Abstract
Abacavir (ABC) is administered either at 600 mg once daily (ABC 600 mg QD) or 300 mg twice daily (ABC 300 mg BID) in anti-human immunodeficiency virus (anti-HIV) combination therapy. Although ABC plasma pharmacokinetics following each regimen has been well defined, no study has directly compared the regimens with respect to pharmacokinetics of ABC's active intracellular anabolite, carbovir-triphosphate (CBV-TP). In an open-label, two-period, crossover study, 34 HIV-infected male and female subjects stabilized on antiretroviral regimens containing either ABC 600 mg QD or ABC 300 mg BID received their usual doses on days −1 and 1 and then switched regimens for days 2 to 11. Serial blood samples collected on days 1 and 11 were assayed for plasma ABC and intracellular CBV-TP concentrations using validated high-performance liquid chromatography-tandem mass spectrometry methods. Pharmacokinetic parameters were calculated using noncompartmental methods. Analysis of variance with a mixed-effect model was performed for treatment and gender comparisons. In 27 evaluable subjects, the regimens provided bioequivalent ABC daily areas under the concentration-time curve from 0 to 24 h (AUC0-24) and comparable CBV-TP concentrations at the end of the dosing interval (Cτ). As expected, ABC QD resulted in 109% higher ABC maximum concentrations of drug in plasma (Cmax) than did ABC BID. ABC QD also resulted in 32% higher CBV-TP AUC0-24 and 99% higher CBV-TP Cmax than did ABC BID. Females had a 38% higher weight-adjusted ABC AUC0-24 and 81% higher weight-adjusted CBV-TP AUC0-24 than did males. Virologic suppression was maintained during regimen switch, and no tolerability differences between regimens were observed. In conclusion, this study showed that ABC 600 mg QD and ABC 300 mg BID regimens led to similar intracellular CBV-TP Cτ values, thus providing pharmacokinetic support for the interchangeability of these two regimens. Women had higher intracellular CBV-TP exposure than did men.
Simplification of antiretroviral treatment (ART) by changing twice-daily (BID) regimens to once-daily (QD) regimens has been shown to contribute to better adherence and patient satisfaction (1, 4, 5, 11, 12, 15, 16), although differences in treatment outcome have not been commonly observed (5, 11). The decision to choose a QD regimen over a BID one, or vice versa, depends on the clinical situation. QD dosing provides an especially practical regimen for subjects who require directly observed therapy, such as those who are incarcerated or in mental health facilities and those attending methadone clinics (10, 14). However, in subjects who are receiving ART drugs that are all administered BID, addition of a further antiretroviral drug with a BID regimen maintains dose symmetry and is easy to remember since all regimen components can be administered at the same time.
Abacavir (ABC) is a nucleoside reverse transcriptase inhibitor that is available both as a single-agent formulation and in fixed-dose combination formulations containing lamivudine (3TC) or 3TC-zidovudine (ZDV) to be used with drugs from other antiretroviral classes in combination ART. QD and BID ABC regimens have demonstrated similar efficacies as initial therapy backbones and in switch studies (5, 11). Switching from a BID regimen of ABC plus 3TC to QD ABC-3TC fixed-dose combination tablets has been shown to improve adherence without changing virologic efficacy or tolerability (11).
Since ABC is a nucleoside analog, its mode of action is to form carbovir-triphosphate (CBV-TP) through serial intracellular phosphorylation to exhibit antiviral activity. To date, no study has compared the QD and BID regimens with respect to intracellular CBV-TP pharmacokinetics (PKs). The estimated 12- to 20-h elimination half-life (t1/2) of CBV-TP is based on three small studies involving mostly male subjects (3, 6, 13). Two studies have shown gender differences in intracellular TP concentrations of 3TC and ZDV in human immunodeficiency virus (HIV)-infected subjects (2, 17). However, the effect of gender on CBV-TP concentrations has not been evaluated. The purpose of the present study was to compare the steady-state PKs of plasma ABC and intracellular CBV-TP by using a crossover design in subjects receiving ART regimens containing ABC at either 600 mg QD or 300 mg BID (ABC 600 mg QD and ABC 300 mg BID, respectively) and to investigate gender differences in intracellular CBV-TP PKs.
MATERIALS AND METHODS
Study design.
This was a phase I open-label, two-period, crossover, PK study conducted at a single center (St. Stephen's Centre, Chelsea and Westminster Hospital, London, United Kingdom) from 5 September 2005 until 22 May 2006. Men or women aged 18 to 65 years with HIV type 1 (HIV-1) infection documented by HIV-1 antibody enzyme-linked immunosorbent assay and confirmed by Western blot detection of HIV-1 antibody were eligible for this study if they had the following characteristics at screening: undetectable viral load (<400 copies/ml), being on an ABC-containing regimen for ≥8 weeks, CD4+ count of ≥250 cells/mm3, weight of 40 to 100 kg, body mass index of 19 to 29 kg/m2, and willingness to temporarily switch their ABC schedule from QD to BID, or vice versa, for 11 days. Females were to have no childbearing potential or were to agree to protocol-specified methods of contraception, including a double-barrier method, complete abstinence from intercourse from 2 weeks prestudy to 2 weeks poststudy, or use of an intrauterine device with a <1% failure rate.
Subjects were excluded if they were receiving tenofovir, hydroxyurea, mycophenolate, or ribavirin; were in another experimental drug trial within 30 days of screening; regularly consumed >83 ml of a 12% strength alcoholic beverage per day; had a medical condition that interfered with the absorption, distribution, metabolism, or excretion of drugs; regularly took drugs of abuse; had liver enzyme test results >3 times the upper limit of normal or bilirubin levels >2 times the upper limit of normal; had a hemoglobin level of <12 g/dl or a platelet count of <50,000/μl; were pregnant or nursing; were positive for hepatitis C virus antibody or hepatitis B virus surface antigen; or had a history of suspected ABC hypersensitivity reaction. All subjects provided written informed consent to participate in the study. The study protocol was approved by the ethics committee at the study site.
The primary objective of this study was to compare CBV-TP exposure values (area under the concentration-time curve from 0 to 24 h [AUC0-24], maximum concentration of drug in serum [Cmax], and concentration at the end of dosing interval [Cτ]) from ABC 600 mg QD and ABC 300 mg BID. The secondary objective included assessing gender differences in PKs of plasma ABC and intracellular CBV-TP and assessing the safety and tolerability of dosing with ABC 300 mg BID and ABC 600 mg QD during the study period.
A sample size of at least 24 evaluable subjects was deemed necessary based on experience with an earlier CBV-TP study, CNA10905, which had investigated the steady-state PKs of intracellular CBV-TP in HIV-infected subjects on an ABC 300 mg BID regimen (13). In that study, intersubject coefficients of variation (CV) of CBV-TP PK parameters were between 62% and 73%. Assuming that intrasubject variability was lower than intersubject variability and that the intrasubject CV was at 40% and assuming two one-sided tests at α = 0.05 for each side and a sample size of 24, it was estimated that the range of 90% confidence interval (CI) for the treatment ratio was within 20% of the point estimates for the PK parameters AUC and Cmax. Due to the potential for subject withdrawal, the complexity of sample processing, and the possibility that some samples may have been unusable, 30 subjects were to be enrolled. If the intrasubject CV was at 55% and the sample size was 24 evaluable subjects, it was estimated that the lower and upper bounds of the 90% CI would be within 28% of the point estimate for AUC and Cmax. It was planned to enroll at least 12 women to assess gender differences.
Study drug dosing.
Subjects were screened between 30 and 8 days before the first study day. In period 1 (day −1 to day 1), subjects continued their usual ABC regimen. In period 2 (day 2 to day 12), subjects stabilized on ABC 300 mg BID were switched to ABC 600 mg QD and those on ABC 600 mg QD were switched to ABC 300 mg BID from days 2 to 11. Subjects continued receiving all other antiretroviral agents. Subjects were discharged on day 12 and were allowed to switch back to their original ABC regimen. Subjects attended a follow-up visit 7 to 10 days afterwards. On the days of PK sampling (day 1 and day 11), subjects underwent fasting for at least 10 h prior to the ABC dose, the doses of ABC were taken under the supervision of clinic staff, and then subjects underwent fasting for 2 h after dosing; when subjects received an ABC BID-containing regimen, the evening dose of ABC was skipped on day 1 or day 11 to better characterize the PKs of intracellular CBV-TP.
Efficacy/safety assessment.
Blood or urine samples for laboratory safety assessments (clinical chemistry, hematology, immunology, urinalysis, and pregnancy test) were taken on day −1, day 5, and day 10 and at follow-up. On day −1 and day 10 and at follow-up, blood samples (approximately 14 ml total) were also collected for measurement of viral load by the HIV-1 Monitor Version 1.0 PCR assay (lower limit of quantitation, 400 copies/ml) (Roche, Nutley, NJ) and of CD4+ lymphocyte cell count by flow cytometry. Vital signs were measured on day 2 and day 11. Samples for viral genotyping were collected on day 2 and day 12 and at follow-up. Adverse events were assessed intermittently throughout the study.
PK sample collection and processing.
Blood samples (16 ml each) were collected in CPT tubes (two tubes, 8 ml per sample) at the time points specified (predose and 2, 4, 6, 8, 12, 16, and 24 h postdose) for measuring intracellular CBV-TP and plasma ABC on day 1 and day 11. In the morning on day 2, subjects had the last 24-hour PK sample on their current regimens collected; this was immediately followed by the ABC regimen switch. An additional 2-ml blood sample for plasma ABC only was collected in a blood collection tube with EDTA at 1 h postdose on day 1 and day 11.
The CPT tubes were centrifuged within 60 min of blood collection at controlled room temperature (18 to 25°C) in a horizontal rotor (swing-out bucket) for 20 min at a relative centrifugal force of 1,500 to 1,800. A 1-ml aliquot of plasma was collected from the tube, transferred to a 1.8-ml Nunc polypropylene storage tube, and stored at ≤−20°C till assayed for ABC concentrations. The peripheral blood mononuclear cell (PBMC) layer was suspended in the remaining plasma, transferred from both CPT tubes to a single graduated 50-ml conical tube, and mixed with isotonic saline (0.9%) to achieve a total volume of 30 ml. A 500-μl aliquot was removed from the suspension for cell counting using a KOVA slide by trained personnel following a validated standard procedure. Cell counting was performed in duplicate and was repeated until cell counts from duplicates were within 25% of each other. The total number of PBMC counts from each PBMC sample (PBMCCT) was calculated and reported in the unit of million cells. The volume of remaining suspension was recorded (accurate to 0.1 ml), and the suspension was centrifuged for 15 min at a relative centrifugal force of 400 to pellet the cells. The supernatant was aspirated and discarded (plasma and platelets), and the cell pellet was well mixed with 1 ml ice-cold 70% methanol solution to lyse and completely redissolve the cell pellet. The lysed PBMC extract was completely transferred to a 1.8-ml Nunc cryostorage vial and stored at −70°C until assayed for CBV-TP concentrations.
Bioanalysis.
PBMC extract samples were analyzed for CBV-TP by Taylor Technology (Princeton, NJ) using a validated analytical method based on anion exchange on Waters Accell QMA solid-phase extraction plates followed by enzymatic hydrolysis to CBV using alkaline phosphatase. The sample was cleaned up on a Varian C18 solid-phase extraction plate and reconstituted with water followed by high-performance liquid chromatography-tandem mass spectrometry (MS/MS) analysis using a YMC octyldecyl silane AQ 2.0- × 50-mm column and positive-ion MS/MS using a TurboIonSpray (MDS Sciex [Mississauga, Canada]) interface and multiple reaction monitoring. This method had a lower limit of quantification of 0.05 ng/ml with a 500-μl aliquot of human PBMC extracts. Bias and precision were calculated using interpolated concentrations of quality control samples at three concentration levels over a calibration range of 0.05 to 10 ng/ml. The interassay precision (CV) and interassay bias were 5.91% and −1.87%, respectively, with CBV-TP at 0.15 ng/ml; 5.58% and −1.39%, respectively, with CBV-TP at 1 ng/ml; and 3.54% and −1.41%, respectively, with CBV-TP at 7.5 ng/ml.
Plasma samples were analyzed for ABC concentration by GlaxoSmithKline (Research Triangle Park, NC) by protein precipitation and high-performance liquid chromatography-MS/MS in the positive-ion TurboIonSpray mode. The calibration curve range for ABC in human plasma was 2.5 to 2,500 ng/ml using a 50-μl sample aliquot of human plasma.
For each analytical method, quality control samples, containing the relevant analytes at three different concentrations and stored with study samples, were analyzed with each batch of samples against separately prepared calibration standards.
PK analyses.
CBV-TP concentrations (C), in fmol/million cells, were calculated based on the CBV-TP concentration in PBMC extracts reported in ng/ml and cell counts (PBMCCT) using the formula C (fmol/million cells) = C (ng/ml) × 106/(molecular mass × PBMCCT/1 ml), where molecular mass is that of CBV-TP, 487.3 Da.
The following PK parameters for plasma ABC and intracellular CBV-TP were estimated using the noncompartmental Model 200 (for extravascular administration) of WinNonlin Professional Edition version 4.1 (Pharsight Corporation, Mountain View, CA) and actual elapsed time from dosing: AUC0-24, area under the drug concentration-time curve over a dosing interval at steady state (AUC0-τ), Cmax at steady state, time of maximum concentration at steady state, Cτ at steady state, and terminal t1/2. AUC0-24 for the BID regimen was calculated as 2 × AUC0-τ. In addition, the ratio of CBV-TP AUC0-24 and ABC AUC0-24 (AUCCBV/AUCABC) was calculated for each subject.
As female subjects enrolled in this study had lower body weights than did male subjects, in order to accurately evaluate the gender difference, weight-normalized (to a 70-kg person) PK parameters, including AUC0-24, Cmax, and Cτ, were estimated for plasma ABC and PBMC CBV-TP using the formula weight-normalized PK = PK × weight (in kg)/70 kg.
Statistical analyses.
Summary statistics were provided for PK parameters by treatment and gender. Statistical analysis was performed to compare exposure differences in ABC and CBV-TP between ABC BID and ABC QD regimens and to assess gender effect. Analysis of variance, considering treatment arm, period, treatment, and gender as fixed effects and subject within treatment arm as a random effect, was performed using the SAS (version 8.2) mixed linear model procedure. The treatment-by-gender interaction was also included as a fixed effect in the model. Ratios of geometric least square means and associated 90% CIs were estimated for the key PK parameters: AUC0-24, Cmax, and Cτ (both original and weight normalized) for intracellular CBV-TP and plasma ABC and AUCCBV/AUCABC. A linear regression analysis, considering intracellular CBV-TP AUC0-24 as the response variable and plasma ABC AUC0-24 as the predictive variable, was also performed by treatment and overall to assess the relationship between exposures of plasma ABC and intracellular CBV-TP. The PK parameters were log transformed before the primary analyses, and treatment comparisons were expressed as ratios on the original scale. There was no formal statistical analysis of safety data.
RESULTS
Study demographics.
Thirty-four subjects were enrolled into the study, of whom 33 were dosed and 29 completed the study. Due to enrollment difficulty, only 10 female subjects participated in this study. Five subjects were withdrawn for the following reasons: alcoholic intoxication impairing the subject's ability to participate (one subject), positive urine test for drugs of abuse (two subjects), or abnormal alanine aminotransferase level in period 1 (two subjects).
Of the 33 subjects who were dosed, 23 (70%) were male and 10 (30%) were female. The mean age of the subjects was 45.1 years (range, 25 to 66 years). Most of the subjects (25 [76%]) were Caucasian, six (18%) were African, one (3%) was American Indian, and one (3%) was Asian. All subjects had undetectable HIV-1 RNA (<400 copies/ml), and the median CD4+ cell count was 657 cells/mm3 (range, 280 to 3,290 cells/mm3).
PKs.
Twenty-seven (18 males and 9 females) of the 33 subjects completed both treatment study periods and were included in the PK analysis and treatment comparison. PK results and the statistical comparison of steady-state plasma ABC and CBV-TP PK parameters are presented in Tables 1 and 2.
TABLE 1.
Steady-state intracellular CBV-TP PK parameters, by treatment and gender, and comparison
| Intracellular CBV-TP PK parameter | Geometric mean (% CV)
|
Geometric least square mean ratio (90% CI)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment A (ABC 300 mg BID regimen)
|
Treatment B (ABC 600 mg QD regimen)
|
|||||||||
| All subjects (n = 27) | Male (n = 18) | Female (n = 9) | All subjects (n = 27) | Male (n = 18) | Female (n = 9) | Treatment B/A (n = 27/27) | Female/male (n = 9/18)
|
|||
| Treatment A | Treatment B | Combinedc | ||||||||
| Original scale | ||||||||||
| AUC0-24(h·fmol/million cells)a | 814 (64) | 688 (53) | 1,138 (72) | 1,051 (71) | 792 (45) | 1,851 (69) | 1.32 (1.07, 1.63) | 1.76 (1.27, 2.45) | 2.21 (1.63, 2.99) | 2.09 (1.69, 2.58) |
| Cmax (fmol/million cells) | 58.5 (54) | 49.3 (49) | 82.5 (42) | 114 (76) | 86.5 (49) | 198 (83) | 1.99 (1.61, 2.45) | 1.76 (1.33, 2.32) | 2.16 (1.52, 3.07) | 2.05 (1.64, 2.57) |
| Cτ (fmol/million cells) | 23.5 (102) | 18.7 (98) | 37.2 (84) | 27.1 (137) | 20.0 (109) | 49.9 (148) | 1.18 (0.82, 1.71) | 2.05 (1.17, 3.59) | 2.21 (1.27, 3.84) | 2.13 (1.36, 3.35) |
| AUCCBV/AUCABCd | 103 (66) | 102 (69) | 105 (66) | 123 (65) | 109 (58) | 158 (74) | 1.22 (0.96, 1.55) | NAe | NA | 1.24 (0.96, 1.62) |
| Wt normalizedb | ||||||||||
| AUC0-24(h·fmol/million cells)1 | 811 (61) | 719 (46) | 1,034 (82) | 1,048 (74) | 827 (57) | 1,683 (74) | 1.32 (1.07, 1.63) | 1.53 (1.09, 2.14) | 1.92 (1.34, 2.74) | 1.81 (1.41, 2.34) |
| Cmax (fmol/million cells) | 58.3 (49) | 51.4 (43) | 75.0 (50) | 114 (76) | 90.3 (62) | 180 (88) | 1.99 (1.58, 2.49) | 1.53 (1.16, 2.01) | 1.87 (1.25, 2.81) | 1.78 (1.35, 2.35) |
| Cτ (fmol/million cells) | 23.4 (98) | 19.5 (91) | 33.8 (99) | 27.1 (136) | 20.9 (113) | 45.3 (152) | 1.18 (0.82, 1.71) | 1.78 (1.01, 3.12) | 1.92 (1.09, 3.36) | 1.85 (1.17, 2.92) |
AUC0-24for BID regimen was calculated as 2 × AUC(0 − τ).
Weight-normalized PK parameters were calculated as PK × weight (kg)/70 (kg).
Average of treatments A and B for each subject was used.
Ratio of CBV-TP AUC0-24and ABC AUC0-24, expressed as (fmol·h/million cells)/(μg·h/ml).
NA, not available.
TABLE 2.
Steady-state plasma ABC PK parameters, by treatment and gender, and comparisons
| Plasma ABC PK parameter | Geometric mean (% CV)
|
Geometric least square mean ratio (90% CI)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment A (ABC 300 mg BID regimen)
|
Treatment B (ABC 600 mg QD regimen)
|
|||||||||
| All subjects (n = 27) | Male (n = 18) | Female (n = 9) | All subjects (n = 27) | Male (n = 18) | Female (n = 9) | Treatment B/A (n = 27/27) | Female/male (n = 9/18)
|
|||
| Treatment A | Treatment B | Combinedf | ||||||||
| Original scale | ||||||||||
| AUC0-24(h·μg/ml)a | 7.90 (46) | 6.74 (41) | 10.9 (35) | 8.52 (43) | 7.26 (40) | 11.7 (27) | 1.08 (1.02, 1.15) | 1.59 (1.22, 2.08) | 1.60 (1.25, 2.05) | 1.60 (1.24, 2.05) |
| Cmax (μg/ml) | 1.84 (40) | 1.59 (34) | 2.48 (32) | 3.85 (37) | 3.43 (40) | 4.83 (11) | 2.09 (1.88, 2.32) | 1.55 (1.23, 1.96) | 1.39 (1.11, 1.74) | 1.43 (1.17, 1.76) |
| Cτ (μg/ml) | 0.018 (105) | 0.015 (106) | 0.026 (89) | 0.009 (102)c | 0.008 (103)d | 0.009 (117)e | 0.374 (0.283, 0.494) | 1.68 (0.944, 3.00) | 1.19 (0.495, 2.84) | 1.84 (0.931, 3.64) |
| Wt normalizedb | ||||||||||
| AUC0-24(h·μg/ml)a | 7.88 (46) | 7.04 (41) | 9.88 (48) | 8.50 (43) | 7.58 (41) | 10.7 (38) | 1.08 (1.02, 1.15) | 1.38 (1.03, 1.85) | 1.39 (1.06, 1.82) | 1.38 (1.05, 1.82) |
| Cmax (μg/ml) | 1.84 (41) | 1.66 (35) | 2.25 (45) | 3.83 (35) | 3.58 (39) | 4.39 (23) | 2.09 (1.88, 2.32) | 1.34 (1.03, 1.75) | 1.21 (0.954, 1.52) | 1.24 (0.994, 1.55) |
| Cτ (μg/ml) | 0.018 (101) | 0.016 (98) | 0.024 (103) | 0.008 (107)c | 0.008 (99)d | 0.009 (144)e | 0.372 (0.282, 0.491) | 1.46 (0.827, 2.58) | 1.18 (0.479, 2.90) | 1.60 (0.813, 3.13) |
AUC0-24for BID regimen was calculated as 2 × AUC(0 − τ).
Weight-adjusted PK parameters were calculated as PK × weight (kg)/70 (kg).
n = 21.
n = 15.
n = 6.
Average of treatments A and B for each subject was used.
Intracellular CBV-TP.
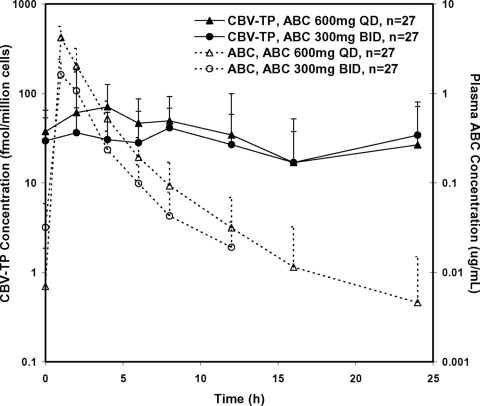
Intracellular CBV-TP concentrations were much more stable over time than were plasma ABC concentrations (Fig. 1). However, considerable variability in intracellular CBV-TP concentrations was observed between and within subjects; therefore, it was difficult to accurately estimate t1/2 of intracellular CBV-TP for both regimens. The reportable t1/2 values averaged 14.1 h and ranged from 4.8 to 39 h. Intracellular CBV-TP exposures were higher from the ABC 600 mg QD regimen than from the ABC 300 mg BID regimen. ABC 600 mg QD provided CBV-TP AUC0-24 and Cmax values that were on average 32% and 99% greater, respectively, than those from the ABC 300 mg BID regimen (Table 1). CBV-TP Cτ from the QD regimen was 18% higher than that from the BID regimen; however, such a difference was not statistically significant, as the 90% CI of the treatment ratio included 1 (Table 1). Intracellular CBV-TP exposures were higher in female subjects than in male subjects, with AUC0-24, Cmax, and Cτ values 109%, 105%, and 113% greater, respectively, in females than in males. When the comparisons were performed based on weight-normalized PK parameters, AUC0-24, Cmax, and Cτ values in females were 81%, 78%, and 85%, respectively, higher than those in males. AUCCBV/AUCABC values were on average 23% higher in females than in males; however, such a difference was not statistically significant, as the 90% CI included 1 (Table 1).
FIG. 1.
Mean concentration-time profiles of plasma ABC (dashed lines) and intracellular CBV-TP (solid lines) following ABC 300 mg BID (circles) and ABC 600 mg QD (triangles). Error bars represent standard deviations.
Plasma ABC.
Plasma ABC concentrations changed more dramatically than did intracellular CBV-TP concentration with the estimated t1/2 averaged at 3 to 4 h (Fig. 1). Due to the short plasma t1/2 of ABC, plasma ABC Cτ values were below the quantification limit for several subjects, especially during the QD treatment period.
Plasma ABC AUC0-24 values were equivalent between the ABC 600 mg QD and 300 mg BID regimens, while the ABC Cmax was 109% higher and Cτ was 62.6% lower with ABC 600 mg QD than with 300 mg BID (Table 2). Plasma ABC exposures were also higher in female subjects than in male subjects. Females had plasma ABC AUC0-24, Cmax, and Cτ values 60%, 43%, and 84% greater than those of males, respectively. When PK parameters were adjusted by weight, plasma AUC0-24, Cmax, and Cτ remained 38%, 24%, and 60% greater in females than in males, respectively (Table 2).
Relationship between plasma ABC and intracellular CBV-TP exposure.
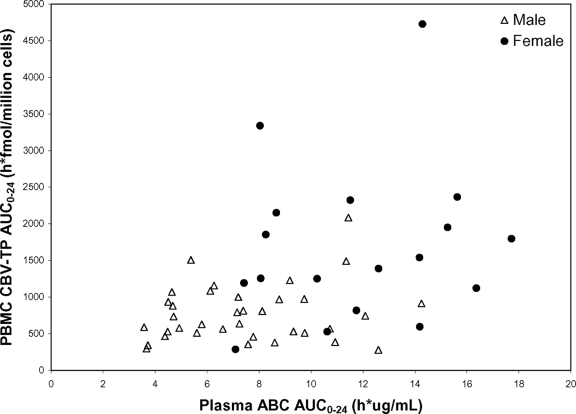
Intracellular CBV-TP AUC0-24 was significantly correlated with plasma ABC AUC0-24 (Fig. 2). However, when gender was included in the regression model, no significant association between plasma ABC and intracellular CBV-TP AUC0-24 was detected, and gender had become a significant predictor for the CBV-TP exposure, where females had a significantly higher CBV-TP exposure than did males. The association between plasma ABC AUC0-24 and intracellular CBV-TP AUC0-24 was also evaluated separately in male and female subjects, and the results showed no significant association in either males or females.
FIG. 2.
Relationship between plasma ABC AUC0-24 and intracellular CBV-TP AUC0-24. Data from both ABC 600 mg QD and ABC 300 mg BID treatments are included. Data from both females (solid circles) and males (open triangles) are presented. A significant correlation between plasma ABC and intracellular CBV-TP AUC0-24 values was found when all data were used (P < 0.01 based on regression of log-transformed data); however, when gender was included in the regression model, no significant association was detected.
Efficacy/safety.
The two treatment arms maintained similar percentages of subjects with undetectable HIV-1 RNA (<40 copies/ml) at screening and follow-up. The median CD4 cell count, 655/mm3 and 660/mm3 at screening in subjects on ABC 300 mg BID and on ABC 600 mg QD, respectively, remained stable during the course of the study, being 580 and 680/mm3 in these arms, respectively, at follow-up.
ABC was equally well tolerated in the subjects on the ABC 600 mg QD and 300 mg BID arms, with the same number (n = 4 [12%]) of subjects reporting any treatment-emergent adverse events. No subjects died or reported serious adverse events, no subjects were withdrawn due to adverse events, and no cases of ABC-associated hypersensitivity syndrome were reported when subjects switched frequency of ABC dosing. No vital sign or laboratory test trends were noted for either treatment arm.
DISCUSSION
The currently approved dosing regimens for ABC are 300 mg BID and 600 mg QD, both given in combination with other antiretroviral drugs. The approval of the 600 mg QD ABC dosing regimen was based on the findings of a randomized clinical study, CNA30021 (12), which demonstrated that ABC 600 mg QD dosing was noninferior to ABC 300 mg BID dosing in combination with QD 3TC and efavirenz. Additionally, CNA10905, a PK study of intracellular CBV-TP, reported an intracellular CBV-TP t1/2 of ∼20 h, therefore supporting QD dosing of ABC (13). However, CNA10905 examined an ABC 300 mg BID dosing regimen only. Another PK study, COL101665, was conducted to characterize the PKs of intracellular CBV-TP following ABC 600 mg QD dosing (3). Unexpectedly low exposures of both intracellular CBV-TP and plasma ABC concentrations were observed. The lower plasma ABC exposure was inconsistent with all previously reported PK studies in adults. The reason for the low concentrations of CBV-TP and ABC in this study has not been established.
Our current study compared intracellular CBV-TP exposure between ABC 300 mg BID and ABC 600 mg QD dosing in a crossover design in the same set of subjects and investigated gender differences in intracellular CBV-TP PKs. We found that plasma ABC AUC0-24 values were equivalent between the two regimens while intracellular CBV-TP AUC0-24 from 600 mg QD was slightly (on average, 32%) higher than that following the 300 mg BID regimen. Most importantly, despite the lower plasma ABC Cτ after ABC 600 mg QD dosing, the intracellular CBV-TP Cτ values were similar between ABC 600 mg QD and ABC 300 mg BID; Cmax values of intracellular CBV-TP were higher for ABC 600 mg QD than for ABC 300 mg BID. Thus, the PK results support the clinical efficacy data that demonstrated that ABC 600 mg QD dosing was noninferior to 300 mg BID dosing (12).
Due to complexity in sample collection and processing or inherent variability in ABC anabolism, high intersubject variability in intracellular CBV-TP concentrations was observed in this study. The intersubject variability in CBV-TP exposure (AUC, Cmax, and Cτ) was greater than 50%. The intersubject variability in t1/2 was even higher, which may have been, in part, a consequence of the short sampling period of only up to 24 h that prevented an accurate estimation of t1/2. Intracellular CBV-TP PKs following ABC 300 mg BID or ABC 600 mg QD dosing have been reported in several studies (3, 6, 7, 9, 13). There was significant variation in intracellular CBV-TP concentrations among different studies, which is in part due to the different assays that were employed. Despite the variability, all studies demonstrated prolonged terminal t1/2 of intracellular CBV-TP: >12 h by Harris et al. (6) and Kewn et al. (9), 18 h by Hawkins et al. (7), and 21 h by Piliero et al. (13). The observed prolonged terminal t1/2 supported the ABC 600 mg QD dosing regimen. Intracellular CBV-TP concentrations following ABC 300 mg BID dosing seen in our study are consistent with those observed in CNA10905 (13), which used similar sample collection/processing procedures and the same bioanalytical assay.
An influence of gender on plasma ABC and intracellular CBV-TP was observed in our study. Female subjects had higher exposure than did male subjects, even after adjusting for body weight. The higher intracellular CBV-TP exposure in female subjects was correlated with higher plasma ABC exposure and higher extent of conversion from plasma ABC to intracellular CBV-TP. To date, one other PK study that involved one female and four males treated with ABC 600 mg QD reported that the female had PBMC CBV-TP concentrations two to eight times greater than those in the male subjects (6). However, a gender-related difference in ABC plasma PKs was not shown in the population PK studies by Weller et al. (18) or Jullien et al. (8). Pivotal clinical trials of ABC-containing regimens (CNA30021, CNA30024, and CNAB3002) that performed a statistical analysis of 48-week efficacy findings (proportion of subjects achieving HIV-1 RNA levels of ≤50 or ≤400 copies/ml) by gender did not find significant differences between responses in women and those in men, although generally fewer than 25% of the study populations were female (data on file, GlaxoSmithKline). Studies of other nucleoside reverse transcriptase inhibitors have reported gender-related differences in both PKs and treatment response. Anderson et al. (2) evaluated 33 antiretroviral-naïve adults, including four women, treated with a regimen of ZDV, 3TC, and indinavir and followed their treatment response and PBMC TP concentrations for over 18 months. Assessment of 310 ZDV-TP and 3TC-TP PBMC samples revealed that ZDV-TP and 3TC-TP concentrations in women were 2.3- to 1.6-fold higher than those in men (P < 0.001). Women achieved virologic suppression (HIV-1 RNA level of <50 copies/ml) twice as fast as did men. Another study, ACTG161 (17), also noted that even when data were corrected for differences in body weight, women (n = 5) on the same ZDV dosage regimen as that of men (n = 16) had a mean ZDV-total phosphate AUC that was 45% greater (P < 0.005). Again, all gender studies of PKs and pharmacodynamics have included a disproportionately smaller number of female subjects than of male subjects, and future studies should target equivalent numbers of subjects of each gender.
In conclusion, similar intracellular CBV-TP Cτ values were observed with ABC 600 mg QD and ABC 300 mg BID dose regimens in this study, providing PK support at the intracellular level for the interchangeability of these two regimens. A gender difference in intracellular phosphorylation of ABC was observed, with women showing higher intracellular CBV-TP exposure than that of men.
Acknowledgments
This work was supported by GlaxoSmithKline.
We thank GSK employees Andrew Preece for study coordination and Gary Pakes for his contribution to the preparation of the manuscript.
Footnotes
Published ahead of print on 2 February 2009.
REFERENCES
- 1.Anderson, P. L. 2004. Pharmacologic perspectives for once-daily antiretroviral therapy. Ann. Pharmacother. 38:1924-1934. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, P. L., T. N. Kakuda, S. Kawle, and C. V. Fletcher. 2003. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS 17:2159-2168. [DOI] [PubMed] [Google Scholar]
- 3.Bellos, N. C., I. H. Song, Y. Lou, G. J. Yuen, C. T. Lancaster, and G. E. Pakes. 2008. Intracellular carbovir-triphosphate (CBV-TP) pharmacokinetics (PK) and plasma abacavir (ABC) PK in HIV+ patients treated with ABC 600 mg once daily (QD) (COL101665), abstr. 310. Mol. Cell. Determinants HIV Pathog. Keystone Symp., Banff, Alberta, Canada, 27 March to 1 April 2008.
- 4.Conway, B. 2007. The role of adherence to antiretroviral therapy in the management of HIV infection. J. Acquir. Immune Defic. Syndr. 45(Suppl. 1):S14-S18. [DOI] [PubMed] [Google Scholar]
- 5.Gatti, A. M., F. Arpinelli, G. Visconà, R. Panebianco, and G. Rizzardini on behalf of the ADEQUA Study Team. 2002. Impact of less complex HIV therapy on adherence and quality of life, abstr. 96. Sixth Int. Congr. Drug Ther. HIV Infect., Glasgow, Scotland, 17 to 21 November 2002.
- 6.Harris, M., D. Back, S. Kewn, S. Jutha, R. Marina, and J. S. G. Montaner. 2002. Intracellular carbovir triphosphate levels in patients taking abacavir once a day. AIDS 16:1196-1197. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins, T., W. Viekley, R. L. St. Claire III, B. Guyer, N. Clark, and B. P. Kearney. 2005. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J. Acquir. Immune Defic. Syndr. 39:405-411. [DOI] [PubMed] [Google Scholar]
- 8.Jullien, V., J.-M. Tréluyer, H. Chappuy, J. Dimet, E. Rey, N. Dupin, D. Salmon, G. Pons, and S. Urien. 2005. Weight related differences in the pharmacokinetics of abacavir in HIV-infected patients. Br. J. Clin. Pharmacol. 59:183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kewn, S., B. Maher, P. G. Hoggard, S. H. Khoo, P. Carey, W. Wilkins, J. Gould, and D. J. Back. 2000. The pharmacokinetics of abacavir phosphorylation in peripheral blood mononuclear cells from HIV positive subjects. AIDS 14(Suppl. 4):S95-S96. [Google Scholar]
- 10.Maggiolo, F., D. Ripamonti, and F. Suter. 2003. Once-a day HAART: dream or reality. HIV Clin. Trials 4:193-201. [DOI] [PubMed] [Google Scholar]
- 11.Maitland, D., A. Jackson, J. Osorio, S. Mandalia, B. Gazzard, and G. Moyle. 2008. Switching from twice-daily abacavir and lamivudine to the once-daily fixed-dose combination tablet of abacavir and lamivudine improves patient adherence and satisfaction with therapy. HIV Med. 9:667-672. [DOI] [PubMed] [Google Scholar]
- 12.Moyle, G., E. DeJesus, P. Cahn, S. Castillo, H. Zhao, D. Gordon, C. Craig, and T. Scott for the ZIAGEN Once-Daily in Antiretroviral Combination Therapy (ZODIAC) Study Team. 2005. Abacavir 600 mg once daily versus 300 mg twice-daily combined with lamivudine in combination with efavirenz (EFV) OAD is well-tolerated and effective in the treatment of antiretroviral therapy (ART) naïve adults with HIV-1 infection (ZODIAC study: CNA30021). J. Acquir. Immune Defic. Syndr. 38:417-425. [DOI] [PubMed] [Google Scholar]
- 13.Piliero, P., A. D. Shachoy-Clark, M. Para, S. Preston, Y. Lou, G. Drusano, D. S. Stein, and G. J. Yuen. 2003. A study examining the pharmacokinetics of abacavir and the intracellular carbovir triphosphate (GSK Protocol CNA10905), abstr. A-1797. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother.
- 14.Pollard, R. B. 2002. Can. HIV infection be treated successfully with a once-daily regimen? AIDS Read. 12:489-500, 508. [PubMed] [Google Scholar]
- 15.Portsmouth, S. D., J. Osorio, J. K. McCormick, B. G. Gazzard, and G. J. Moyle. 2005. Better maintained adherence on switching from twice-daily to once-daily therapy for HIV: a 24-week randomized trial of treatment simplification using stavudine prolonged-release capsules. HIV Med. 6:185-190. [DOI] [PubMed] [Google Scholar]
- 16.Stone, V. E., J. Jordan, J. Tolson, R. Miller, and T. Pilon. 2004. Perspectives on adherence and simplicity for HIV-infected patients on antiretroviral therapy: self-report of the relative importance of multiple attributes of highly active antiretroviral therapy (HAART) regimens in predicting adherence. J. Acquir. Immune Defic. Syndr. 36:808-816. [DOI] [PubMed] [Google Scholar]
- 17.Stretcher, B. N., A. J. Pesce, P. T. Frame, and D. S. Stein. 1994. Pharmacokinetics of zidovudine phosphorylation in peripheral blood mononuclear cells from patients infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 38:1541-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weller, S., K. M. Radomski, Y. Lou, and D. S. Stein. 2000. Population pharmacokinetics and pharmacodynamic modeling of abacavir (1592U89) from a dose-ranging double-blind, randomized monotherapy trial with human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 44:2052-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]