Abstract
Oxazolidinone antibiotics have activity against Mycobacterium tuberculosis. Linezolid, the only marketed oxazolidinone, has been used off-label in combination regimens to treat multidrug-resistant tuberculosis, but its precise contribution to the efficacy of such combinations is unclear. Another oxazolidinone, PNU-100480, has been demonstrated to have more potent activity in vitro and in a murine model of tuberculosis. In this study, we compared the pharmacokinetics and the antituberculosis activities of these two oxazolidinones over a range of doses and found that linezolid has limited activity at clinically relevant doses in the murine model compared to that of PNU-100480, which has potent bactericidal activity, even at lower drug exposures. These findings were unexpected, given the similar in vitro activities of PNU-100480, its major metabolites, and linezolid. Moreover, the incorporation of PNU-100480 dramatically improved the bactericidal activities of regimens containing current first-line antituberculosis drugs and moxifloxacin. For example, the addition of PNU-100480 (100 mg/kg of body weight/day) to the standard daily regimen of rifampin (rifampicin), isoniazid, and pyrazinamide resulted in an additional 2.0-log10-unit reduction in lung CFU counts during the first 2 months of treatment. The combination of PNU-100480, moxifloxacin, and pyrazinamide, which does not contain either rifampin or isoniazid, was also more active than rifampin, isoniazid, and pyrazinamide. These results suggest that PNU-100480 may have the potential to significantly shorten the duration of therapy for drug-susceptible as well as multidrug-resistant tuberculosis.
New drugs with potent antituberculosis (anti-TB) activities, especially against persisters, are needed to shorten the duration of treatment for TB and thereby facilitate the global implementation of directly observed therapy (18). If such drugs retain their activities against tubercle bacilli resistant to existing anti-TB drugs, they would be expected to improve the treatment of multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) as well.
The oxazolidinones comprise a new class of protein synthesis inhibitors that block translation through a novel mechanism by preventing the formation of the initiation complex. Linezolid (LZD; Zyvox), the only marketed oxazolidinone, has broad-spectrum activity against gram-positive bacteria and is currently approved for use for the treatment of complicated skin and skin structure infections and hospital-acquired pneumonia (Zyvox package insert). However, it is also active against many mycobacterial species, including Mycobacterium tuberculosis, for which its MIC ranges from 0.125 to 1 μg/ml and whose MIC50 is 0.5 μg/ml and MIC90 is 1 μg/ml (1, 5, 8). As a result, LZD has been used outside of labeled indications to treat recalcitrant cases of MDR- and XDR-TB. Although several case series suggest that LZD may contribute to successful sputum culture conversion in such cases, its activity in individual TB patients and its precise contribution to combination regimens remain unclear. Those studies also demonstrate that the duration of LZD administration may be limited by hematologic and neurologic toxicity that can occur with long-term administration (3, 9, 19, 25, 26). Therefore, new oxazolidinones with more potent in vivo activity against M. tuberculosis and a lower risk of toxicity with prolonged administration are desirable.
Oxazolidinones with more potent activity against M. tuberculosis have been described previously (2, 21, 24). The antituberculosis activity of PNU-100480 (PNU) was first reported in 1996 (2). Subsequent experiments with a murine model found that PNU was more active than LZD when both drugs were administered at 100 mg/kg of body weight, but the clinical relevance of this LZD dose was not established and the activities of PNU and LZD were not clearly different when their activities were compared when they were used at lower doses (5). Moreover, although PNU appeared to have modest activity when it was combined with rifampin (RIF; rifampicin) but not when it was combined with isoniazid (INH), the activity of PNU in combinations containing three or four drugs was not assessed (5).
In the present series of experiments, we compared the anti-TB activities of PNU and LZD over a range of doses, including clinically relevant LZD exposures, and also evaluated PNU in combination with first-line TB drugs and moxifloxacin (MXF) to determine its potential contribution to the activities of novel treatment regimens for drug-susceptible or MDR-TB. The results reveal that in comparison to the activity of LZD, which does not demonstrate clear-cut bactericidal activity, PNU has very potent bactericidal activity that dramatically increases the activity of the standard short-course treatment regimen and the activities of other combinations of anti-TB drugs.
MATERIALS AND METHODS
Bacterial strain.
Mycobacterium tuberculosis H37Rv was passaged in mice, frozen in 1-ml aliquots, and stored at −80°C before use. For each infection, an aliquot was thawed and subcultured in Middlebrook 7H9 broth supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC; Difco, Detroit, MI) and 0.05% Tween 80 (Sigma, St. Louis, MO).
Antimicrobials.
LZD, PNU, the sulfoxide metabolite of PNU (PNU-101603), and the sulfone metabolite of PNU (PNU-101244) were provided by Pfizer (Groton, CT). Due to the poor solubility of PNU in water, both LZD and PNU were suspended in a solution composed of 5% polyethylene glycol 200 (PEG 200; Sigma) and 95% methylcellulose (0.5%; Fisher, Suwanee, GA) in distilled water for administration to mice.
MXF was donated by Bayer (Rolling Meadows, IL). Pyrazinamide (PZA) was purchased from Fisher. INH and RIF were purchased from Sigma. Stock solutions were prepared weekly with distilled water, as described previously (16). All antibiotic solutions were stored at 4°C.
Determination of MIC.
The MIC was determined by the agar proportion method (13). Middlebrook 7H11 agar plates supplemented with 10% OADC and containing serial twofold concentrations of LZD, PNU, or the sulfoxide or sulfone metabolite of PNU ranging from 0.125 to 4 μg/ml were inoculated with 0.5 ml of the 10−2, 10−4, and 10−6 dilutions of a broth suspension of M. tuberculosis H37Rv with an optical density at 600 nm corresponding to approximately 108 CFU/ml. The numbers of CFU were counted after 21 days of incubation at 37°C with 5% ambient CO2. The MIC was defined as the lowest concentration at which the CFU count on drug-containing plates was <1% of the CFU count on drug-free plates.
Aerosol infection.
Female BALB/c mice (Charles River, Wilmington, MA) aged 4 to 6 weeks were infected by the aerosol route with an inhalation exposure system (Glas-col Inc., Terre Haute, IN) and a log-phase broth culture with an optical density at 600 nm of approximately 1.0. The mice were randomized to treatment groups (five mice per group per time point) after aerosol infection. Untreated mice were routinely killed (i) on the day after infection to determine the numbers of CFU implanted in the lungs and (ii) on the day of treatment initiation to determine the pretreatment CFU count. Quantitative lung cultures were performed on selective 7H11 plates (Becton Dickinson), as described previously (15). All procedures involving animals were approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Drug treatment.
Except where otherwise indicated, antibiotics were administered once daily 5 days per week in 0.2 ml by gavage. Both oxazolidinone suspensions were sonicated briefly prior to use and were shaken between doses. RIF was given 1 h prior to administration of the other drugs to avoid an adverse pharmacokinetic interaction (6, 7, 12).
Dose-ranging activities of PNU-100480 and LZD.
Beginning 13 days after aerosol infection, the test mice received (i) PNU (25, 50, or 100 mg/kg) or LZD (25, 50, 100, 130, or 260 mg/kg) once daily or (ii) PNU (25 or 50 mg/kg) or LZD (130 mg/kg) twice daily. The twice-daily treatments were separated by 8 h. Negative control mice received the PEG 200 and methylcellulose vehicle alone, while positive control mice received INH at 25 mg/kg. After 4 weeks of treatment, the mice were killed for assessment of spleen weights and lung CFU counts.
Pharmacokinetics of PNU and linezolid.
To determine the single-dose and steady-state pharmacokinetic profiles of PNU and LZD in the murine model, a substudy was nested in the dose-ranging study described above by using infected mice treated with PNU at 100 mg/kg once daily or LZD at 130 mg/kg once or twice daily. Three mice per group were killed at 0.5, 1, 2, 4, 8, and 24 h after administration of the first dose of treatment (day 1 [D1]) and at the aforementioned time points plus the addition of a time point at 9 h (i.e., 1 h after administration of the second daily dose) on day 24 (D24) of treatment. The mice were anesthetized with chloroform and were exsanguinated by cardiac puncture. Whole blood was collected on ice and was then centrifuged to obtain serum. Acetonitrile was added to the samples before storage in gasketed screw-cap tubes at −20°C. The D24 samples were collected and processed in the same manner and stored at −20°C before being sent together with the D1 samples to Pfizer (Groton, CT) for determination of the drug concentrations. The supernatant was injected (injection volume, 10 μl) onto a liquid chromatograph (SCL-10A; Shimadzu, Kyoto, Japan)-tandem mass spectrometer (Sciex API 3000; Applied Biosystems Group, Foster City, CA). A Hypersil C18 column (particle size, 5 μm; 50 by 2.1 mm; Thermo Electron Corp., Waltham, MA) and a step gradient consisting of mobile phase A (water with 0.05% 5 mM ammonium formate) and mobile phase B (acetonitrile, water, 5 mM ammonium formate [80:20:0.05%]) were used. Ionization was in the electrospray positive ion mode. Bioanalytical data were captured with the Analyst program (version 1.4.1; Applied Biosystems Group). Calculations of the pharmacokinetic data were based on the mean serum concentrations and were performed by use of the noncompartmental approach (linear trapezoidal rule for calculation of the area under the concentration-time curve [AUC] with the aid of the Watson [version 7.2] bioanalytical Laboratory Information Management Systems program [Thermo Electron Corp.]). For the AUC calculations for D24, the levels of the analytes in plasma at time zero were set to the concentration at 24 h. Concentrations of 0 were used for kinetic calculations for all results below the lower limit of quantitation (5 ng/ml).
Activities of drug combinations containing PNU.
Beginning 14 days after aerosol infection, positive control mice received one of the following treatments: INH (25 mg/kg) alone, RIF (10 mg/kg) alone, RIF-PZA (150 mg/kg), RIF-INH-PZA, MXF (100 mg/kg)-PZA or RIF-MXF-PZA. The rationale for the use of these drug doses has been described previously (12, 17). Test mice received PNU (100 mg/kg) alone or PNU added to each of the control regimens just described. Negative controls went untreated. The mice were killed after 4 and 8 weeks of treatment for assessment of spleen weights and lung CFU counts.
Data analysis.
Lung CFU counts (x) were log transformed as log10(x + 1) before analysis. Group mean CFU counts were compared by one-way analysis of variance with Dunnett's or Bonferroni's posttest (GraphPad Prism software, version 4; GraphPad Software, San Diego, CA) to control for multiple comparisons, as appropriate.
RESULTS
Determination of MICs.
The MICs of LZD, PNU, the sulfoxide metabolite of PNU, and the sulfone metabolite of PNU against M. tuberculosis H37Rv, the challenge strain used in this study, were found to be 0.25 μg/ml for each drug.
Dose-ranging activity of PNU and LZD.
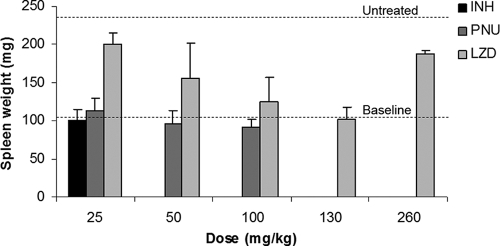
Mice were infected by the aerosol route with 4.44 ± 0.04 log10 CFU. Treatment was initiated 13 days later, when the mean lung CFU count was 7.49 ± 0.11 log10 and the mean spleen weight was 105 ± 11 mg. At the end of the treatment period, 28 days later, only two of five untreated mice remained alive. The mean spleen weight among those living mice had increased to 237 ± 16 mg (Fig. 1). In contrast, monotherapy with INH at 25 mg/kg prevented splenomegaly (mean spleen weight, 100 ± 1 mg) and death. A dose-dependent decrease in the spleen weight relative to that for the spleens of the untreated controls was observed with increasing doses of LZD between 25 mg/kg (200 ± 47 mg) and 130 mg/kg (102 ± 5 mg). Surprisingly, however, the spleens from mice treated with LZD at 260 mg/kg (whether it was given as a single dose or at 130 mg/kg twice daily) were larger than expected (190 ± 52 mg) and were similar in size to the spleens from mice treated with LZD at 25 mg/kg/day. Because the numbers and the sizes of lung lesions decreased with increasing daily doses of LZD, including 260 mg/kg, the splenomegaly observed in mice receiving 260 mg/kg/day was not felt to be due to reduced anti-TB activity. Splenomegaly has not previously been observed with LZD in toxicity studies but could be a dose-dependent effect of myelotoxicity and extramedullary hematopoiesis. Treatment with PNU prevented splenomegaly at all doses administered, including the 25-mg/kg dose (mean spleen weight, 113 ± 17 mg).
FIG. 1.
Spleen weights at the baseline and after treatment with INH, PNU, or LZD compared to those after no treatment.
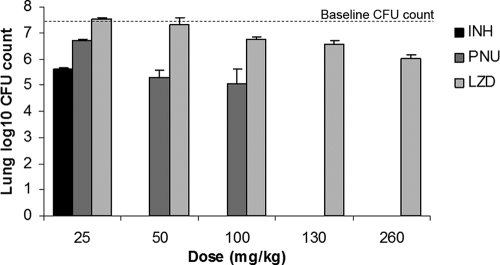
The lung CFU counts for mice treated once daily are presented in Fig. 2. The surviving untreated mice experienced an increase in CFU counts to nearly 8 log10 units. Treatment with INH reduced the mean lung CFU count to 5.62 log10, for a log killing of 1.87 compared to the baseline value. All PNU regimens resulted in a significant reduction in the mean CFU count from that at the baseline (P < 0.01), starting with a 0.78-log10-unit reduction with the 25-mg/kg dose. If bactericidal activity is defined by a ≥2-log10-unit reduction in CFU counts compared to the baseline value after 4 weeks of treatment (23), PNU at doses of 50 and 100 mg/kg exhibited bactericidal activity by reducing the mean CFU count to 5.28 and 5.07 log10 units, respectively, for log killing values of 2.21 and 2.42, respectively. The effects of these PNU doses were greater than those observed with INH. Although LZD also displayed dose-dependent activity, its activity was more limited than that of PNU. Only at LZD doses of ≥100 mg/kg was a significant reduction from the baseline CFU count demonstrated (P < 0.01). Even at the highest dose tested, 260 mg/kg, LZD did not exhibit bactericidal activity, producing a log killing of only 1.46. PNU was significantly more active than LZD at each dose tested. Treatment with PNU at 25 mg/kg resulted in a CFU count lower than that observed after treatment with LZD at 25 or 50 mg/kg (P < 0.01), and the count was not significantly different from the counts observed after treatment with LZD at 100 or 130 mg/kg. At 50 and 100 mg/kg, PNU was more active than any dose of LZD (P < 0.001). Dividing the total daily dose of PNU and LZD into two daily doses had no clear effect (i.e., positive or negative) on activity (Table 1).
FIG. 2.
Lung CFU counts at the baseline and after treatment with INH, PNU, or LZD.
TABLE 1.
Effect of dividing the daily oxazolidinone dose on activity
| Drug (total daily dose [mg/kg]) | Daily regimen | Lung log10 CFU count on:
|
|
|---|---|---|---|
| Day 0 | Day 28 | ||
| None | NAa | 7.47 ± 0.11 | 7.92 ± 0.03 |
| PNU | |||
| 50 | 25 mg/kg twice | 5.51 ± 0.14 | |
| 50 | 50 mg/kg once | 5.28 ± 0.53 | |
| 100 | 50 mg/kg twice | 4.89 ± 0.27 | |
| 100 | 100 mg/kg once | 5.07 ± 0.14 | |
| LZD | |||
| 260 | 130 mg/kg twice | 6.02 ± 0.17 | |
| 260 | 260 mg/kg once | 6.03 ± 0.12 | |
NA, not applicable.
Pharmacokinetics of PNU and LZD.
Selected pharmacokinetic parameter values for PNU and LZD are presented in Table 2. The 130-mg/kg daily dose of LZD produced a steady-state AUC of 379 μg · h/ml, approximately 50% higher than the anticipated steady-state AUC observed in humans administered 600 to 625 mg by mouth twice daily (215 to 294 μg · h/ml) (10, 22). On the basis of the linear kinetics in this dose range (Pfizer, data on file), the 100-mg/kg dose of LZD in the mouse represents the upper end of the steady-state AUC range in humans, albeit with a maximum concentration in serum (Cmax) that is approximately three times higher than that obtained in humans. As expected, there was significant first-pass metabolism of PNU to the major sulfoxide metabolite and the minor sulfone metabolite (2). Because PNU and its metabolites have the same MIC against the test strain, the concentration of each metabolite was added to that of the parent for the pharmacokinetic analyses. The steady-state AUC for PNU and its metabolites observed with the 100-mg/kg daily dose was approximately threefold lower than that observed with LZD at 130 mg/kg. The fact that the 25-mg/kg dose of PNU was as active as this dose of LZD suggests that PNU is 15 times more potent in vivo than LZD, a finding that is not explained by the similar MICs of LZD, PNU, and the PNU metabolites.
TABLE 2.
Pharmacokinetics of PNU and LZD in infected mice
| Regimen (dose [mg/kg]) and day | Cmax (μg/ml) | AUC0-24a (μg · h/ml) |
|---|---|---|
| PNU (100) | ||
| D1 | 6.07 | 7.33 |
| D24 | 4.32 | 8.74 |
| Sulfoxide metabolite | ||
| D1 | 20.0 | 37.8 |
| D24 | 16.9 | 98.8 |
| Sulfone metabolite | ||
| D1 | 0.79 | 2.58 |
| D24 | 0.66 | 9.73 |
| PNU + metabolitesb | ||
| D1 | 24.1 | 47.8 |
| D24 | 21.7 | 117 |
| LZD 130 mg/kg | ||
| D1 | 64.9 | 164 |
| D24c | 58.4 | 379 |
AUC0-24, AUC from time zero to 24 h.
The sum of the concentration of the parent drug plus the concentrations of the sulfoxide and sulfone metabolites was used to calculate the AUC.
Activities of drug combinations containing PNU.
To investigate the potential contribution of PNU to the combined chemotherapy of TB, mice were aerosol infected with 3.89 ± 0.19 log10 CFU. Treatment began 14 days later, when the mean lung CFU count was 7.37 ± 0.05 log10 units. Four of 10 untreated control mice died before the 1-month treatment time point, when the remainder were killed. The beneficial effect on the lung CFU counts of adding PNU to a variety of one-, two-, and three-drug regimens is evident in Table 3. Monotherapy with PNU resulted in a 2.65-log10-unit reduction in the CFU count from that at the baseline to 4.72 log10 units over the first 28 days, whereas INH and RIF monotherapy reduced the lung CFU counts by 1.58 and 1.63 log10 to 5.79 and 5.74 log10 units, respectively. Remarkably, PNU continued to exert bactericidal activity during the second month of treatment, reducing the bacterial burden to 2.70 log10 CFU over the first 2 months, greater than the activity of INH or RIF alone and similar to the CFU count in mice treated with the standard first-line combination regimen of RIF-INH-PZA, which reduced the CFU count to 2.47 log10 units. The combination of INH and PNU was no more active than PNU alone, while the combination of RIF and PNU had a multiplicative effect, resulting in a mean lung CFU count nearly 30 times lower than that observed with PNU alone. The combination of RIF and PNU had activity approaching that of the potent sterilizing combination of RIF and PZA, which reduced the mean lung CFU count to 1.05 log10 units, although longer studies using relapse as an endpoint will be necessary to determine whether the activity of PNU can replace the sterilizing activity of PZA.
TABLE 3.
Additive activity of PNU in combination with first-line drugs and MXF
| Regimen | Mean lung log10 CFU count ±SD
|
||||
|---|---|---|---|---|---|
| Before treatment (D0) | After treatment with the indicated regimen
|
After treatment with the indicated regimen plus PNU at 100 mg/kg/day
|
|||
| Day 28 | Day 56 | Day 28 | Day 56 | ||
| No treatment | 7.37 ± 0.12 | 7.76 ± 0.09 | NDa | 4.72 ± 0.17 | 2.70 ± 0.29 |
| INH | 5.79 ± 0.14 | 4.53 ± 0.24 | 4.33 ± 0.29 | 2.87 ± 0.23b | |
| RIF | 5.74 ± 0.17 | 4.65 ± 0.24 | 3.73 ± 0.19 | 1.29 ± 0.22b | |
| RIF-PZA | 3.97 ± 0.11 | 1.05 ± 0.44 | 3.04 ± 0.22 | 0.50 ± 0.33c,d | |
| RIF-INH-PZA | 4.88 ± 0.09 | 2.47 ± 0.18 | 3.54 ± 0.24 | 0.47 ± 0.20b | |
| RIF-MXF-PZA | 3.72 ± 0.20 | 0.61 ± 0.38c | 3.41 ± 0.18 | 0.12 ± 0.27e,f | |
| MXF-PZA | 5.10 ± 0.13 | 3.17 ± 0.28 | 3.33 ± 0.33 | 0.93 ± 0.34b | |
ND, not determined.
P < 0.001 versus the results for the corresponding regimen without PNU.
One of five mice were culture negative.
P < 0.05 versus the results for the corresponding regimen without PNU.
Four of five mice were culture negative.
P = 0.05 versus the results for the corresponding regimen without PNU.
In this experiment, as in previous studies (14, 15, 17, 20), the addition of INH to RIF-PZA had a significant antagonistic effect, resulting in mean lung CFU counts of 1.05 ± 0.44 and 2.47 ± 0.18 after 2 months of treatment with RIF-PZA and RIF-INH-PZA, respectively. This effect could falsely suggest that the substitution of a new drug for INH in the RIF-INH-PZA regimen has a significant beneficial effect, even if the new drug is completely inactive itself, simply because the antagonistic effect of INH has been removed. In this experiment, however, PNU had similarly strong effects whether it was added to RIF-INH-PZA or substituted for INH (i.e., as RIF-PZA-PNU). After 2 months of treatment, the mean lung CFU counts were 0.47 ± 0.20 and 0.50 ± 0.33 in mice treated with RIF-INH-PZA-PNU and RIF-PZA-PNU, respectively, both of which were lower than the mean lung CFU count in mice receiving RIF-PZA alone (P < 0.05), providing further evidence that the benefit of substituting PNU for INH does not come from the removal of the antagonistic influence of INH. PNU also improved the activity of the experimental RIF-MXF-PZA regimen. Treatment with RIF-MXF-PZA for 2 months resulted in a mean lung CFU count of 0.61 ± 0.38, with one of five mice being culture negative, while treatment with RIF-MXF-PZA-PNU resulted in a mean CFU count of 0.12 ± 0.27, with four of five mice being culture negative (P = 0.05 for the difference in the mean CFU counts). The difference was of marginal statistical significance, likely due to the low CFU counts, but implies that PNU may further improve the regimen's sterilizing activity. Finally, treatment with MXF-PZA-PNU for 2 months resulted in a mean lung CFU count of 0.93 ± 0.34, demonstrating that PNU is able to replace RIF in the treatment-shortening RIF-MXF-PZA regimen without diminishing the regimen's activity.
DISCUSSION
In the series of experiments described here, we have built upon initial observations of the activity of the oxazolidinone PNU against M. tuberculosis (5) and demonstrated that PNU has more potent in vivo anti-TB activity than was previously recognized. At comparable doses, it is much more active than the human-equivalent dose of LZD, the only clinically available oxazolidinone. The enhanced activity of PNU in comparison to that of LZD is not explained by the similar in vitro activities of the two drugs, even when the metabolites of PNU are considered. Most importantly, we found that combining PNU with existing first- and second-line anti-TB drugs results in dramatic increases in bactericidal activity, suggesting that it may have the potential to shorten the duration of chemotherapy for drug-susceptible TB as well as MDR-TB.
Because of its in vitro activity against M. tuberculosis, LZD is increasingly used off-label to treat patients with MDR- and XDR-TB (9, 19, 25, 26). However, because such patients are treated concomitantly with other anti-TB drugs, often including aminoglycosides and/or fluoroquinolones, it has been difficult to assess the contribution of LZD to their treatment outcomes. Similarly, it has been difficult to gauge the clinical significance of reducing the LZD dose from 600 mg twice daily to 600 mg daily, which is sometimes attempted to avert the hematologic toxicity that may occur with long-term administration (19). While the potential contribution of LZD to the treatment of TB was not the primary objective of this study, our results may provide insight into the potential contribution of LZD. The multidose pharmacokinetic study of LZD at 130 mg/kg daily demonstrated an AUC of 379 μg · h/ml, which is approximately 150% of the average steady-state AUC in humans receiving 600 to 625 mg twice daily, i.e., 215 to 294 μg · h/ml (10, 22). The linear kinetics of LZD over this dose range permit us to estimate that the 100-mg/kg dose in the mouse produced an AUC of approximately 292 μg · h/ml, an exposure that lies at the high end of the range for humans. LZD demonstrated limited bacterial killing at this dose (i.e., a 0.74-log10-unit reduction in lung CFU counts over 4 weeks), suggesting that it is not likely to have a great bactericidal effect in humans. While this effect is limited, it is not necessarily inconsistent with observations that LZD may help promote sputum culture conversion in patients with MDR-TB. However, it does dampen enthusiasm for the routine use of 600 mg once daily, given the absence of any detectable bactericidal activity of the 50-mg/kg dose in mice. In light of these findings, it is encouraging that PNU exhibited such promising bactericidal activity, even though it achieved lower exposures than LZD.
Cynamon and colleagues first reported the anti-TB activity of PNU in a murine model following intravenous infection of outbred CD-1 mice, the initiation of treatment at between 1 and 7 days after infection, and treatment for 4 weeks (5). In their model, the spontaneous decline in CFU counts among the untreated controls prevented a definitive characterization of PNU's activity as bacteriostatic or bactericidal. In the current study, the dose-ranging activities of PNU and LZD were assessed against a more established infection in a murine model, and the activity of PNU alone was also compared to the activities of INH and RIF after 8 weeks of treatment. When treatment was initiated 2 weeks after aerosol infection, PNU proved significantly more active than LZD as well as INH and RIF over the first 4 weeks of treatment. Moreover, PNU unexpectedly continued to exert greater bactericidal activity than INH and RIF through the second month of treatment. Such activity against the nonmultiplying, drug-tolerant bacilli capable of surviving the first 4 weeks of treatment indicates that PNU may have significant sterilizing activity, but proper studies based on relapse as an outcome will be necessary to confirm this.
It is not enough, however, to develop new drugs with anti-TB activity. A new drug must have a beneficial effect when it is combined with existing TB drugs and, possibly, other new drugs to form new drug regimens that improve the treatment of drug-susceptible TB, MDR-TB, and/or XDR-TB. In this regard, we found PNU to be highly active in various multidrug combinations. For example, the addition of PNU to the first-line regimen of RIF-INH-PZA increased the bactericidal activity by 2 orders of magnitude, which is more than a simple additive effect. This result suggests that the addition of PNU is capable of shortening the duration of treatment in the murine model. If such results are confirmed by long-term experiments with relapse as an end point, they could prove useful in the design of the phase II evaluation of PNU or another potent oxazolidinone. We also found that PNU added to the activity of MXF-PZA to form a three-drug combination that was more active than RIF-INH-PZA, even thought the combination did not contain either RIF or INH. Considering that the current treatment of MDR-TB requires complex regimens of 18 to 24 months' duration in which parenteral administration of the agents figures prominently, the contribution of PNU to the MXF-PZA-PNU combination suggests that PNU or another potent oxazolidinone may have the potential to radically improve the treatment of MDR-TB.
There are two major limitations to this study. First, there can be no certainty that the results observed in mice will translate to humans because the disease has different histopathological features in the two species that could differentially affect the susceptibility of the organisms to PNU. Despite this, the results of experiments evaluating combination chemotherapeutic regimens in this model have been consistent with the results of clinical trials with existing first-line TB drugs (11). Also, it is unknown if the PNU doses tested in mice are relevant for humans. However, we have documented pharmacokinetic and pharmacodynamic profiles for the 100-mg/kg dose in mice that could inform future dose-finding studies with PNU. It is also encouraging that the 50-mg/kg dose retained most of the bactericidal activity of the 100-mg/kg dose. Second, these studies with the mouse do not provide much useful information about the toxicity profile of PNU in humans. LZD may be associated with adverse hematologic or neuropathic events that occur with increasing frequency as the treatment duration increases, although a recent report suggests that the risk of such toxicity may be lessened by reducing the treatment dose to 600 mg per day (19). Whether PNU will have such effects remains to be seen. Our finding that the activities of both LZD and PNU are the same whether the total daily dose is given as one or two doses suggests that activity against slowly growing M. tuberculosis may correlate with the AUC/MIC, as observed against other pathogens (4), and supports dosing with these drugs once daily or even less frequently if such a strategy is effective in limiting potential toxicity.
In conclusion, our studies demonstrate that PNU has strong bactericidal activity against established infections with M. tuberculosis in a murine model. The facts that it continues to exert such bactericidal activity through the second month of treatment and dramatically improves the activity of the RIF-INH-PZA and MXF-PZA regimens justify further preclinical evaluations to determine whether PNU has sterilizing activity capable of shortening the duration of treatment necessary to prevent relapses.
Acknowledgments
This study was supported by Pfizer.
Michael Barbachyn, Donn Wishka, Manjinder Lall, and Steve Brickner of Pfizer prepared the oxazolidinones used in the study.
Footnotes
Published ahead of print on 15 December 2008.
REFERENCES
- 1.Alcala, L., M. J. Ruiz-Serrano, T. C. Perez-Fernandez, D. Garcia, V. M. Diaz-Infantes, M. Marin-Arriaza, and E. Bouza. 2003. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis that are susceptible or resistant to first-line antituberculous drugs. Antimicrob. Agents Chemother. 47:416-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbachyn, M. R., D. K. Hutchinson, S. J. Brickner, M. H. Cynamon, J. O. Kilburn, S. P. Klemens, S. E. Glickman, K. C. Grega, S. K. Hendges, D. S. Toops, C. W. Ford, and G. E. Zurenko. 1996. Identification of a novel oxazolidinone (U-100480) with potent antimycobacterial activity. J. Med. Chem. 39:680-685. [DOI] [PubMed] [Google Scholar]
- 3.Condos, R., N. Hadgiangelis, E. Leibert, G. Jacquette, T. Harkin, and W. N. Rom. 2008. Case series report of a linezolid-containing regimen for extensively drug-resistant tuberculosis. Chest 134:187-192. [DOI] [PubMed] [Google Scholar]
- 4.Craig, W. A. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 17:479-501. [DOI] [PubMed] [Google Scholar]
- 5.Cynamon, M. H., S. P. Klemens, C. A. Sharpe, and S. Chase. 1999. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob. Agents Chemother. 43:1189-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhillon, J., J. M. Dickinson, K. Sole, and D. A. Mitchison. 1996. Preventive chemotherapy of tuberculosis in Cornell model mice with combinations of rifampin, isoniazid, and pyrazinamide. Antimicrob. Agents Chemother. 40:552-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickinson, J., A. Guy, and D. A. Mitchison. 1992. Bioavailability of rifampin in experimental murine tuberculosis. Antimicrob. Agents Chemother. 36:2066-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fattorini, L., D. Tan, E. Iona, M. Mattei, F. Giannoni, L. Brunori, S. Recchia, and G. Orefici. 2003. Activities of moxifloxacin alone and in combination with other antimicrobial agents against multidrug-resistant Mycobacterium tuberculosis infection in BALB/c mice. Antimicrob. Agents Chemother. 47:360-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortun, J., P. Martin-Davila, E. Navas, M. J. Perez-Elias, J. Cobo, M. Tato, E. G. De la Pedrosa, E. Gomez-Mampaso, and S. Moreno. 2005. Linezolid for the treatment of multidrug-resistant tuberculosis. J. Antimicrob. Chemother. 56:180-185. [DOI] [PubMed] [Google Scholar]
- 10.Gee, T., R. Ellis, G. Marshall, J. Andrews, J. Ashby, and R. Wise. 2001. Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob. Agents Chemother. 45:1843-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosset, J., and B. Ji. 1998. Experimental chemotherapy of mycobacterial diseases, p. 51-97. In P. R. J. Gangadharam and P. A. Jenkins (ed.), Mycobacteria, vol. 2, Chemotherapy. Chapman & Hall, New York, NY. [Google Scholar]
- 12.Grosset, J., C. Truffot-Pernot, C. Lacroix, and B. Ji. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob. Agents Chemother. 36:548-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inderlied, C. B., and M. Salfinger. 1999. Antimycobacterial agents and susceptibility tests, p. 1601-1623. In: P. R. Murray, E. J. Baron, M. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, DC.
- 14.Lalande, V., C. Truffot-Pernot, A. Paccaly-Moulin, J. Grosset, and B. Ji. 1993. Powerful bactericidal activity of sparfloxacin (AT-4140) against Mycobacterium tuberculosis in mice. Antimicrob. Agents Chemother. 37:407-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecoeur, H. F., C. Truffot-Pernot, and J. H. Grosset. 1989. Experimental short-course preventive therapy of tuberculosis with rifampin and pyrazinamide. Am. Rev. Respir. Dis. 140:1189-1193. [DOI] [PubMed] [Google Scholar]
- 16.Nuermberger, E., I. Rosenthal, S. Tyagi, K. N. Williams, D. Almeida, C. A. Peloquin, W. R. Bishai, and J. H. Grosset. 2006. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 50:2621-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuermberger, E. L., T. Yoshimatsu, S. Tyagi, R. J. O'Brien, A. N. Vernon, R. E. Chaisson, W. R. Bishai, and J. H. Grosset. 2004. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am. J. Respir. Crit. Care Med. 169:421-426. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien, R. J., and P. P. Nunn. 2001. The need for new drugs against tuberculosis. Obstacles, opportunities, and next steps. Am. J. Respir. Crit. Care Med. 163:1055-1058. [DOI] [PubMed] [Google Scholar]
- 19.Park, I. N., S. B. Hong, Y. M. Oh, M. N. Kim, C. M. Lim, S. D. Lee, Y. Koh, W. S. Kim, D. S. Kim, W. D. Kim, and T. S. Shim. 2006. Efficacy and tolerability of daily-half dose linezolid in patients with intractable multidrug-resistant tuberculosis. J. Antimicrob. Chemother. 58:701-704. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal, I. M., K. Williams, S. Tyagi, C. A. Peloquin, A. A. Vernon, W. R. Bishai, J. H. Grosset, and E. L. Nuermberger. 2006. Potent twice-weekly rifapentine-containing regimens in murine tuberculosis. Am. J. Respir. Crit. Care Med. 174:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sood, R., M. Rao, S. Singhal, and A. Rattan. 2005. Activity of RBx 7644 and RBx 8700, new investigational oxazolidinones, against Mycobacterium tuberculosis infected murine macrophages. Int. J. Antimicrob. Agents 25:464-468. [DOI] [PubMed] [Google Scholar]
- 22.Stalker, D. J., G. L. Jungbluth, N. K. Hopkins, and D. H. Batts. 2003. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J. Antimicrob. Chemother. 51:1239-1246. [DOI] [PubMed] [Google Scholar]
- 23.Tyagi, S., E. Nuermberger, T. Yoshimatsu, K. Williams, I. Rosenthal, N. Lounis, W. Bishai, and J. Grosset. 2005. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob. Agents Chemother. 49:2289-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vera-Cabrera, L., J. Castro-Garza, A. Rendon, J. Ocampo-Candiani, O. Welsh, S. H. Choi, K. Blackwood, and C. Molina-Torres. 2005. In vitro susceptibility of Mycobacterium tuberculosis clinical isolates to garenoxacin and DA-7867. Antimicrob. Agents Chemother. 49:4351-4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von der Lippe, B., P. Sandven, and O. Brubakk. 2006. Efficacy and safety of linezolid in multidrug resistant tuberculosis (MDR-TB)—a report of ten cases. J. Infect. 52:92-96. [DOI] [PubMed] [Google Scholar]
- 26.Yew, W. W., C. H. Chau, and K. H. Wen. 2008. Linezolid in the treatment of ‘difficult’ multidrug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 12:345-346. [PubMed] [Google Scholar]