Chlorhexidine digluconate (CHG) is widely used in the clinical setting for skin antisepsis prior to incision or insertion of medical devices, e.g., central venous catheters (11-13); however, its permeation into skin is limited (6, 7, 9, 10, 16). In a recent study, we demonstrated the limited penetration of CHG in a skin model comprising full-thickness excised human skin following the application of 2% (wt/vol) aqueous CHG (9). The aim of this current study was to compare the penetration of chlorhexidine into skin following the topical application of 2% (wt/vol) CHG in 70% (vol/vol) isopropyl alcohol (IPA) with that of aqueous CHG.
Skin permeation studies were performed on full-thickness excised human skin as described previously (9). Many differences between animal and human skin absorption have been shown to be permeant specific, and due to the applied nature of this work, it was determined that human skin must be used (3, 5). Due to the limitation of the availability of fresh human skin, frozen skin is most often used as a model to minimize the interpersonal variability of the donor skin permeability. Human skin has benefits over animal skin, and the storage of human skin for a prolonged time has been shown not to have a significant effect on skin permeability (3, 5). In brief, excised human skin was exposed to aqueous and alcoholic CHG for clinically relevant time periods of 2 min and 30 min in a Franz cell diffusion model. The concentration of CHG in serial skin sections was determined by high-performance liquid chromatography.
Overall, following 2- and 30- min exposures, the skin penetration of CHG from both aqueous and alcoholic solutions was limited (Fig. 1 and 2). At skin depths of ≥300 μm, the concentration of CHG detected from both solutions was negligible (<0.0008 μg/mg tissue). In addition, CHG did not completely permeate through the full thickness of the human skin model.
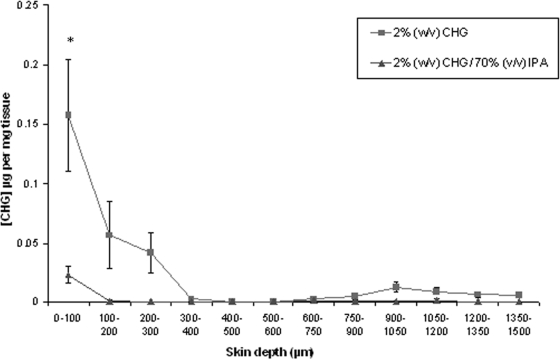
FIG. 1.
Penetration profile showing the location and concentration of chlorhexidine (μg/mg tissue) in excised human skin after 2-min (n = 15) exposure to 2% (wt/vol) CHG in 70% (vol/vol) IPA and aqueous 2% (wt/vol) CHG (mean ± SE). *, P = 0.008.
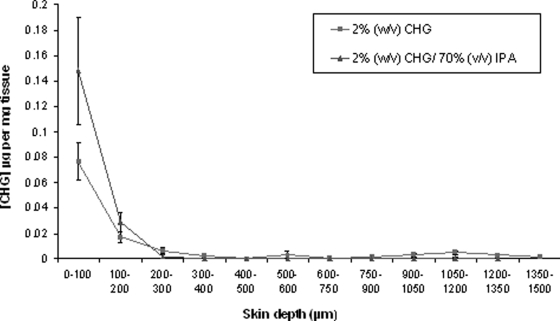
FIG. 2.
Penetration profile showing the location and concentration of chlorhexidine (μg/mg tissue) in excised human skin after 30-min (n = 15) exposure to 2% (wt/vol) CHG in 70% (vol/vol) IPA and aqueous 2% (wt/vol) CHG (mean ± SE).
The concentration of CHG recovered within the top 100-μm skin sections was significantly less following a 2-min exposure to alcoholic CHG than that following similar exposure to aqueous CHG (mean CHG concentration [± standard error (SE)] of 0.023 [± 0.007] μg and 0.157 [± 0.047] μg per mg tissue for CHG/IPA and CHG, respectively [P = 0.008]). Following a 30-min exposure, there was no significant difference in the skin penetration of the CHG from alcoholic and aqueous solutions within the model (P > 0.05).
The results from this study clearly demonstrate the limited permeation of CHG within a human skin model following the application of either alcoholic or aqueous solutions. Moreover, the negligible concentrations of CHG detected at skin depths of >300 μm may indeed allow for microorganisms residing in the deeper layers, such as around hair follicles, to survive the skin antisepsis procedures recommended in the current EPIC (evidence-based practice in infection control) guidelines (13).
While chlorhexidine in alcoholic solution has clearly been shown to have superior antimicrobial activity compared to aqueous CHG (1, 8), their efficacy in reducing catheter colonization and infection is comparable (14). Alcohol, at a concentration of 70% (vol/vol) has rapid antimicrobial activity against a broad spectrum of microorganisms (2). However, it has also been shown to extract important lipid components of the stratum corneum and to cause the dehydration of stratum corneum proteins, thus potentially compromising the permeation of CHG within the skin (4, 15). These results clearly lay the foundation for further research within the field of skin antisepsis with a view to developing improved formulation strategies for the use of CHG in clinical practice.
Acknowledgments
This work was supported by EPSRC case reward CNA/05/09.
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Adams, D., M. Quayum, T. Worthington, P. Lambert, and T. Elliott. 2005. Evaluation of a 2% chlorhexidine gluconate in 70% isopropyl alcohol skin disinfectant. J. Hosp. Infect. 61:287-290. [DOI] [PubMed] [Google Scholar]
- 2.Adams, D. H. 2006. An evaluation of three strategies to reduce device related infections associated with hypodermic needles and peripheral vascular catheters. Aston University, Birmingham, United Kingdom.
- 3.Bronaugh, R. L., R. F. Stewart, and M. Simon. 1986. Methods for in vitro percutaneous absorption studies. VII: Use of excised human skin. J. Pharm. Sci. 75:1094-1097. [DOI] [PubMed] [Google Scholar]
- 4.dos Anjos, J. L., D. de Sousa Neto, and A. Alonso. 2007. Effects of ethanol/l-menthol on the dynamics and partitioning of spin-labeled lipids in the stratum corneum. Eur. J. Pharm. Biopharm. 67:406-412. [DOI] [PubMed] [Google Scholar]
- 5.Harrison, S. M., B. W. Barry, and P. H. Dugard. 1984. Effects of freezing on human skin permeability. J. Pharm. Pharmacol. 36:261-262. [DOI] [PubMed] [Google Scholar]
- 6.Hendley, J. O., and K. M. Ashe. 1991. Effect of topical antimicrobial treatment on aerobic bacteria in the stratum corneum of human skin. Antimicrob. Agents Chemother. 35:627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendley, J. O., and K. M. Ashe. 2003. Eradication of resident bacteria of normal human skin by antimicrobial ointment. Antimicrob. Agents Chemother. 47:1988-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hibbard, J. S., G. K. Mulberry, and A. R. Brady. 2002. A clinical study comparing the skin antisepsis and safety of ChloraPrep, 70% isopropyl alcohol, and 2% aqueous chlorhexidine. J. Infus. Nurs. 25:244-249. [DOI] [PubMed] [Google Scholar]
- 9.Karpanen, T. J., T. Worthington, B. R. Conway, A. C. Hilton, T. S. Elliott, and P. A. Lambert. 2008. Penetration of chlorhexidine into human skin. Antimicrob. Agents Chemother. 52:3633-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lafforgue, C., L. Carret, F. Falson, M. E. Reverdy, and J. Freney. 1997. Percutaneous absorption of a chlorhexidine digluconate solution. Int. J. Pharm. 147:243-246. [Google Scholar]
- 11.McDonnell, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Grady, N. P., M. Alexander, E. P. Dellinger, J. L. Gerberding, S. O. Heard, D. G. Maki, H. Masur, R. D. McCormick, L. A. Mermel, M. L. Pearson, I. I. Raad, A. Randolph, and R. A. Weinstein. 2002. Guidelines for the prevention of intravascular catheter-related infections. MMWR Recommend. Rep. 51:1-29. [PubMed] [Google Scholar]
- 13.Pratt, R. J., C. M. Pellowe, J. A. Wilson, H. P. Loveday, P. J. Harper, S. R. Jones, C. McDougall, and M. H. Wilcox. 2007. epic2: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J. Hosp. Infect. 65(Suppl. 1):S1-S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valles, J., I. Fernandez, D. Alcaraz, E. Chacon, A. Cazorla, M. Canals, D. Mariscal, D. Fontanals, and A. Moron. 2008. Prospective randomized trial of 3 antiseptic solutions for prevention of catheter colonization in an intensive care unit for adult patients. Infect. Control Hosp. Epidemiol. 29:847-853. [DOI] [PubMed] [Google Scholar]
- 15.Van der Merwe, D., and J. E. Riviere. 2005. Comparative studies on the effects of water, ethanol and water/ethanol mixtures on chemical partitioning into porcine stratum corneum and silastic membrane. Toxicol. In Vitro 19:69-77. [DOI] [PubMed] [Google Scholar]
- 16.Wang, J. C., R. R. Williams, L. Wang, and J. Loder. 1990. In vitro skin permeation and bioassay of chlorhexidine phosphanilate, a new antimicrobial agent. Pharm. Res. 7:995-1002. [DOI] [PubMed] [Google Scholar]