Abstract
To facilitate mode of action studies on antibacterial inhibitors of early-stage cell wall biosynthesis (CWB), we determined the transcriptional response of Staphylococcus aureus to depletion/inhibition of enzymes in this pathway by DNA microarray analysis. We identified a transcriptional signature distinct from that previously observed following exposure to inhibitors of late-stage CWB.
Defining the mode of action (MOA) of a new antibacterial agent is essential for guiding the further development process, including optimization of structure-activity relationships (3, 11). Transcriptional profiling using DNA microarrays has emerged as a powerful technique for MOA studies, since it can provide a genome-wide overview of the cellular response to antibacterial inhibitors at the level of transcription (3, 4, 11). By analyzing the genes deregulated following exposure to a novel antimicrobial, the MOA can be predicted by comparison with profiles obtained with established antibiotics with known MOAs (3, 4, 11).
Cell wall biosynthesis (CWB) is an important target for antibiotic action in Staphylococcus aureus (12). Substantial efforts have been directed toward the discovery of antibacterial inhibitors of early-stage CWB, mediated by the Mur enzymes (1, 2, 12) (Fig. 1). This portion of the pathway, also called stage I or the cytoplasmic stage of peptidoglycan synthesis (12), remains largely unexploited as a target for antibacterial chemotherapy (2, 12). To assist analysis of novel candidate anti-CWB inhibitors and to build on earlier studies that have identified transcriptional responses to antibiotics targeting the later stages of CWB (e.g., vancomycin, oxacillin) (7, 14), we sought to establish a universal transcriptional signature of S. aureus following inhibition of stage I CWB.
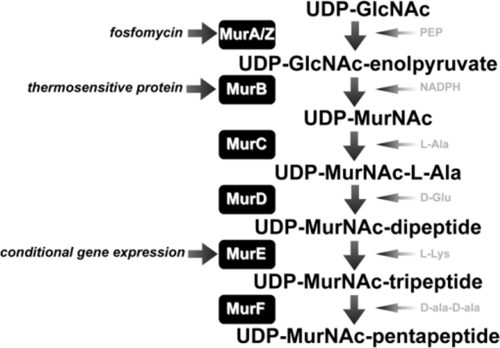
FIG. 1.
The stage I cell wall biosynthesis pathway in Staphylococcus aureus involves the biosynthesis of UDP-MurNAc-pentapeptide from UDP-GlcNAc, mediated by the Mur enzymes. The three points at which inhibition of the pathway was achieved in this study are shown in italics. PEP, phosphoenolpyruvate.
Unfortunately, there is a lack of characterized control inhibitors that specifically inhibit this stage of the pathway. Of the Mur enzyme inhibitors reported in the literature, only fosfomycin unequivocally mediates antibacterial activity specifically through inhibition of this portion of the pathway by interfering with the function of the UDP-N-acetylglucosamine enolpyruvyl transferase isoenzymes, MurA and MurZ (12). To circumvent this problem, in addition to using fosfomycin as a test inhibitor, we also employed genetic and posttranslational challenge to specifically inhibit/deplete the cell of other individual enzymes active in early-stage CWB (MurB and MurE).
S. aureus RN4220 and derivatives were used throughout this study. Strain TS2557 (8) carries a temperature-sensitive mutation in murB. S. aureus CYL368 (5) has been engineered to place murE under the control of the Pspac promoter, rendering expression of this gene conditional upon the presence of isopropyl-β-d-1-thiogalactopyranoside (IPTG). Since strain CYL368 required the presence of tetracycline in the growth medium to ensure maintenance of the lacI repressor plasmid (pMJ8246), pMJ8246 was also introduced into S. aureus RN4220 to enable both conditional and control strains to be cultured under identical conditions in the presence of tetracycline.
Strains were cultured in tryptone soya broth (TSB) with aeration. Conditional mutants were cultured under conditions that resulted in ca. 80% inhibition of growth in the mutant relative to the wild type, while drug-treated cultures were exposed to a concentration of antibiotic causing ca. 25% reduction in growth relative to untreated cultures after 40 min (3). Cells were harvested by adding 2 volumes of RNAprotect solution (Qiagen) directly to the culture and then processed according to the manufacturer's instructions.
S. aureus CYL368 and RN4220(pMJ8246) were grown overnight at 37°C in the presence of 3 μg tetracycline/ml and 0.3 mM IPTG. Cells were harvested, washed extensively to remove IPTG, and resuspended to an optical density at 600 nm (OD600) of 0.05 in fresh broth lacking IPTG. Cultures were then grown at 37°C and harvested at an OD600 of 0.25. Strains TS2557 and RN4220 were grown overnight at 30°C. Both strains were resuspended to an OD600 of 0.075 in fresh broth, grown at 42°C, and harvested at an OD600 of 0.25.
Fosfomycin treatment was conducted as follows. An overnight culture of S. aureus RN4220 grown at 37°C was used to inoculate fresh, prewarmed TSB to an OD600 of ∼0.02 and grown at 37°C to an OD600 of 0.1. The culture was split into prewarmed flasks, one of which contained fosfomycin (20 μg/ml), and incubation continued for 40 min before harvesting cells.
RNA was prepared using the RNA midi kit (Qiagen) from cells treated with lysostaphin. Control RNA and sample RNA were used to make differentially labeled cDNA by reverse transcription in the presence of fluorescent dyes Cy3 and Cy5. Both RNAs were then cohybridized, scanned, and analyzed as previously described (16). Cultures were grown in triplicate and hybridized in duplicate for a total of six arrays per condition. Microarray feature extraction was undertaken using ImaGene software (BioDiscovery), and the resulting fluorescent intensities were further processed using MAVI Pro software (MWG Biotech). Normalization and statistical analysis were performed using GeneSpring v7.3.1 software (Agilent Technologies). Differentially expressed genes for each condition were identified; these genes had normalized ratios that were >2-fold up- or downregulated with a P value of <0.05 by t test with Benjamini and Hochberg false discovery rate correction.
Genes subject to the same level of deregulation (≥2-fold up- or downregulated with a P of <0.05) under all three conditions (inhibition/depletion of MurA or MurZ, MurB, and MurE), were considered members of the transcriptional signature for inhibition of CWB (Table 1). This transcriptional signature primarily involved upregulation of genes involved in providing precursors essential for CWB (e.g., gltAB [glutamate biosynthesis], oppABCDF; [oligopeptide transport], dapABD, asd, thrBC, dhoM, ilvBCD, and leuABCD [amino acid biosynthesis]) and genes involved in the response to environmental stress (e.g., ctsR [transcriptional regulator of stress response], clpB [stress response-related chaperone], msrA2 [methionine sulfoxide reductase], and katA [catalase]) (Table 1).
TABLE 1.
Genes deregulated following inhibition/depletion of enzymes of stage I cell wall biosynthesis in S. aureusa
| Locus tag designation (S. aureus N315d) | Gene designation (S. aureus N315) | Fold change in expression following inhibition/depletion ofb:
|
Transcriptional response from previous studiesc
|
|||
|---|---|---|---|---|---|---|
| MurA and MurZ | MurB | MurE | Conditional depletion of MurF | Inhibitors of stages II and III of CWB pathway | ||
| Upregulated genes | ||||||
| SA0011 | 3.47 | 3.09 | 6.69 | U | ||
| SA0122 | butA | 3.67 | 5.39 | 9.02 | U | U |
| SA0346 | 3.23 | 3.81 | 2.78 | U | ||
| SA0422 | 2.72 | 3.10 | 3.60 | U | ||
| SA0430 | gltA | 13.75 | 14.41 | 33.41 | U | U |
| SA0431 | gltB | 7.74 | 10.09 | 11.18 | U | U |
| SA0480 | ctsR | 2.13 | 5.13 | 2.73 | ||
| SA0481 | 2.40 | 4.38 | 2.48 | U | ||
| SA0482 | 2.37 | 4.83 | 2.30 | |||
| SA0612 | 2.22 | 2.69 | 2.88 | U | ||
| SA0707 | 3.19 | 2.96 | 5.18 | U | ||
| SA0818 | rocD | 2.46 | 4.43 | 6.20 | U | |
| SA0835 | clpB | 4.05 | 10.40 | 3.39 | U | |
| SA0845 | oppB | 11.66 | 13.41 | 134.41 | U | U |
| SA0846 | oppC | 12.64 | 13.17 | 149.31 | U | U |
| SA0847 | oppD | 11.81 | 12.95 | 52.95 | U | U |
| SA0848 | oppF | 13.48 | 17.55 | 118.00 | U | U |
| SA0849 | oppA | 10.59 | 15.31 | 65.31 | U | U |
| SA0871 | 3.40 | 3.59 | 3.45 | U | ||
| SA0883 | 5.42 | 4.69 | 11.23 | U | ||
| SA0886 | 2.50 | 3.40 | 2.96 | |||
| SA0958 | 2.98 | 2.12 | 2.00 | U | ||
| SA1019 | 2.57 | 2.45 | 3.25 | U | ||
| SA1163 | 4.21 | 4.45 | 5.60 | U | ||
| SA1164 | dhoM | 7.27 | 5.14 | 17.50 | U | U |
| SA1165 | thrC | 7.58 | 5.17 | 12.94 | U | U |
| SA1166 | thrB | 4.39 | 3.63 | 3.43 | U | U |
| SA1170 | katA | 2.98 | 2.27 | 2.39 | ||
| SA1226 | asd | 9.32 | 5.99 | 27.16 | U | U |
| SA1227 | dapA | 8.40 | 5.58 | 17.73 | U | U |
| SA1228 | dapB | 7.19 | 4.64 | 13.00 | U | U |
| SA1229 | dapD | 5.96 | 4.26 | 10.58 | U | U |
| SA1230 | 4.84 | 3.11 | 6.83 | U | U | |
| SA1231 | dal | 4.73 | 3.20 | 4.76 | U | |
| SA1235 | 2.09 | 2.01 | 2.02 | |||
| SA1236 | 4.33 | 2.58 | 3.26 | U | U | |
| SA1238 | 3.88 | 2.62 | 2.96 | U | ||
| SA1254 | 5.23 | 3.50 | 2.52 | U | ||
| SA1255 | 9.90 | 3.80 | 2.83 | U | ||
| SA1256 | 9.30 | 3.29 | 3.10 | U | ||
| SA1257 | msrA2 | 10.54 | 3.35 | 2.92 | U | |
| SA1532 | 4.58 | 3.05 | 7.69 | U | U | |
| SA1545 | serA | 6.24 | 6.00 | 6.67 | U | U |
| SA1546 | 3.93 | 2.39 | 3.60 | U | U | |
| SA1691 | sgtB | 7.24 | 4.34 | 2.15 | U | U |
| SA1858 | ilvD | 5.89 | 12.47 | 6.90 | U | |
| SA1859 | ilvB | 8.40 | 12.71 | 10.63 | U | |
| SA1860 | ilvH | 7.48 | 16.51 | 6.31 | U | |
| SA1861 | ilvC | 11.90 | 16.00 | 20.74 | U | U |
| SA1862 | leuA | 9.05 | 14.46 | 12.70 | U | |
| SA1863 | leuB | 8.89 | 13.35 | 8.69 | U | |
| SA1864 | leuC | 8.92 | 14.99 | 10.40 | U | |
| SA1865 | leuD | 8.58 | 14.77 | 9.84 | U | |
| SA1866 | ilvA | 5.81 | 11.57 | 6.06 | U | |
| SA2112 | 2.75 | 2.16 | 3.10 | |||
| SA2221 | 14.49 | 3.57 | 2.12 | U | ||
| SA2235 | opuCC | 2.48 | 3.77 | 3.80 | U | |
| SA2236 | opuCB | 2.44 | 3.89 | 4.23 | U | |
| SA2237 | opuCA | 2.60 | 5.98 | 4.82 | U | |
| SA2240 | 2.15 | 2.40 | 3.38 | U | ||
| SA2346 | 4.35 | 2.45 | 4.50 | U | ||
| SA2347 | 4.51 | 2.95 | 5.68 | U | ||
| SA2357 | 4.26 | 4.86 | 5.02 | U | U | |
| SA2396 | 3.05 | 4.08 | 4.99 | U | U | |
| SA2397 | 4.12 | 13.88 | 15.47 | U | U | |
| SA2473 | 2.56 | 3.43 | 3.56 | |||
| SA2475 | 2.82 | 4.43 | 4.37 | U | U | |
| SA2476 | 3.76 | 5.61 | 7.00 | U | U | |
| SA2477 | 5.24 | 7.64 | 13.11 | |||
| SA2478 | 6.19 | 8.12 | 19.76 | |||
| SA2490 | 2.97 | 3.81 | 2.92 | U | ||
| Downregulated genes | ||||||
| MW0552 | 0.49 | 0.46 | 0.33 | |||
| SA0009 | serS | 0.49 | 0.26 | 0.34 | ||
| SA0085 | 0.50 | 0.42 | 0.44 | D | ||
| SA0100 | 0.21 | 0.09 | 0.20 | U | ||
| SA0111 | sirA | 0.26 | 0.23 | 0.47 | D | |
| SA0183 | glcA | 0.17 | 0.23 | 0.29 | D | |
| SA0213 | 0.36 | 0.29 | 0.47 | |||
| SA0268 | 0.31 | 0.36 | 0.18 | |||
| SA0269 | 0.06 | 0.11 | 0.21 | |||
| SA0270 | 0.20 | 0.38 | 0.28 | D | ||
| SA0325 | glpT | 0.47 | 0.32 | 0.29 | ||
| SA0423 | 0.36 | 0.29 | 0.31 | D | ||
| SA0497 | rplJ | 0.47 | 0.28 | 0.27 | ||
| SA0498 | rplL | 0.49 | 0.22 | 0.25 | D | |
| SA0654 | fruB | 0.18 | 0.34 | 0.34 | U | |
| SA0655 | fruA | 0.17 | 0.25 | 0.31 | ||
| SA0820 | glpQ | 0.44 | 0.32 | 0.27 | D | |
| SA0905 | atl | 0.46 | 0.18 | 0.38 | D | |
| SA0912 | qoxB | 0.33 | 0.36 | 0.49 | D | |
| SA0913 | qoxA | 0.35 | 0.35 | 0.49 | D | |
| SA0951 | potB | 0.50 | 0.35 | 0.38 | D | |
| SA1056 | 0.22 | 0.49 | 0.25 | |||
| SA1160 | nucI | 0.42 | 0.32 | 0.30 | D | |
| SA1502 | rplT | 0.46 | 0.21 | 0.23 | D | |
| SA1665 | 0.50 | 0.37 | 0.31 | D | D | |
| SA1850 | 0.43 | 0.37 | 0.48 | U | ||
| SA1921 | tdk | 0.44 | 0.33 | 0.45 | D | |
| SA1979 | 0.35 | 0.32 | 0.40 | D | ||
| SA2016 | rpsI | 0.49 | 0.35 | 0.40 | D | |
| SA2093 | ssaA | 0.24 | 0.05 | 0.09 | D | D |
| SA2097 | ssaA | 0.37 | 0.05 | 0.08 | D | |
| SA2300 | 0.36 | 0.24 | 0.36 | D | ||
| SA2332 | 0.37 | 0.19 | 0.16 | |||
| SA2356 | isaA | 0.29 | 0.09 | 0.10 | D | |
| SA2430 | aur | 0.31 | 0.28 | 0.40 | D | |
| SA2486 | 0.49 | 0.36 | 0.39 | |||
Genes deregulated following inhibition/depletion of MurA and MurZ, MurB, MurE, and MurF, but not upon exposure to any inhibitors of stages II and III of CWB, are considered members of the universal transcriptional response to inhibition of stage I and are shown in bold type.
Only those genes showing ≥2-fold deregulation in the same direction (up- or downregulation) under all three experimental conditions employed in this study (inhibition/depletion of MurA and MurZ, MurB, and MurE) are shown.
The transcriptional responses of these genes in previous studies (following conditional depletion of MurF [13] or following inhibition of stages II and III of the CWB pathway [10]) are also shown. U, upregulated; D, downregulated.
Except in cases where genes are not present or have not been designated in S. aureus N315. In this case, locus tag designations are from S. aureus MW2.
Little deregulation was detected in genes encoding enzymes directly involved in CWB or cell wall turnover, with the exception of upregulation of dal (alanine racemase), sgtB (penicillin-binding protein homologue), and downregulation of atl (autolysin) (Table 1). No consistent deregulation in expression of the Mur enzymes was observed, suggesting that as for Escherichia coli (9, 15), expression of genes involved in stage I peptidoglycan synthesis in S. aureus is constitutive, and CWB is unrestricted up to, and including, synthesis of the UDP-MurNAc pentapeptide.
We subsequently obtained a universal transcriptional signature specifically for inhibition of stage I CWB (Table 1). This was derived from our transcriptional signature for inhibition of CWB by including only those genes similarly deregulated following conditional depletion of MurF in a previous study (13) and subtracting all genes known to be deregulated following exposure to inhibitors of stage II/III CWB (bacitracin, vancomycin, oxacillin, and cefoxitin) (6, 7, 10, 14) (Table 1). Our results suggest that transcriptional profiling can be employed not only to identify inhibitors of CWB but also to establish whether they act on early or late stages in the biosynthetic pathway.
Microarray data accession number.
Fully annotated microarray data have been deposited in BμG@Sbase (accession number E-BUGS-71) (http://bugs.sgul.ac.uk/E-BUGS-71) and also ArrayExpress (accession number E-BUGS-71).
Acknowledgments
We are grateful to C. Y. Lee (University of Kansas) and K. Kurokawa (University of Tokyo) for providing strains CYL368 and TS2557, respectively.
We acknowledge The Wellcome Trust for funding BμG@S (the Bacterial Microarray Group at St. George's University of London).
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Dunsmore, C. J., K. Miller, K. L. Blake, S. G. Patching, P. J. F. Henderson, J. A. Garnett, W. J. Stubbings, S. E. V. Phillips, D. J. Palestrant, J. De Los Angeles, J. A. Leeds, I. Chopra, and C. W. G. Fishwick. 2008. 2-Aminotetralones: novel inhibitors of MurA and MurZ. Bioorg. Med. Chem. Lett. 18:1730-1734. [DOI] [PubMed] [Google Scholar]
- 2.El Zoeiby, A., F. Sanschagrin, and R. C. Levesque. 2003. Structure and function of the Mur enzymes: development of novel inhibitors. Mol. Microbiol. 47:1-12. [DOI] [PubMed] [Google Scholar]
- 3.Freiberg, C., H. P. Fischer, and N. A. Brunner. 2005. Discovering the mechanism of action of novel antibacterial agents through transcriptional profiling of conditional mutants. Antimicrob. Agents Chemother. 49:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutter, B., C. Schaab, S. Albrecht, M. Borgmann, N. A. Brunner, C. Freiberg, K. Ziegelbauer, C. O. Rock, I. Ivanov, and H. Loferer. 2004. Prediction of mechanisms of action of antibacterial compounds by gene expression profiling. Antimicrob. Agents Chemother. 48:2838-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jana, M., T. T. Luong, H. Komatsuzawa, M. Shigeta, and C. Y. Lee. 2000. A method for demonstrating gene essentiality in Staphylococcus aureus. Plasmid 44:100-104. [DOI] [PubMed] [Google Scholar]
- 6.Kuroda, H., M. Kuroda, L. Cui, and K. Hiramatsu. 2007. Subinhibitory concentrations of beta-lactam induce haemolytic activity in Staphylococcus aureus through the SaeRS two-component system. FEMS Microbiol. Lett. 268:98-105. [DOI] [PubMed] [Google Scholar]
- 7.Kuroda, M., H. Kuroda, T. Oshima, F. Takeuchi, H. Mori, and K. Hiramatsu. 2003. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol. Microbiol. 49:807-821. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo, M., K. Kurokawa, S. Nishida, Y. Li, H. Takimura, C. Kaito, N. Fukuhara, H. Maki, K. Miura, K. Murakami, and K. Sekimizu. 2003. Isolation and mutation site determination of the temperature-sensitive murB mutants of Staphylococcus aureus. FEMS Microbiol. Lett. 222:107-113. [DOI] [PubMed] [Google Scholar]
- 9.Mengin-Lecreulx, D., E. Siegel, and J. van Heijenoort. 1989. Variations in UDP-N-acetylglucosamine and UDP-N-acetylmuramyl-pentapeptide pools in Escherichia coli after inhibition of protein synthesis. J. Bacteriol. 171:3282-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagarajan, V., and M. O. Elasri. 2007. SAMMD: Staphylococcus aureus Microarray Meta-Database. BMC Genomics 8:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Neill, A. J., and I. Chopra. 2004. Preclinical evaluation of novel antibacterial agents by microbiological and molecular techniques. Expert Opin. Investig. Drugs 13:1045-1063. [DOI] [PubMed] [Google Scholar]
- 12.Silver, L. L. 2006. Does the cell wall of bacteria remain a viable source of targets for novel antibiotics? Biochem. Pharmacol. 71:996-1005. [DOI] [PubMed] [Google Scholar]
- 13.Sobral, R. G., A. E. Jones, S. G. Des Etages, T. J. Dougherty, R. M. Peitzsch, T. Gaasterland, A. M. Ludovice, H. de Lencastre, and A. Tomasz. 2007. Extensive and genome-wide changes in the transcription profile of Staphylococcus aureus induced by modulating the transcription of the cell wall synthesis gene murF. J. Bacteriol. 189:2376-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Utaida, S., P. M. Dunman, D. Macapagal, E. Murphy, S. J. Projan, V. K. Singh, R. K. Jayaswal, and B. J. Wilkinson. 2003. Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cell-wall-active antibiotics reveals a cell-wall-stress stimulon. Microbiology 149:2719-2732. [DOI] [PubMed] [Google Scholar]
- 15.van Heijenoort, J. 2001. Recent advances in the formation of the bacterial peptidoglycan monomer unit. Nat. Prod. Rep. 18:503-519. [DOI] [PubMed] [Google Scholar]
- 16.Witney, A. A., G. L. Marsden, M. T. Holden, R. A. Stabler, S. E. Husain, J. K. Vass, P. D. Butcher, J. Hinds, and J. A. Lindsay. 2005. Design, validation, and application of a seven-strain Staphylococcus aureus PCR product microarray for comparative genomics. Appl. Environ. Microbiol. 71:7504-7514. [DOI] [PMC free article] [PubMed] [Google Scholar]