corynebacterium spp., and Propionibacterium spp. Cases were classified according to the site of acquisition into nosocomial, health care associated, and community acquired (11). The primary source of bacteremia was defined using the Centers for Disease Control and Prevention criteria (12).
We defined single-antibiotic therapy as the administration of only beta-lactam antibiotics with activity against gram-negative bacilli within 24 h of obtaining blood cultures that was continued for a minimum duration of 48 h. Combination antibiotic therapy was defined as the use of two antibiotics, with one being a beta-lactam with activity against gram-negative bacilli and the other a fluoroquinolone, with both started within 24 h of obtaining blood cultures and given for a minimum duration of 48 h. Beta-lactam antibiotics with activity against gram-negative bacilli in this study included piperacillin-tazobactam, ticarcillin-clavulanate, aztreonam, imipenem-cilastin, meropenem, ertapenem, cefepime, ceftazidime, ceftriaxone, cefotaxime, cefixime, and cefoxitin. Fluoroquinolone antibiotics included ciprofloxacin, levofloxacin, gatifloxacin, and moxifloxacin.
Critically ill patients were defined as those who had Pitt bacteremia scores of ≥4, and less severely ill patients were defined as those with Pitt bacteremia scores of <4 (20).
Case ascertainment.
A cohort of 2,362 consecutive episodes of monomicrobial bacteremia caused by gram-negative bacilli from 1 January 2001 to 31 October 2006 was retrospectively identified using the Mayo Clinic microbiology laboratory database, and all were considered for inclusion in the study. We excluded patients without valid research authorization (n = 57), patients with recurrent episodes of bacteremia caused by gram-negative bacilli (n = 252), patients with Haemophilus sp. bacteremia (n = 34), patients younger than 18 years old (n = 118), and those who were treated in the outpatient setting (n = 44). The computer-based antimicrobial-monitoring (CBAM) program at our institution was used to identify hospitalized patients who met our definitions of single- and combination antibiotic therapy. Patients who did not meet the criteria for either single or combination therapy, including those for whom either beta-lactams or fluoroquinolones were started more than 24 h after blood cultures were obtained (n = 249) and patients to whom either class of antibiotics was given for less than a total duration of 48 h (n = 348), were excluded. Patients who received single-fluoroquinolone treatment (n = 362) and those who received classes of antibiotics with activity against gram-negative bacilli other than beta-lactams and fluoroquinolones, excluding trimethoprim-sulfamethoxazole prophylaxis (n = 196), were also excluded. The principal investigator (M.N.A.-H.) reviewed the antibiotic database generated by the CBAM program during the index admission for each patient to confirm that they fulfilled the criteria for inclusion in the study. The principal investigator (M.N.A.-H.) also reviewed the dosages of antibiotic regimens to ensure that included patients received at least the minimum recommended dose based on the respective creatinine clearance, if renally cleared, according to the institution's CBAM guidelines. The computer programmer was blinded to the study hypothesis, and both the principal investigator and the computer programmer were blinded to the primary outcome of patients during the case ascertainment phase of the study. We identified 398 and 304 unique patients with first episodes of monomicrobial bacteremia caused by gram-negative bacilli during the study period who met our definitions for single and combination therapy, respectively.
Statistical analysis.
Descriptive statistics were used to summarize the data: means and standard deviations for continuous variables and counts and percentages for categorical variables. The chi-square or Fisher's exact test was used to assess for associations between categorical variables and treatment arm, while the Wilcoxon rank-sum test was used to assess for differences in continuous variables between the two treatment groups. Because the Pitt bacteremia score has been shown to be a reliable predictor of mortality in patients with bacteremia caused by gram-negative bacilli (1, 17, 20), all analyses were stratified on acute severity of illness as measured by the Pitt bacteremia score.
The primary objective was to compare the 28-day all-cause mortality between patients who received a combination of beta-lactam and fluoroquinolone antibiotics within 48 h of the onset of bacteremia and those who received single-beta-lactam treatment. Patients were followed for 28 days from the onset of bacteremia caused by gram-negative bacilli or until the last health care encounter. Death was confirmed by reviewing medical records and the Minnesota death registry database. Patients who were lost to follow up within 28 days of the onset of bacteremia were censored at the date of the last health care encounter. To compare 28-day mortality between single and combination therapies for both strata (Pitt bacteremia scores of <4 and ≥4) separately, a propensity score approach was used to adjust for differences in baseline clinical characteristics across the two treatment groups. First, for each stratum, multivariable logistic regression was utilized to estimate the propensity for being treated with a combination of beta-lactams and fluoroquinolones. Baseline clinical characteristics were included in the multivariable logistic model if the P value for a univariate association with the treatment arm was less than or equal to 0.10. Although the identification of a gram-negative blood culture isolate, the results of in vitro antimicrobial susceptibility tests, and the primary source of bacteremia may not have been available to physicians at the time a treatment decision was made for all patients in the cohort, these variables were included in the propensity score model, as they could be indirectly implied from a patient's past medical history or past exposures. Subsequently, the logit-transformed predicted probabilities of receiving combination therapy were used as a covariate in the Cox proportional-hazards regression models. Cox proportional-hazards regression was used to compare 28-day all-cause mortality rates between the two treatment arms, adjusting for the propensity for being treated with combination therapy. Equivalently, 14-day all-cause mortality was also assessed. Hazard ratios (HR) and 95% confidence intervals (CI) were reported. SAS software version 8 (SAS Institute Inc., Cary, NC) was used for all statistical analyses, and an alpha level was set at 0.05 for statistical significance. All aspects of the study's primary aim were prespecified prior to data collection.
To determine whether the effect of combination therapy was due to a greater likelihood of receiving at least one antibiotic with in vitro activity against a gram-negative isolate in a blood culture or to a possible additive or synergistic effect of two active antibiotics, we performed an additional post hoc analysis. We compared the 28-day mortality in patients with bacteremia caused by gram-negative bacilli who received two antibiotics with in vitro activity against the gram-negative isolate to that in patients who received only one antibiotic with in vitro activity. We used the same propensity score model and stratification method discussed above for this analysis. Finally, we compared the incidences of Clostridium difficile colitis and infections due to resistant gram-positive organisms, including methicillin-resistant Staphylococcus aureus, and multidrug-resistant gram-negative bacilli during the index admission for patients who received single and combination therapy.
RESULTS
Tables 1 and 2 show the baseline clinical characteristics of patients with bacteremia caused by gram-negative bacilli with Pitt bacteremia scores of <4 and ≥4, respectively, by type of therapy. Patients who received single- and combination antibiotic therapy in both Pitt bacteremia score strata were comparable in age, site of acquisition of bacteremia caused by gram-negative organisms, and most chronic underlying medical conditions, including the Charlson comorbidity score (5). There was a significant association between therapy type and the gram-negative organism isolated in the blood culture in patients with Pitt bacteremia scores of <4, but not in patients with Pitt bacteremia scores of ≥4. For patients with Pitt bacteremia scores of <4, combination therapy patients had a higher percentage of E. coli and Pseudomonas species, whereas single-therapy patients had a higher percentage of Klebsiella species. Likewise, there was a significant association between therapy type and the primary source of bacteremia only in patients with Pitt bacteremia scores of <4. For patients with Pitt bacteremia scores of <4, the most common source of bacteremia for patients who received combination therapy was urinary, whereas the most common source for patients who received single therapy was gastrointestinal. In addition, patients with Pitt bacteremia scores of <4 who received combination therapy were significantly less likely to have a current diagnosis of cancer and had higher serum creatinine levels at the onset of bacteremia than those who received single therapy. On the other hand, patients with Pitt bacteremia scores of ≥4 who received combination therapy were more likely to be immunocompromised than patients who received single therapy.
TABLE 1.
Baseline clinical characteristics of patients with bacteremia caused by gram-negative bacilli with Pitt bacteremia scores of <4 by therapy type
| Variable | Value
|
P value | |
|---|---|---|---|
| Single therapy (n = 319) | Combination therapy (n = 214) | ||
| Age (yr) [median (IQRa)] | 64 (51-76) | 68 (52-77) | 0.35 |
| Gender [n (%)] | 0.10 | ||
| Female | 145 (45) | 82 (38) | |
| Male | 174 (55) | 132 (62) | |
| Diabetes mellitus [n (%)] | 73 (23) | 53 (25) | 0.62 |
| End stage renal disease [n (%)] | 9 (3) | 8 (4) | 0.55 |
| End stage liver disease [n (%)] | 23 (7) | 10 (5) | 0.23 |
| Active cancer [n (%)] | 160 (50) | 86 (40) | 0.02 |
| Cancer type [n (%b)] | 0.36 | ||
| Hematologic tumor | 65 (37) | 39 (45) | |
| Solid tumor | 95 (54) | 47 (55) | |
| Immunocompromised host [n (%)] | 114 (36) | 76 (36) | 0.96 |
| Type of immunosuppression | |||
| Neutropenia [n (%)] | 52 (16) | 27 (13) | 0.24 |
| Recent chemotherapy [n (%)] | 66 (21) | 40 (19) | 0.57 |
| Corticosteroids [n (%)] | 31 (10) | 28 (13) | 0.22 |
| Transplant recipient [n (%)] | 37 (12) | 30 (14) | 0.41 |
| Immunosuppressive therapy [n (%)] | 30 (9) | 28 (13) | 0.18 |
| Recent surgery [n (%)] | 112 (35) | 64 (30) | 0.21 |
| Central venous catheter [n (%)] | 113 (35) | 68 (32) | 0.38 |
| Foley catheter [n (%)] | 57 (18) | 41 (19) | 0.71 |
| Charlson comorbidity score [median (IQR)] | 4.0 (2.0-8.0) | 4.0 (2.0-7.0) | 0.89 |
| Serum creatinine (mg/dl) [median (IQR)] | 1.2 (1.0-1.7) | 1.4 (1.0-1.9) | <0.01 |
| Prior antibiotic therapy [n (%)] | 72 (23) | 58 (27) | 0.23 |
| Gram-negative organism [n (%)] | <0.01 | ||
| E. coli | 120 (38) | 105 (49) | |
| Klebsiella species | 89 (28) | 38 (18) | |
| Pseudomonas species | 27 (8) | 37 (17) | |
| Enterobacter species | 29 (9) | 15 (7) | |
| Others | 54 (17) | 19 (9) | |
| Acquisition site of bacteremia [n (%)] | 0.59 | ||
| Nosocomial | 96 (30) | 66 (31) | |
| Health care associated | 125 (39) | 75 (35) | |
| Community acquired | 98 (31) | 73 (34) | |
| Primary source of bacteremia [n (%)] | <0.01 | ||
| Urinary | 62 (19) | 105 (49) | |
| Gastrointestinal | 98 (31) | 21 (10) | |
| Respiratory | 13 (4) | 13 (6) | |
| Line related | 17 (5) | 14 (7) | |
| Skin and soft tissue | 15 (5) | 5 (2) | |
| Other | 2 (0) | 3 (1) | |
| Unknown primary source | 112 (35) | 53 (25) | |
| Resistance to beta-lactam antibiotic [n (%)] | 13 (4) | 12 (6) | 0.46 |
IQR, interquartile range.
Among subjects with active cancer.
TABLE 2.
Baseline clinical characteristics of patients with bacteremia caused by gram-negative bacilli with Pitt bacteremia scores of ≥4 by therapy type
| Variable | Value
|
P value | |
|---|---|---|---|
| Single therapy (n = 79) | Combination therapy (n = 90) | ||
| Age (yr) [median (IQRa)] | 67 (53-78) | 67 (54-76) | 0.69 |
| Gender [n (%)] | 0.23 | ||
| Female | 23 (29) | 34 (38) | |
| Male | 56 (71) | 56 (62) | |
| Diabetes mellitus [n (%)] | 17 (22) | 22 (24) | 0.65 |
| End stage renal disease [n (%)] | 2 (3) | 6 (7) | 0.21 |
| End stage liver disease [n (%)] | 10 (13) | 7 (8) | 0.29 |
| Active cancer [n (%)] | 34 (43) | 39 (43) | 0.97 |
| Cancer type [n (%b)] | 0.02 | ||
| Hematologic tumor | 8 (24) | 21 (54) | |
| Solid tumor | 26 (76) | 18 (46) | |
| Immunocompromised host [n (%)] | 13 (16) | 29 (32) | 0.02 |
| Type of immunosuppression | |||
| Neutropenia [n (%)] | 3 (4) | 12 (13) | 0.03 |
| Recent chemotherapy [n (%)] | 8 (10) | 17 (19) | 0.11 |
| Corticosteroids [n (%)] | 5 (6) | 10 (11) | 0.28 |
| Transplant recipient [n (%)] | 4 (5) | 11 (12) | 0.10 |
| Immunosuppressive therapy [n (%)] | 4 (5) | 9 (10) | 0.23 |
| Recent surgery [n (%)] | 45 (58) | 42 (47) | 0.15 |
| Central venous catheter [n (%)] | 37 (48) | 46 (51) | 0.69 |
| Foley catheter [n (%)] | 45 (57) | 39 (43) | 0.08 |
| Charlson comorbidity score [median (IQR)] | 4.0 (1.0-8.0) | 4.0 (2.0-7.0) | 0.95 |
| Serum creatinine (mg/dl) [median (IQR)] | 1.5 (1.1-2.1) | 1.6 (1.1-2.5) | 0.18 |
| Prior antibiotic therapy [n (%)] | 26 (33) | 26 (29) | 0.57 |
| Gram-negative organism [n (%)] | 0.76 | ||
| E. coli | 19 (24) | 23 (26) | |
| Klebsiella species | 15 (19) | 21 (23) | |
| Pseudomonas species | 11 (14) | 16 (18) | |
| Enterobacter species | 15 (19) | 13 (14) | |
| Other | 19 (24) | 17 (19) | |
| Acquisition site of bacteremia [n (%)] | 0.28 | ||
| Nosocomial | 47 (59) | 44 (49) | |
| Health care associated | 18 (23) | 30 (33) | |
| Community acquired | 14 (18) | 16 (18) | |
| Primary source of bacteremia [n (%)] | 0.68 | ||
| Urinary | 17 (22) | 29 (32) | |
| Gastrointestinal | 17 (22) | 13 (14) | |
| Respiratory | 18 (23) | 18 (20) | |
| Line related | 6 (8) | 7 (8) | |
| Skin and soft tissue | 1 (1) | 1 (1) | |
| Unknown primary source | 20 (25) | 22 (24) | |
| Resistance to beta-lactam antibiotic [n (%)] | 5 (6) | 1 (1) | 0.10 |
IQR, interquartile range.
Among subjects with active cancer.
The most frequent empirically used beta-lactam antibiotics within 48 h from onset of bacteremia in this study were piperacillin-tazobactam (38.8%), cefepime (36.2%), meropenem (9.2%), ceftriaxone (4.4%), imipenem-cilastin (2.1%), ceftazidime (1.8%), and ertapenem (1.0%), while the most frequently used fluoroquinolone antibiotics in combination therapy were levofloxacin (52.5%) and ciprofloxacin (46.2%). Combination antimicrobial therapy with beta-lactams and fluoroquinolones was used for a median duration of 3 days (range, 2 to 21 days). Only 31 of 304 patients (10.1%) who received fluoroquinolone antibiotic combinations in our study had bacteremia due to fluoroquinolone-resistant gram-negative bacilli.
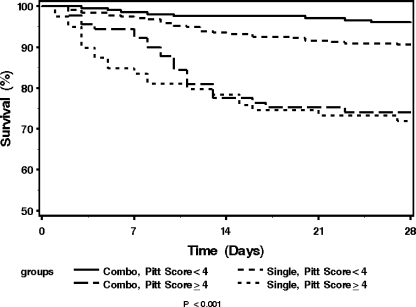
The unadjusted 28-day all-cause mortalities for patients with bacteremia caused by gram-negative bacilli who received single and combination therapy were 8.8% (28 of 319) and 4.2% (9 of 214), respectively, for patients with Pitt bacteremia scores of <4 and 27.8% (22 of 79) and 25.6% (23 of 90) for patients with Pitt bacteremia scores of ≥4 who received single and combination therapy, respectively (Fig. 1). In order to adjust for differences in baseline clinical characteristics between the two treatment groups, the baseline variables that showed different distributions between the two treatment groups were included in a propensity score multivariate logistic regression model (Table 3). After adjusting for the propensity to treat with combination therapy, the 28-day all-cause mortality was lower in patients who received combination therapy than in those who received single therapy in less severely ill patients with Pitt bacteremia scores of <4 (HR, 0.44; 95% CI, 0.20 to 0.98; P = 0.044). For critically ill patients with Pitt bacteremia scores of ≥4, the 28-day mortality was not different between patients who received combination therapy and those who received single therapy (HR, 0.87; 95% CI, 0.47 to 1.62; P = 0.660). These results were consistent when 14-day all-cause mortalities were compared. The 14-day all-cause mortality was significantly lower in patients who received combination therapy than in those who received single therapy in less severely ill patients with Pitt bacteremia scores of <4 (HR, 0.34; 95% CI, 0.12 to 0.92; P = 0.035). For critically ill patients with Pitt bacteremia scores of ≥4, the risk of 14-day mortality was not different for patients who received combination therapy and those who received single therapy (HR, 0.99; 95% CI, 0.50 to 1.96; P = 0.966).
FIG. 1.
Kaplan-Meier survival curves for patients with bacteremia caused by gram-negative bacilli who received single- and combination antibiotic therapy based on Pitt bacteremia scores. Combo, combination therapy with beta-lactams and fluoroquinolones; Single, beta-lactam alone.
TABLE 3.
Multivariable logistic regression modeling the propensity to treat with combination antibiotic therapy
| Variable | Pitt score < 4 (n = 533)
|
Pitt score ≥ 4 (n = 169)
|
||
|---|---|---|---|---|
| ORa (95% CI) | P value | ORa (95% CI) | P value | |
| Female gender | 0.56 (0.37-0.86) | <0.01 | 1.44 (0.65-3.22) | 0.37 |
| Active cancer | 0.81 (0.51-1.30) | 0.39 | 0.73 (0.35-1.55) | 0.42 |
| Immunocompromised host | 1.11 (0.67-1.85) | 0.68 | 2.42 (0.86-6.83) | 0.09 |
| Neutropenia | 0.97 (0.47-2.02) | 0.94 | 5.13 (0.86-30.47) | 0.07 |
| Foley catheter | 0.50 (0.26-0.96) | 0.04 | 0.47 (0.18-1.27) | 0.14 |
| Serum creatinine | 1.05 (0.87-1.28) | 0.60 | 1.30 (0.90-1.89) | 0.17 |
| Admission to ICUb | 3.09 (1.86-5.11) | <0.01 | 5.95 (1.78-19.84) | <0.01 |
| Prior antibiotic therapy | 1.49 (0.91-2.43) | 0.12 | 0.64 (0.28-1.48) | 0.30 |
| Gram-negative organism | ||||
| E. coli | 2.69 (1.29-5.60) | <0.01 | 0.96 (0.32-2.90) | 0.94 |
| Klebsiella species | 1.64 (0.76-3.54) | 0.21 | 1.30 (0.42-4.04) | 0.66 |
| Pseudomonas species | 4.60 (1.99-10.60) | <0.01 | 1.12 (0.34-3.69) | 0.86 |
| Enterobacter species | 2.31 (0.91-5.89) | 0.08 | 0.71 (0.22-2.27) | 0.56 |
| Other | 1.00 (reference) | 1.00 (reference) | ||
| Nosocomial or health care-associated acquisition | 1.05 (0.61-1.81) | 0.86 | 1.17 (0.43-3.14) | 0.76 |
| Primary source of bacteremia | ||||
| Urinary | 3.67 (2.06-6.52) | <0.01 | 2.34 (0.77-7.07) | 0.13 |
| Gastrointestinal | 0.37 (0.19-0.72) | <0.01 | 0.63 (0.19-2.07) | 0.45 |
| Respiratory | 1.85 (0.73-4.70) | 0.12 | 1.48 (0.48-4.51) | 0.50 |
| Line-related | 1.77 (0.72-4.35) | 0.22 | 1.46 (0.33-6.52) | 0.62 |
| Skin and soft tissue | 0.77 (0.28-2.13) | 0.62 | 0.90 (0.05-17.94) | 0.95 |
| Unknown primary source | 1.00 (reference) | 1.00 (reference) | ||
| Resistance to beta-lactam antibiotic | 2.51 (0.96 6.54) | 0.06 | 0.29 (0.03-2.85) | 0.29 |
OR, odds ratio, of receiving combination antibiotic therapy.
ICU, intensive care unit.
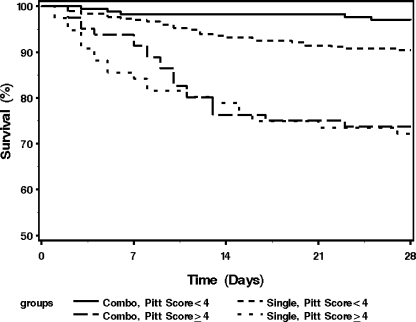
Less severely ill patients with a Pitt bacteremia score of <4 who received two antibiotics with in vitro activity against the gram-negative isolate also had lower 28-day mortality than those who received one antibiotic with in vitro activity (HR, 0.32; 95% CI, 0.12 to 0.84; P = 0.021). For critically ill patients with Pitt bacteremia scores of ≥4, the risk of 28-day mortality was not different between patients who received one or two antibiotics with in vitro activity against the respective gram-negative isolate (HR, 0.89; 95% CI, 0.47 to 1.71; P = 0.728) (Fig. 2).
FIG. 2.
Kaplan-Meier survival curves for patients with bacteremia caused by gram-negative bacilli who received one and two in vitro active antibiotics based on Pitt bacteremia scores. Combo, combination therapy with two in vitro active antibiotics; Single, single therapy with one in vitro active antibiotic.
We did not observe an increased incidence of adverse events in patients who received combination therapy compared to those who received single therapy. Eleven (3.6%) of 304 patients developed C. difficile colitis with combination therapy during the index admission compared to 9 (2.3%) of 398 with single therapy (P = 0.361). There was also no significant difference in the incidence of infections due to multidrug-resistant gram-negative organisms in patients who received combination and single therapy (10/304 [3.3%] and 13/398 [3.3%], respectively; P > 0.999). Similarly, there was no significant increase in the incidence of infections due to resistant gram-positive organisms, such as methicillin-resistant S. aureus, following combination and single therapy (3/304 [1.0%] and 3/398 [0.8%], respectively; P > 0.999).
DISCUSSION
To our knowledge, no previously reported studies have examined the utility of combination antibiotic therapy with beta-lactams and fluoroquinolones in patients with bacteremia caused by gram-negative bacilli. In the current investigation, combination therapy was associated with lower 14-day and 28-day all-cause mortality rates among less severely ill patients with Pitt bacteremia scores of <4, and they represented 75.9% (533 of 702) of the entire cohort. There was a suggestion of a synergistic or additive effect of combination therapy, because less severely ill patients who received two antibiotics with in vitro activity against the respective blood isolate had lower mortality than did those who received one antibiotic with in vitro activity against the blood culture isolate.
Despite the substantial decline in mortality from bacteremia caused by gram-negative bacilli over the past 3 decades, our results are consistent with those of an investigation from 30 years ago that showed improved clinical response with combination therapy compared to single therapy in patients with bacteremia due to different gram-negative bacilli (2). Other studies that reported a benefit from combination therapy were, for the most part, limited to selected gram-negative organisms (4, 14, 17). Our survey included patients with all types of bacteremia caused by gram-negative bacilli. The findings of our work have greater clinical application because they are not restricted in microbiological scope, and they are useful because treatment is begun in most patients before the identification of a blood culture isolate is done.
Unlike studies that showed a survival benefit from combination therapy only in critically ill patients with bacteremia caused by gram-negative bacilli who had very high mortality (17), the effect of combination therapy on mortality in our study was seen in less severely ill patients with Pitt bacteremia scores of <4 who had relatively low mortality, but not in critically ill patients with Pitt bacteremia scores of ≥4. One possible explanation is that mortality in critically ill patients might be impacted by factors other than bacteremia, such as systemic inflammatory response, multiorgan failure, and other acute comorbidities. On the other hand, mortality in less severely ill patients should be limited to bacteremia caused by gram-negative bacilli and its immediate complications.
Based on the sizable difference in mortality between patients with Pitt bacteremia scores of <4 and those with scores of ≥4 (6.9% versus 26.6%, respectively, in our study), stratifying patients by acute severity of illness is crucial in studies of bacteremia caused by gram-negative bacilli. Without such stratification, the overall mortality would be a reflection of the proportion of critically ill patients included in the study. Moreover, if patients in a certain treatment arm were more likely to be critically ill than those in the other treatment arm, not stratifying by acute severity of illness would mask a clinical benefit for that specific therapy. This lack of assessment is commonplace, as 6 (35%) of 17 studies included in a recent meta-analysis did not include an assessment of severity of illness in patients with bacteremia caused by gram-negative bacilli (25). Finally, it is unlikely that the clinical benefit of adding fluoroquinolones in our investigation was due to overcoming resistance to beta-lactam antibiotics because the overall resistance of gram-negative bacilli to beta-lactam antibiotics was low.
The strength of our study is mainly its large sample size and the inclusion of adequate power in the primary overall analysis. Nevertheless, the study was not empowered to detect a difference in mortality in subgroups of patients divided on the basis of bacteremia due to a particular gram-negative organism, primary source of infection, or an individual class of beta-lactam antibiotics received. Using all-cause mortality as a primary outcome, blinding during the case ascertainment phase of the study, and defining the study hypothesis and aims prior to data collection reduced subjectivity and added strength to the study. Additionally, only 42 of 702 patients (6.0%) were lost to follow-up within 28 days of the onset of bacteremia, which is very impressive for a retrospective cohort study.
A propensity score analysis was utilized in order to adjust for the bias that can result in a retrospective design in which the treatment allocation is not randomized. Selection bias, or confounding due to important prognostic baseline differences among patients, often leads to biased estimates in observational studies (13). For example, bias can result from unmeasured interactions in the diagnostic and treatment process, such as patients' or physicians' preferences. An individual's propensity score is defined as his/her conditional probability of a particular exposure versus another (i.e., combination versus single therapy), given the observed confounders. Therefore, akin to a randomized trial, there is balance of the measured confounders between exposure groups after adjusting for the propensity score (7, 23, 24). To our knowledge, this is the first study of bacteremia caused by gram-negative organisms to use a propensity score analysis to examine the effect of combination antibiotic therapy on outcome.
The study has some limitations. First, this is a retrospective cohort study, so treatment allocation was not randomized. Despite adjusting for a large number of potential known confounders using propensity score analysis, it remains possible that there are unknown confounders that are not accounted for in the study. Other limitations are related to the generalizability of the study results. We used strict inclusion and exclusion criteria in the study. This allowed the elimination of considerable background noise by excluding patients with polymicrobial and recurrent bacteremia caused by gram-negative bacilli. In addition, excluding patients who received antimicrobial regimens that did not conform to our study's definitions of single- and combination antibiotic therapy limited the number of potential confounders and ultimately strengthened the statistical model. On the other hand, using strict criteria limited the study to a subset of patients with bacteremia caused by gram-negative bacilli, rather than the entire group. For example, many critically ill patients with P. aeruginosa bacteremia were excluded from the study due to the administration of aminoglycosides. Furthermore, in vitro resistance rates of gram-negative bacillus blood culture isolates to fluoroquinolone antibiotics at each institution need to be considered when interpreting the study results. There may be less benefit with fluoroquinolone combinations at institutions where higher resistance rates exist. Finally, we did not collect data on all adverse events, including QT interval prolongation. Our analysis of adverse events was limited to those events that occurred during the index hospital admission for bacteremia caused by gram-negative bacilli.
A double-blinded randomized clinical trial is the gold standard design to attempt to definitely confirm the notion that combination therapy for bacteremia caused by gram-negative bacilli is beneficial. Nonetheless, performing such a study requires a large budget and a prolonged period to enroll enough patients to secure an adequately powered study. During this time, resistance patterns of gram-negative bacilli could change, and new antimicrobials that make the study results clinically less relevant may become available. In addition, in an intention-to-treat analysis, many patients can receive alternative regimens to what they were randomly allocated to receive. For example, attending physicians may override the study protocol and administer a preferred antimicrobial regimen, particularly if patients clinically deteriorate.
Historically, studies of bacteremia caused by gram-negative bacilli have included beta-lactams with or without another class of antimicrobials. An examination of fluoroquinolone monotherapy for treatment of bacteremia caused by gram-negative bacilli has not been generally recommended; therefore, we excluded this group of patients. Based on the popularity of fluoroquinolone use in the adult population, however, subsequent investigations should include an examination of fluoroquinolone monotherapy in the treatment of this syndrome.
In summary, our results show that the empirical use of combination beta-lactam and fluoroquinolone antibiotics for bacteremia caused by gram-negative bacilli in the first 48 to 72 h after obtaining blood cultures, while awaiting identification of a gram-negative organism and the results of in vitro antimicrobial susceptibility tests, is beneficial in areas where gram-negative isolates have relatively low rates of resistance to fluoroquinolones. Considering the nephrotoxicity and minimal overall benefit from aminoglycoside antibiotic combinations in previous studies, combination therapy with fluoroquinolone and beta-lactam antibiotics for bacteremia caused by gram-negative bacilli appears more promising. Further examination is required before combination therapy can be routinely recommended.
Acknowledgments
We thank Randy Wendt and the CBAM program staff at the Mayo Clinic, Rochester, MN, for their help in generating the antibiotic database for this study.
The study received funding from the Baddour Family funds and the Small Grants program at the Mayo Clinic, Rochester, MN.
M.N.A.-H., J.W.W., B.D.L., K.M.T., J.E.E., E.A.V., I.M.T., and L.M.B. have no conflict of interest.
M.N.A. and B.D.L. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Al-Hasan, M. N., J. W. Wilson, B. D. Lahr, J. E. Eckel-Passow, and L. M. Baddour. 2008. Incidence of Pseudomonas aeruginosa bacteremia: a population-based study. Am. J. Med. 121:702-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, E. T., L. S. Young, and W. L. Hewitt. 1978. Antimicrobial synergism in the therapy of gram-negative rod bacteremia. Chemotherapy 24:45-54. [DOI] [PubMed] [Google Scholar]
- 3.Bliziotis, I. A., A. Michalopoulos, S. K. Kasiakou, G. Samonis, C. Christodoulou, S. Chrysanthopoulou, and M. E. Falagas. 2005. Ciprofloxacin vs an aminoglycoside in combination with a beta-lactam for the treatment of febrile neutropenia: a meta-analysis of randomized controlled trials. Mayo Clin. Proc. 80:1146-1156. [DOI] [PubMed] [Google Scholar]
- 4.Chamot, E., E. Boffi El Amari, P. Rohner, and C. Van Delden. 2003. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob. Agents Chemother. 47:2756-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charlson, M. E., P. Pompei, K. L. Ales, and C. R. MacKenzie. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373-383. [DOI] [PubMed] [Google Scholar]
- 6.Chow, J. W., and V. L. Yu. 1999. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int. J. Antimicrob. Agents 11:7-12. [DOI] [PubMed] [Google Scholar]
- 7.D'Agostino, R. B., Jr. 1998. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 17:2265-2281. [DOI] [PubMed] [Google Scholar]
- 8.Drago, L., E. De Vecchi, L. Nicola, D. Legnani, A. Lombardi, and M. R. Gismondo. 2005. In vitro synergy and selection of resistance by fluoroquinolones plus amikacin or beta-lactams against extended-spectrum beta-lactamase-producing Escherichia coli. J. Chemother. 17:46-53. [DOI] [PubMed] [Google Scholar]
- 9.Drago, L., E. De Vecchi, L. Nicola, L. Tocalli, and M. R. Gismondo. 2005. In vitro selection of resistance in Pseudomonas aeruginosa and Acinetobacter spp. by levofloxacin and ciprofloxacin alone and in combination with beta-lactams and amikacin. J. Antimicrob. Chemother. 56:353-359. [DOI] [PubMed] [Google Scholar]
- 10.EORTC. 1987. Ceftazidime combined with a short or long course of amikacin for empirical therapy of gram-negative bacteremia in cancer patients with granulocytopenia. N. Engl. J. Med. 317:1692-1698. [DOI] [PubMed] [Google Scholar]
- 11.Friedman, N. D., K. S. Kaye, J. E. Stout, S. A. McGarry, S. L. Trivette, J. P. Briggs, W. Lamm, C. Clark, J. MacFarquhar, A. L. Walton, L. B. Reller, and D. J. Sexton. 2002. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 137:791-797. [DOI] [PubMed] [Google Scholar]
- 12.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control. 16:128-140. [DOI] [PubMed] [Google Scholar]
- 13.Grimes, D. A., and K. F. Schulz. 2002. Bias and causal associations in observational research. Lancet 359:248-252. [DOI] [PubMed] [Google Scholar]
- 14.Hilf, M., V. L. Yu, J. Sharp, J. J. Zuravleff, J. A. Korvick, and R. R. Muder. 1989. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am. J. Med. 87:540-546. [DOI] [PubMed] [Google Scholar]
- 15.Kang, C. I., S. H. Kim, W. B. Park, K. D. Lee, H. B. Kim, E. C. Kim, M. D. Oh, and K. W. Choe. 2005. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob. Agents Chemother. 49:760-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klibanov, O. M., R. H. Raasch, and J. C. Rublein. 2004. Single versus combined antibiotic therapy for gram-negative infections. Ann. Pharmacother. 38:332-337. [DOI] [PubMed] [Google Scholar]
- 17.Korvick, J. A., C. S. Bryan, B. Farber, T. R. Beam, Jr., L. Schenfeld, R. R. Muder, D. Weinbaum, R. Lumish, D. N. Gerding, M. M. Wagener, et al. 1992. Prospective observational study of Klebsiella bacteremia in 230 patients: outcome for antibiotic combinations versus monotherapy. Antimicrob. Agents Chemother. 36:2639-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leibovici, L., M. Paul, O. Poznanski, M. Drucker, Z. Samra, H. Konigsberger, and S. D. Pitlik. 1997. Monotherapy versus beta-lactam-aminoglycoside combination treatment for gram-negative bacteremia: a prospective, observational study. Antimicrob. Agents Chemother. 41:1127-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Micek, S. T., A. E. Lloyd, D. J. Ritchie, R. M. Reichley, V. J. Fraser, and M. H. Kollef. 2005. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob. Agents Chemother. 49:1306-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paterson, D. L., W. C. Ko, A. Von Gottberg, S. Mohapatra, J. M. Casellas, H. Goossens, L. Mulazimoglu, G. Trenholme, K. P. Klugman, R. A. Bonomo, L. B. Rice, M. M. Wagener, J. G. McCormack, and V. L. Yu. 2004. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann. Intern. Med. 140:26-32. [DOI] [PubMed] [Google Scholar]
- 21.Paul, M., I. Silbiger, S. Grozinsky, K. Soares-Weiser, and L. Leibovici. 25 January 2006. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst. Rev. CD003344. [DOI] [PubMed]
- 22.Piccoli, L., M. Guerrini, A. Felici, and F. Marchetti. 2005. In vitro and in vivo synergy of levofloxacin or amikacin both in combination with ceftazidime against clinical isolates of Pseudomonas aeruginosa. J. Chemother. 17:355-360. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum, P. R., and D. B. Rubin. 1983. The central role of the propensity score in observational studies for causal effects. Biometrika 70:41-55. [Google Scholar]
- 24.Rubin, D. B. 1997. Estimating causal effects from large data sets using propensity scores. Ann. Intern. Med. 127:757-763. [DOI] [PubMed] [Google Scholar]
- 25.Safdar, N., J. Handelsman, and D. G. Maki. 2004. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect. Dis. 4:519-527. [DOI] [PubMed] [Google Scholar]
- 26.Zager, R. A., and R. B. Prior. 1986. Gentamicin and gram-negative bacteremia. A synergism for the development of experimental nephrotoxic acute renal failure. J. Clin. Investig. 78:196-204. [DOI] [PMC free article] [PubMed] [Google Scholar]