Abstract
In an attempt to augment the efficacy of 7-chloro 4-aminoquinoline analogs and also to overcome resistance to antimalarial agents, we synthesized three cyclen (1,4,7,10-tetraazacyclododecane) analogs of chloroquine [a bisquinoline derivative, 7-chloro-4-(1,4,7,10-tetraaza-cyclododec-1-yl)-quinoline HBr, and a 7-chloro-4-(1,4,7,10-tetraaza-cyclododec-1-yl)-quinoline-Zn2+ complex]. The bisquinoline displays the most potent in vitro and in vivo antimalarial activities. It displays 50% inhibitory concentrations (IC50s) of 7.5 nM against the D6 (chloroquine-sensitive) clone of Plasmodium falciparum and 19.2 nM against the W2 (chloroquine-resistant) clone, which are comparable to those of artemisinin (10.6 and 5.0 nM, respectively) and lower than those of chloroquine (10.7 and 87.2 nM, respectively), without any evidence of cytotoxicity to mammalian cells, indicating a high selectivity index (>1,333 against D6 clone and >521 against W2 clone). Potent antimalarial activities of the bisquinoline against chloroquine- and mefloquine-resistant strains of P. falciparum were also confirmed by in vitro [3H]hypoxanthine incorporation assay. The in vivo antimalarial activity of the bisquinoline, as determined in P. berghei-infected mice, is comparable to that of chloroquine (50% effective dose, ≤1.1 mg/kg when given orally); no apparent toxicity has been observed up to the highest dose tested (3 × 30 mg/kg). The bisquinoline inhibits in vitro hemozoin (β-hematin) formation with an IC50 of 1.1 μM, which is about 10-fold more potent than chloroquine (IC50 9.5 μM). Overall, this article describes the discovery of a new class of cyclen 4-aminoquinoline analogs as potent antimalarial drugs.
Macrocyclic polyamines have proven to be extremely valuable as scaffolds for incorporating metal ions into larger molecular fragments. Specifically, the kinetic and thermodynamic metal binding characteristics of the 12-membered tetraazamacrocycle cyclen (1,4,7,10-tetraazacyclododecane; compound 1 in Fig. 1) are well suited to a wide range of biological applications. In addition, the versatility of this macrocycle continues to grow as new methodology for selective functionalization has appeared. Thus, derivatized forms of cyclen are becoming increasingly popular as standard building blocks in applications requiring highly selective metal ion chelation (1, 6, 7, 9, 11). Within the past decade, cyclen has become an important structural moiety for a variety of diagnostic and therapeutic pharmaceutical agents (3) and in the development of magnetic resonance imaging contrast agents (4). More recently, advances in targeted cancer agents such as antibodies and peptides have revived interest in cyclen-based bifunctional chelating agents for therapy (12). The biochemical and pharmacological properties of macrocyclic polyamines have been partially exploited. Important DNA-recognizing behavior and anti-human immunodeficiency virus activity of some derivatives of these macrocycles have been reported (1, 8, 10). However, owing to their unique capacity to bind some of the biologically significant metals, including Zn, these macrocyclic polyamines have opened a new era in bioorganic and medicinal chemistry research.
FIG. 1.
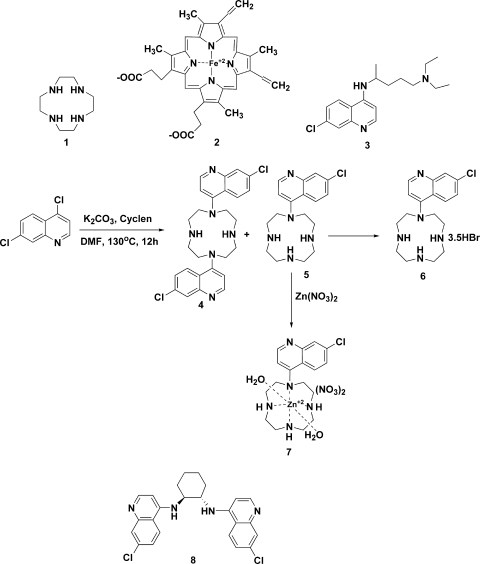
Structures of the compounds described in this report. DMF, N,N-dimethylformamide.
The general structural similarity of cyclen and heme (compound 2 in Fig. 1) and its metal binding property attracted our interest in incorporating this ring system into antimalarial chemotherapy to augment the efficacy of chloroquine (compound 3 in Fig. 1) against drug-susceptible and -resistant strains of malarial parasites. First, the cyclen moiety may be considered the cyclic analog of the alkylamino side chain of chloroquine. Second, it may be considered a simpler structural framework of heme (compound 2), which can be another potential means to augment the toxicity of chloroquine for parasites. It is generally accepted that chloroquine exerts its toxic effects by interfering with the conversion of free heme to hemozoin, thus accumulating in the food vacuole a large quantity of heme, which lyses membranes, generates reactive oxygen intermediates, and inhibits many other processes. Thus, heme is quite toxic to malarial parasites. The structural similarity between cyclen and heme would possibly provide a better means to inhibit hemozoin formation from heme by malarial parasites. Thus, three cyclen analogs of chloroquine, a bisquinoline derivative, an HBr salt, and a Zn2+ complex (compounds 4, 6, and 7, respectively, in Fig. 1), were synthesized and evaluated for antimalarial activity. In addition, the inhibitory potency of compound 4 (Fig. 1) to β-hematin formation has also been determined to understand its mechanism of antimalarial action.
MATERIALS AND METHODS
Synthesis.
The syntheses of compounds 4, 6, and 7 were performed as outlined in the central part of Fig. 1. Amination of 2 equivalents of 4,7-dichloroquinoline with 1 equivalent of cyclen at 130°C for 12 h furnished 35% of the bisquinoline (compound 4 in Fig. 1) and 25% of the monosubstituted quinoline (compound 5 in Fig. 1) cyclen derivatives after purification by column chromatography over silica gel using CHCl3-CH3OH-NH4OH (9:0.9:0.1). A trace of what appeared to be a trisubstituted product was also detected by mass spectroscopy but was not characterized further. However, no tetraquinoline product was observed by mass spectroscopy, possibly due to steric hindrance. The bisquinoline derivative (compound 4) was crystallized from ethanol to yield fluffy, needle-shaped crystals. The monosubstituted product (compound 5) was converted to its HBr salt (compound 6 in Fig. 1) by treating a methanolic solution with concentrated HBr diluted with methanol. The product crystallized from methanol yielded a salt (compound 6) containing 3.5 mol of HBr per mol of the compound, as indicated by elemental analysis. This unusual stoichiometry of the HBr salt of compound 6 may explain the folding of the cyclen ring and the production of steric hindrance on the ring nitrogen atoms. Potentiometric titration revealed that two of the cyclen N atoms become protonated with pKa values of 10.0 and 7.5, respectively, and the third one being the quinoline ring N atom with a pKa value of 4.6 (unpublished observation). Since the salt was formed in methanol (with little water), the trihydrobromide salt thus formed quickly precipitated out of the solution with only partial protonation of the sterically hindered fourth nitrogen, which appears to be one of the 2° amines of the cyclen ring. A portion of compound 5 was converted to the Zn2+ complex (compound 7 in Fig. 1) by overnight treatment of an equimolar amount of the ethanol solution with 50:50 ethanol-aqueous Zn(NO3)2 · 6H2O at 60°C, which was then crystallized from ethanol to yield compound 7 (60%). All of the compounds were characterized by 1H nuclear magnetic resonance (NMR), electron impact mass spectrometry (EIMS), and elemental analyses.
Bisquinoline derivative of cyclen (compound 4).
1H NMR analysis (CDCl3): 8.7 (4H, m, Ar), 8.0, (H, m, Ar) 7.3 (H, m, Ar) 7.0 (H, m, Ar), 3.5 (8H, m, 4 × CH2), 3.0 (8H, m, 4 × CH2), 2.4 (2H, brs, 2 × NH). EIMS analysis: 494 (M), 495 (M + 1), 496 (M + 2), 497 (M + 3), 498 (M + 4). Elemental analysis: calc. (C26H28Cl2N6), C, 63.03; H, 5.7; N, 16.96; found, C, 61.99; H, 5.79; N, 16.4. It contains 1 mol of ethanol as a crystallization solvent, which is also evident in the NMR spectrum.
7-Chloro-4-(1,4,7,10-tetraaza-cyclododec-1-yl)-quinoline HBr (compound 6).
1H NMR analysis (free base in CDCl3): 8.6 to 8.8 (2H, m, Ar), 8.0 (1H, m, Ar), 7.5, (1H, m, Ar), 7.0, (1H, m, Ar), 3.4 (4H, m, 2 × CH2), 2.7 to 3.0 (12H, m, 6 × CH2), 2.3 to 2.6 (3H, brs, overlapped with CH2 peaks, 3 × NH). EIMS analysis: 333 (M), 334 (M + 1), 335 (M + 2), 336 (M + 3) 337 (M + 4). Elemental analysis: calc. (C17H24ClN5 · 3HBr), C, 35.4; H, 4.7; N, 12.2; calc. (C17H24ClN5 · 4HBr), C, 31.1; H, 4.3; N, 10.7; found, C, 32.9; H, 4.8; N, 12.2, which matches well when the salt contains 3.5 mol of HBr.
7-Chloro-4-(1,4,7,10-tetraaza-cyclododec-1-yl)-quinoline-Zn2+ complex (compound 7).
1H NMR analysis (D2O): 8.6 (1H, m, Ar), 7.6 to 7.9 (2H, m, Ar), 7.3 to 7.5 (2H, m, Ar), 2.4 to 3.3 (19H, m, 8 × CH2 + 3 × NH). Elemental analysis: calc. (C17H25ClN7O8Zn), C, 36.7; H, 4.5; N, 17.6; found, C, 35.7; H, 4.5; N, 17.5. Calculated based on the structure shown in the central part of Fig. 1.
Bioassays. (i) In vitro antimalarial activity and cytotoxicity.
Compounds 4, 6, and 7 were tested for in vitro antimalarial activity against the D6 (chloroquine sensitive) and W2 (chloroquine-resistant) strains of Plasmodium falciparum, which were obtained from the Division of Experimental Therapeutics, Walter Reed Army Institute of Research (WRAIR), Washington, DC. The assay is based on the determination of plasmodial lactate dehydrogenase (LDH) activity. A suspension of red blood cells infected with strain D6 or W2 of P. falciparum (200 μl, with 2% parasitemia and 2% hematocrit in RPMI 1640 medium supplemented with 10% human serum and 60 μg/ml amikacin) is added to the wells of a 96-well plate containing 10 μl of serially diluted test samples. The plate is incubated at 37°C for 72 h in a modular incubation chamber flushed with a gas mixture of 90% N2, 5% O2, and 5% CO2. Parasite LDH activity is determined according to the procedure of Makler and Hinrichs (13). Twenty microliters of the incubation mixture is mixed with 100 μl of Malstat reagent (Flow Inc., Portland, OR) and incubated at room temperature for 30 min. Twenty microliters of a 1:1 mixture of nitroblue tetrazolium and phenazine ethosulfate (Sigma, St. Louis, MO) is then added, and the plate is further incubated in the dark for 1 h. The reaction is stopped by adding 100 μl of 5% acetic acid. The plate is read at 650 nm. Percent growth is plotted versus test concentrations. IC50s are obtained from the dose-response curves. Artemisinin and chloroquine are included as drug controls, and dimethyl sulfoxide is included as a vehicle control. To determine the selectivity index (SI) of the antimalarial activity of test compounds, their in vitro cytotoxicity to mammalian cells is also determined. The assay is performed with 96-well tissue culture-treated plates. Vero cells (monkey kidney fibroblasts [American Type Culture Collection, Manassas, VA]) are seeded into the wells of 96-well plates at a density of 25,000 cells/well and incubated for 24 h. Samples at different concentrations are added, and the plates are again incubated for 48 h. The number of viable cells is determined by Neutral Red assay (2). IC50s are obtained from dose-response curves.
(ii) Inhibition of β-hematin formation.
The effect on the formation of β-hematin in vitro was evaluated with a spectrophotometric 96-well microplate assay (16). A 10 mM stock of the compound was dissolved in 0.1 N HCl, and the solution was diluted further with acetate buffer (100 mM, pH 4.5). The β-hematin formation assay was performed with 96-well Millipore Multiscreen filtration plates. The assay mixture, in a total volume of 200 μl, contained acetate buffer (100 mM, pH 4.5), hemin HCl (100 μM), and oleoylglycerol (2 μg). Each assay was performed at least in triplicate. The compound and chloroquine (as a positive control) were tested at different concentrations. The plates were incubated at room temperature (24 to 28°C) for 14 h with constant shaking; the plate was then put on the filtration manifold, and a vacuum was applied to stop the reaction and remove the buffers. The contents of each well were sequentially washed with at least 1 ml of Tris-HCl-sodium dodecyl sulfate solution and alkaline bicarbonate buffer (100 mM, pH 9.0) prewarmed to 37°C. To dissolve the final pellet, 20 μl of 1 N NaOH was added to each well and the plates were incubated at 37°C for 30 min. One hundred eighty microliters of Tris-HCl (100 mM, pH 7.4) containing 2.5% sodium dodecyl sulfate was added to each well. The contents were transferred to a clear, flat-bottom 96-well plate, and the plate was read at 405 nm on a microplate reader. The data were computed as percent inhibition of β-hematin formation in the presence of different concentrations of the compound, and a dose-response curve was obtained.
(iii) In vivo antimalarial activity.
The in vivo antimalarial activity of the compounds was determined in mice infected with Plasmodium berghei (NK-65 strain) according to the Peters 4-day suppressive test (14), which was modified to a 3-day treatment schedule. Male mice (Swiss Webster strain) weighing 18 to 20 g were intraperitoneally inoculated with 2 × 107 parasitized red blood cells obtained from a highly infected donor mouse. Mice were divided into different groups with five mice in each group. Test compounds and chloroquine were prepared in 0.1 N HCl and diluted further in distilled water to obtain appropriate concentrations. The compounds were administered orally to P. berghei-infected mice about 2 h after infection (day 0). The test compounds were administered to the mice once a day for 3 consecutive days (days 0 to 2). A control group was treated with an equal volume of vehicle, while another control group was treated with the standard antimalarial drug chloroquine. The mice were closely observed after every dose for any signs of toxicity. Blood smears were prepared on different days starting at 5 days postinfection (until day 28 postinfection) by clipping the tail end, stained with Giemsa, and observed under a microscope for determination of parasitemia. Mice without parasitemia until day 28 postinfection were considered cured.
RESULTS
All of the compounds tested display potent in vitro antimalarial activity (Table 1). Compound 4 was the most potent one against both chloroquine-sensitive and -resistant strains of P. falciparum, with IC50s of 7.5 and 19.2 nM, respectively. Its activity is comparable to that of artemisinin (IC50s, 10.6 and 5.0 nM, respectively) and better than that of chloroquine (IC50s, 10.7 and 87.2 nM, respectively), with no cytotoxicity (SI, >1,333 for the D6 clone and >521 for the W2 clone). The potent antimalarial efficacy of compound 4 against chloroquine- and mefloquine-resistant strains of P. falciparum was also confirmed by [3H]hypoxanthine incorporation assays (Table 1). Compound 4 is equally active against strains D6 (a chloroquine-susceptible strain with marginal resistance to mefloquine), W2 (a chloroquine-resistant strain), and TM91C235 (a laboratory-generated mefloquine-resistant strain). It is important to mention that the antimalarial activity of all of the analogs was determined at the National Center for Natural Products Research, where the LDH assay is routinely utilized for screening against strains D6 and W2 of P. falciparum by employing chloroquine and artemisinin as control drugs. The most active analog (compound 4) was sent for further testing against the mefloquine-resistant analog (TM91C235) at WRAIR, where the [3H]hypoxanthine incorporation assay is routinely utilized by employing chloroquine and mefloquine as control drugs. Both of these methods had been reported to be directly comparable (13), and that was found to be the case in the present study as well. As shown in Table 1, both of these assays produced comparable IC50s of both chloroquine (10.7 and 8.2 nM) and compound 4 (7.5 and 4.1 nM) against the D6 strain. However, the difference in the IC50s for resistant strain W2 could be due to different levels of drug resistance in the cultures used at the two different laboratories.
TABLE 1.
In vitro antimalarial activities of cyclen 4-aminoquinoline analogs against different strains of P. falciparuma
| Test and compound or drug | IC50 (nM) (SI) for parasite strain:
|
Cytotoxicity | ||
|---|---|---|---|---|
| D6 | W2 | TM91C235 | ||
| Parasitic LDH assay | ||||
| Compound 4 | 7.5 (>1,333) | 19.2 (>521) | NC | |
| Compound 6 | 64.8 (>154) | 210.7 (>48) | NC | |
| Compound 7 | 80.5 (>124) | 1234 (>8) | NC | |
| Chloroquine | 10.7 (>943) | 87.2 (>115) | NC | |
| Artemisinin | 10.6 (>943) | 5.0 (>2,000) | NC | |
| Hypoxanthine incorporation assayb | ||||
| Compound 4 | 4.1 | 5.8 | 2.5 | |
| Chloroquine | 8.2 | 283.5 | 90.0 | |
| Mefloquine | 19.9 | 7.2 | 55.8 | |
NC, no cytotoxicity up to about 10 μM; SI = selectivity index (IC50 for Vero cells/IC50 for P. falciparum); D6, chloroquine-susceptible strain; W2-chloroquine-resistant strain; TM91C235, mefloquine-resistant strain.
Data obtained from WRAIR, Bethesda, MD.
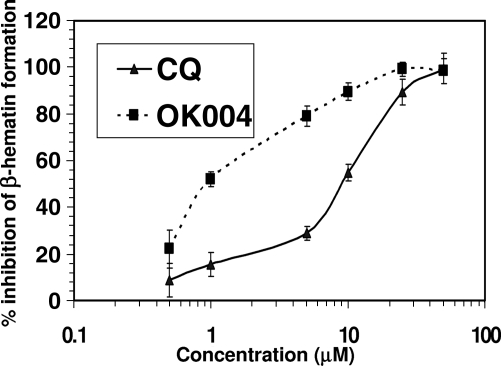
Compound 4 causes significantly greater inhibition of β-hematin formation than does chloroquine, with an IC50 of 1.1 μM in comparison to the chloroquine IC50 of 9.5 μM (Fig. 2).
FIG. 2.
Effects of the cyclen 4-aminoquinoline analog compound 4 (OK004) and chloroquine (CQ) on in vitro β-hematin formation. Each point represents the mean of three observations ± the standard error of the mean.
The in vivo antimalarial activity of compound 4, which was found to be most potent in vitro, was determined in mice infected with P. berghei (NK-65 strain) according to the Peters 4-day suppressive test as described in Materials and Methods. As shown in Table 2, the compound showed activity comparable to that of the standard drug chloroquine (50% effective dose, ≤1.1 mg/kg; 90% effective dose, 5.6 mg/kg). No apparent toxicity was observed up to the highest dose tested (3 × 30 mg/kg), and thus the compound warrants further development as a potential clinical candidate.
TABLE 2.
In vivo antimalarial activity of cyclen 4-aminoquinoline analog compound 4
| Compound or drug | Dose (days × amt [mg/kg]) | % Suppression of parasitemiaa
|
MSTb (no. of survivors/total) | No. cured/total (%)c | ||
|---|---|---|---|---|---|---|
| Day 5 | Day 7 | Day 10 | ||||
| Vehicled | 22.2 (0/5) | 0/5 | ||||
| Compound 4 | 3 × 1.1 | 65.2 | 33.3 | 22.7 | 23.8 (0/5) | 0/5 |
| 3 × 3.3 | 84.6 | 44.1 | 43.9 | 22.8 (0/5) | 0/5 | |
| 3 × 10 | 96.3 | 59.7 | 40.9 | 26.2 (2/5) | 0/5 | |
| 3 × 30 | 100 | 100 | 100 | >28 (5/5) | 3/5 (60) | |
| Chloroquine | 3 × 30 | 100 | 100 | 100 | >28 (4/4) | 3/4 (75) |
Percent suppression of parasitemia compared to the vehicle control.
MST, mean survival time in days(number of mice that survived until 28th day postinfection/total number of mice in group).
Mice with no parasitemia until day 28 postinfection/total mice in group(% cured).
The vehicle was 0.1 N HCl.
DISCUSSION
As evident in the results of in vitro antimalarial assays, cyclen substitution appears to overcome the resistance of the parasite to 4-aminoquinolines, perhaps due to a greater interaction of the analog than that of chloroquine with heme. This may result in decreased efflux of the drug, a common mechanism responsible for chloroquine resistance in malaria (17).
During the intraerythrocytic growth and proliferation of the malaria parasite, hemoglobin is utilized as the predominant source of nutrition. Digestion of hemoglobin by the malaria parasite occurs through a sequential metabolic process and leads to continuous release of heme as a toxic by-product. The malaria parasite, however, has developed a distinct process for detoxification of heme through its conversion into an insoluble, crystalline form known as hemozoin or malaria pigment. The antimalarial action of several drugs such as chloroquine, mefloquine, halofantrine, pyronaridine, and artemisinins has been attributed to their interaction with heme and inhibition of hemozoin formation (15). The antimalarial action of 4-aminoquiniline analogs has been correlated with their accumulation in the parasite food vacuole and inhibition of hemozoin synthesis. Thus, the effect of the most potent cyclen analog (compound 4) on the formation of β-hematin has also been evaluated and compared with those of chloroquine and artemisinin as standards. The assay of inhibition of β-hematin formation, as shown in Results, indicates that the cyclen moiety causes a significant increase in the potency of hemozoin synthesis inhibition. However, the antimalarial activity of compound 4 against the chloroquine-susceptible strain P. falciparum D6 is almost the same as that of chloroquine. This indicates that the better activity of compound 4 against the chloroquine-resistant strain P. falciparum W2 may be due to its higher accumulation in the resistant parasite. Further mechanistic studies are needed to understand the mechanism and also for further development of antimalarial agents of this class of compounds.
It should be noted that by using isotherm titration microcalorimetry of eight quinoline antimalarials, including a bisquinoline (compound 8 in Fig. 1), it has been demonstrated that these compounds mediate antimalarial activity by inhibiting hemozoin formation through binding to a dimer of hematin (5).
In that study, the bisquinoline derivative was shown to have the most potent such activity with the highest association constant. While the stoichiometry of drug-hematin dimer binding was 1:2 for the quinoline antimalarials chloroquine, quinacrine, and amodiaquine, it was 1:10 for bisquinoline compound 8, suggesting that it has a greater capacity to bind the hematin dimer. The result presented here, that the bisquinoline compound 4 is about ninefold more potent than chloroquine at inhibiting hemozoin synthesis, correlates well with those observations.
Acknowledgments
We are grateful to the Centers for Disease Control and Prevention (1U01 CI000211-01) and the USDA (cooperative agreement 58-6408-2-0009 for antimalarial assays) for financial support.
We are also grateful to John Trott of the National Center for Natural Products Research, University of Mississippi, for performing the antimalarial assays. The in vitro [3H]hypoxanthine incorporation assays were conducted at WRAIR, Bethesda, MD.
Footnotes
Published ahead of print on 26 January 2009.
REFERENCES
- 1.Aoki, S., and E. Kimura. 2004. Zinc-nucleic acid interaction. Chem. Rev. 104:769-787. [DOI] [PubMed] [Google Scholar]
- 2.Borenfreund, E., H. Babich, and N. Martin-Alguacil. 1990. Rapid chemosensitivity assay with human normal and tumor cells in vitro. In Vitro Cell Dev. Biol. 26:1030-1034. [DOI] [PubMed] [Google Scholar]
- 3.Bradshaw, J. S., K. E. Krakowiak, and R. M. Izatt. 1993. The chemistry of heterocyclic compounds, p 16-21, 83-85, 157-165. Wiley & Sons, Inc., New York, NY.
- 4.Caravan, P., J. J. Ellison, T. J. McMurray, and R. B. Lauffer. 1999. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem. Rev. 99:2293-2352. [DOI] [PubMed] [Google Scholar]
- 5.Dorn, A., S. R. Vippagunta, H. Matile, C. Jaquet, J. L. Vennerstrom, and R. G. Ridley. 1998. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerisation by quinoline antimalarials. Biochem. Pharmacol. 55:727-736. [DOI] [PubMed] [Google Scholar]
- 6.Kikuta, E., S. Aoki, and E. Kimura. 2001. A new type of potent inhibitors of HIV-1 TAR RNA-Tat peptide binding by zinc(II)-macrocyclic tetraamine complexes. J. Am. Chem. Soc. 123:7911-7912. [DOI] [PubMed] [Google Scholar]
- 7.Kikuta, E., S. Aoki, and E. Kimura. 2002. New potent agents binding to a poly(dT) sequence in double-stranded DNA: bis(Zn2+-cyclen) and tris(Zn2+-cyclen) complexes. J. Biol. Inorg. Chem. 7:473-482. [DOI] [PubMed] [Google Scholar]
- 8.Kimura, E., N. Katsube, T. Koike, M. Shiro, and S. Aoki. 2002. Effects of bis(aromatic) pendants on recognition of nucleobase thymine by Zn2+-1,4,7,10-tetraazacyclododecane (Zn2+-cyclen). Supramol. Chem. 14:95-102. [Google Scholar]
- 9.Kimura, E. 2001. Model studies for molecular recognition of carbonic anhydrase and carboxypeptidase. Acc. Chem. Res. 34:171-179. [DOI] [PubMed] [Google Scholar]
- 10.Kimura, E., and E. Kikuta. 2000. Why zinc in zinc enzymes? From biological roles to DNA base-selective recognition. J. Biol. Inorg. Chem. 5:139-155. [DOI] [PubMed] [Google Scholar]
- 11.Kumar, K., and M. Tweedle. 1993. Macrocyclic polyaminocarboxylate complexes of lanthanides as magnetic resonance imaging contrast agents. Pure Appl. Chem. 65:515-520. [Google Scholar]
- 12.Liu, S., and D. S. Edwards. 2001. Bifunctional chelators for therapeutic lanthanide radiopharmaceuticals. Bioconjug. Chem. 12:7-34. [DOI] [PubMed] [Google Scholar]
- 13.Makler, M. T., and D. J. Hinrichs. 1993. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am. J. Trop. Med. Hyg. 48:205-210. [DOI] [PubMed] [Google Scholar]
- 14.Peters, W. 1975. Chemotherapy of rodent malaria. XXII. Value of drug-resistant strains of Plasmodium berghei in screening for blood schizontocidal activity. Ann. Trop. Med. Parasitol. 69:155-171. [PubMed] [Google Scholar]
- 15.Tekwani, B. L., and L. A. Walker. 2005. Targeting the hemozoin synthesis pathway for new antimalarial drug discovery: technologies for in vitro β-hematin formation assay. Comb. Chem. High Throughput Screen. 8:61-77. [DOI] [PubMed] [Google Scholar]
- 16.Tripathi, A. K., S. I. Khan, L. A. Walker, and B. L. Tekwani. 2004. Spectrophotometric determination of de novo hemozoin/β-hematin formation in an in vitro assay. Anal. Biochem. 325:85-91. [DOI] [PubMed] [Google Scholar]
- 17.Woodrow, C. J., and S. Krishna. 2006. Antimalarial drugs: recent advances in molecular determinants of resistance and their clinical significance. Cell. Mol. Life Sci. 63:1586-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]