Abstract
Candida albicans frequently develops resistance to treatment with azole drugs due to the acquisition of gain-of-function mutations in the transcription factor Tac1p. Tac1p hyperactivation in azole-resistant isolates results in the constitutive overexpression of several genes, including CDR1 and CDR2, which encode two homologous transporters of the ATP-binding cassette family. Functional studies of Cdr1p and Cdr2p have been carried out so far by heterologous expression in the budding yeast Saccharomyces cerevisiae and by gene deletion or overexpression in azole-sensitive C. albicans strains in which CDR1 expression is low and CDR2 expression is undetectable. Thus, the direct demonstration that CDR1 and CDR2 overexpression causes azole resistance in clinical strains is still lacking, as is our knowledge of the relative contribution of each transporter to clinical azole resistance. In the present study, we used the SAT1 flipper system to delete the CDR1 and CDR2 genes from clinical isolate 5674. This strain is resistant to several azole derivatives due to a strong hyperactive mutation in Tac1p and expresses high levels of Cdr1p and Cdr2p. We found that deleting CDR1 had a major effect, reducing resistance to fluconazole (FLC), ketoconazole (KTC), and itraconazole (ITC) by 6-, 4-, and 8-fold, respectively. Deleting CDR2 had a much weaker effect, reducing FLC or KTC resistance by 1.5-fold, and had no effect on ITC resistance. These results demonstrate that Cdr1p is a major determinant of azole resistance in strain 5674 and potentially in other clinical strains overexpressing Cdr1p and Cdr2p, while Cdr2p plays a more minor role.
Candida albicans is one of the leading causes of fungal infections affecting immunocompromised individuals. Candida infections range from chronic superficial infections of the skin and mucosal surfaces to invasive, life-threatening systemic infections (21, 38). Many antifungal drugs used to treat Candida infections target the biosynthesis of ergosterol, the major sterol in the fungal cell membranes (24). Polyenes, such as amphotericin B (AMB), directly bind to ergosterol and form pores in the cell membrane, resulting in low selectivity and high toxicity (24). Azoles, a class of well-tolerated antifungal drugs that includes fluconazole (FLC), ketoconazole (KTC), itraconazole (ITC), and new-generation derivatives such as voriconazole and posaconazole, target the enzyme lanosterol 14α-demethylase (Erg11p), which is involved in ergosterol biosynthesis, blocking the production of ergosterol and causing the accumulation of toxic intermediate sterol species (24). As a consequence, the fluidity and permeability of the fungal cell membrane are changed and the activity of membrane-bound proteins, such as enzymes involved in cell wall synthesis, is altered (24).
However, the fungistatic rather than fungicidal action of azole drugs leads to the frequent emergence of azole-resistant (Ar) C. albicans strains (1, 44). One mechanism of azole resistance consists of increased levels of ERG11 RNA, resulting in increased production of the Erg11p enzyme, or point mutations in the ERG11 gene, producing an enzyme with a reduced binding affinity for azole drugs (1, 44). Also, several Ar clinical isolates overexpress the CDR1 and CDR2 genes, which encode two homologous transporters of the ATP-binding cassette (ABC) family, and/or the MDR1 gene, which encodes a major facilitator (1, 44). A number of Ar strains overexpress CDR1 and CDR2 but not MDR1, whereas other strains overexpress only MDR1 (34), suggesting the involvement of two distinct transcriptional pathways. Also, some Ar strains overexpress the three genes, probably due to the accumulation of independent mutations in the two pathways, leading to high levels of resistance in response to stepwise drug selection (44). The overexpression of transporter genes in Ar isolates suggested that a reduced accumulation of azoles in the cell was responsible for the observed azole resistance phenotype (1, 44). By using a dominant selectable marker, it was shown that deleting MDR1 from Ar clinical isolates overexpressing this gene reduced the resistance of the cells to FLC, providing a direct demonstration that MDR1 is involved in clinical FLC resistance (45). However, the direct contribution of CDR1 and CDR2 to clinical azole resistance remained to be determined.
Recent progress has been made in deciphering the regulatory circuitry that governs the regulation of CDR1, CDR2, MDR1, and ERG11 in C. albicans clinical strains. It was shown that the upregulation of the CDR1 and CDR2 genes in Ar isolates is due to gain-of-function mutations in the zinc cluster transcription factor Tac1p (5, 6). Most of these mutations consist of C-terminal amino acid substitutions or small in-frame deletions (4, 5). Tac1p was also shown to activate the transcription of CDR1 and CDR2 upon cell treatment with different compounds such as fluphenazine (FPZ) and steroids (estrogen, progesterone) (6), but the mechanisms by which these compounds trigger Tac1p activity are still unknown. Similarly, gain-of-function mutations in two other zinc cluster transcription factors, Mrr1p and Upc2p, have recently been shown to be responsible for the constitutive upregulation of Mdr1p and Erg11p, respectively, in clinical Ar isolates (10, 22). These data confirmed the involvement of different transcriptional pathways in the upregulation of the CDR1/CDR2, MDR1, and ERG11 genes in Ar isolates.
The Cdr1p and Cdr2p transporters show 84% amino acid sequence identity and are close homologs of Saccharomyces cerevisiae Pdr5p, a major effector of cell tolerance to xenobiotic compounds in budding yeast (2, 25, 32). These transporters are formed by two similar halves, each with an N-terminal hydrophilic domain that contains an ATP-binding motif followed by a C-terminal hydrophobic domain with six predicted transmembrane segments that presumably contain the drug binding sites, a structure characteristic of the pleiotropic drug resistance subfamily of ABC transporters found in fungi and plants (41, 43). Because of the advanced genetics of S. cerevisiae, most studies of Cdr1p and Cdr2p have been carried out with heterologous expression systems in S. cerevisiae, where Cdr1p and Cdr2p were expressed under the control of a strong promoter, leading to very high levels of azole resistance (∼100-fold) (12, 31). It was shown that the two transporters localize at the plasma membrane (35, 37), bind rhodamine 6G (R6G) (12), export their substrates in an energy-dependent manner, and possess ATPase and phospholipid translocase activities (15, 39). Expression systems in S. cerevisiae have also proved useful for structure-function studies of the transmembrane and ATP-binding domains of Cdr1p (14, 26, 31).
However, there is evidence supporting the importance of studying these transporters in a potentially more relevant host, namely, C. albicans Ar cells. First, the function of ABC transporters is influenced by their lipid environment (29, 40) and, since S. cerevisiae and C. albicans have different plasma membrane lipid compositions (17, 42), the function of Cdr1p and Cdr2p could be altered in S. cerevisiae. Also, the S. cerevisiae strains used for these studies carry deletions in (up to seven) genes which encode endogenous ABC transporters (12, 15). Since these ABC transporters can function as sterol transporters or phospholipid flippases (8, 27), the deletion of these genes may affect the plasma membrane composition of the recipient yeast and thus Cdr1p and Cdr2p function. On the other hand, Cdr1p and Cdr2p function has been studied by gene deletion, by using the URA-blaster system, in C. albicans azole-sensitive (As) strains, in which CDR1 expression is low and CDR2 expression is undetectable (32). Deletion of CDR1 resulted in increased azole susceptibility, whereas deletion of CDR2 did not render the cells more susceptible to azoles, due to the lack of CDR2 expression in these cells (32). Interestingly, the cdr1Δ/cdr1Δ cdr2Δ/cdr2Δ double mutant was found to be more susceptible to azole drugs and other toxic compounds than the cdr1Δ/cdr1Δ single mutant (32). Another study showed that the inducible expression of CDR1 in an As strain carrying a homozygous deletion of the gene resulted in resistance to several antifungal agents, including azole drugs (23). Also, genome-wide expression and location analyses of the Tac1p regulon have shown that, in Ar strains, CDR1 and CDR2 are coregulated with many other genes, some of them predicted to be involved in membrane protein trafficking and lipid metabolism and potentially modulating Cdr1p and Cdr2p function (16). Taken together, these observations emphasized the need for directly studying Cdr1p and Cdr2p in Ar C. albicans clinical isolates. In the present study, we addressed this question by deleting the two genes, individually and in combination, from a well-characterized Ar clinical isolate in which the Tac1p pathway is activated (30, 46).
MATERIALS AND METHODS
Strains and growth media.
The C. albicans strains used in this study are listed in Table 1; for details of their construction, see the supplemental material. Strains were routinely grown at 30°C in YPD medium containing 1% yeast extract (EMD Biosciences, Darmstadt, Germany), 2% Bacto peptone (BD Biosciences, Sparks, MD), and 2% glucose (Sigma, St. Louis, MO). For solid medium, 2% agar (Difco, BD) was added.
TABLE 1.
C. albicans strains used in this study
| Strain | Genotype or phenotype | Parent | Reference |
|---|---|---|---|
| Clinical isolates | |||
| SC5314 | As | 13 | |
| 5457 | As | 30 | |
| 5674 | Ar (CDR1 CDR2 overexpression) | 5457 | 30 |
| SZY31 | tac1Δ::FRT/tac1Δ::FRT | 5674 | 46 |
| 5674 cdr2 mutant derivatives | |||
| STY1 | cdr2AΔ::SAT1-FLP/CDR2B | 5674 | This study |
| STY2 | CDR2A/cdr2BΔ::SAT1-FLP | 5674 | This study |
| STY3 | cdr2AΔ::FRT/CDR2B | STY1 | This study |
| STY4 | CDR2A/cdr2BΔ::FRT | STY2 | This study |
| STY5 | cdr2AΔ::FRT/cdr2BΔ::SAT1-FLP | STY3 | This study |
| STY6 | cdr2AΔ::SAT1-FLP/cdr2BΔ::FRT | STY4 | This study |
| STY7 | cdr2AΔ::FRT/cdr2BΔ::FRT | STY5 | This study |
| STY8 | cdr2AΔ::FRT/cdr2BΔ::FRT | STY6 | This study |
| 5674 cdr1 mutant derivatives | |||
| STY13 | cdr1AΔ::SAT1-FLPa/CDR1B | 5674 | This study |
| STY14 | cdr1AΔ::SAT1-FLPa/CDR1B | 5674 | This study |
| STY37 | CDR1A/cdr1BΔ::SAT1-FLPb | 5674 | This study |
| STY38 | CDR1A/cdr1BΔ::SAT1-FLPb | 5674 | This study |
| STY15 | cdr1AΔ::FRT/CDR1B | STY13 | This study |
| STY16 | cdr1AΔ::FRT/CDR1B | STY14 | This study |
| STY41 | CDR1A/cdr1BΔ::FRT | STY37 | This study |
| STY42 | CDR1A/cdr1BΔ::FRT | STY38 | This study |
| STY17 | cdr1AΔ::FRT/cdr1BΔ::SAT1-FLPb | STY15 | This study |
| STY18 | cdr1AΔ::FRT/cdr1BΔ::SAT1-FLPb | STY16 | This study |
| STY19 | cdr1AΔ::FRT/cdr1BΔ::FRT | STY17 | This study |
| STY20 | cdr1AΔ::FRT/cdr1BΔ::FRT | STY18 | This study |
| 5674 cdr1, cdr2 mutant derivatives | |||
| STY25 | cdr2AΔ::FRT/cdr2BΔ::FRT cdr1AΔ::SAT1-FLPa/CDR1B | STY7 | This study |
| STY26 | cdr2AΔ::FRT/cdr2BΔ::FRT cdr1AΔ::SAT1-FLPa/CDR1B | STY7 | This study |
| STY27 | cdr2AΔ::FRT/cdr2BΔ::FRT cdr1AΔ::FRT/CDR1B | STY25 | This study |
| STY28 | cdr2AΔ::FRT/cdr2BΔ::FRT cdr1AΔ::FRT/CDR1B | STY26 | This study |
| STY29 | cdr2AΔ::FRT/cdr2BΔ::FRT cdr1AΔ::FRT/cdr1BΔ::SAT1-FLPb | STY27 | This study |
| STY30 | cdr2AΔ::FRT/cdr2BΔ::FRT cdr1AΔ::FRT/cdr1BΔ::SAT1-FLPb | STY28 | This study |
| STY31 | cdr2AΔ::FRT/cdr2BΔ::FRT cdr1AΔ::FRT/cdr1BΔ::FRT | STY29 | This study |
| STY32 | cdr2AΔ::FRT/cdr2BΔ::FRT cdr1AΔ::FRT/cdr1BΔ::FRT | STY30 | This study |
| STY45 | CDR2A-SAT1-FLP/cdr2BΔ::FRT cdr1AΔ::FRT/cdr1BΔ::FRT | STY31 | This study |
| STY47 | CDR2A-FRT/cdr2BΔ::FRT cdr1AΔ::FRT/cdr1BΔ::FRT | STY45 | This study |
Disruption cassette derived from pCDR1koA.
Disruption cassette derived from pCDR1koB.
Reagents and antifungal compounds.
Molecular biology reagents and restriction enzymes were purchased from Invitrogen (Carlsbad, CA) or from New England BioLabs (Ipswich, MA). Hot-Start KOD+ DNA polymerase (Novagen, La Jolla, CA) was used for the amplification and cloning of PCR products. DNA primers were purchased from Integrated DNA Technologies (San Diego, CA). PCR and plasmid DNA fragments were purified with DNA purification kits from Qiagen (Mississauga, Ontario, Canada). Acid-washed glass beads (425 to 600 nm) used for genomic DNA, and total protein extracts were purchased from Sigma. All chemical and antifungal compounds were purchased from Sigma unless otherwise stated.
RNA preparation and Northern blotting.
Cell growth, total RNA extraction by the hot phenol method, and Northern blot hybridizations were carried out as described previously (30). The CDR1 and CDR2 probes used in this experiment were also described previously (30). The resulting blots were exposed to a Fujifilm Imagine Plate screen and analyzed with the MultiGauge software (version 2.3; Fujifilm).
Membrane protein preparation and Western blotting.
Total membrane extracts from C. albicans cells were prepared as follows. Overnight cultures were diluted into 100 ml of fresh YPD medium to an optical density at 600 nm (OD600) of 0.1. Logarithmically growing cells (OD600 of 1.0) were harvested, washed once with ice-cold distilled H2O, and resuspended in 5 ml of extraction buffer (10 mM Tris-HCl [pH 7.5]; 400 mM NaCl; 10% glycerol; 1 mM sodium orthovanadate; 50 mM sodium fluoride; 50 mM sodium β-glycerophosphate; 10 mM β-mercaptoethanol; 1 μM MG132 and protease inhibitors; 1 mM phenylmethylsulfonyl fluoride; leupeptin, pepstatin, and aprotinin at 5 μg/ml each). Cell suspensions were then frozen in liquid nitrogen and stored at −80°C. Frozen cell pellets were disrupted with a Freezer Mill (SPEX CetriPrep, Metuchen, NJ) with four cycles of successive grinding and cooling periods, each cycle consists of 2 min of grinding at an impact frequency of 14 times/s followed by a 2-min cooling interval. Total protein extracts were cleared by centrifugation at 1,000 × g for 5 min at 4°C. Total membrane extracts were harvested by centrifuging the total protein extracts at 260,000 × g for 45 min at 4°C. Protein concentrations were determined with the micro-BCA protein assay kit from Pierce (Rockford, IL), and total protein extracts (20 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (7.5% acrylamide, 37.5:1 acrylamide/bisacrylamide ratio). The gels were either stained with Coomassie or transferred to a nitrocellulose membrane with a Trans Blot SD Semi-Dry transfer apparatus (Bio-Rad). The membrane was stained with Ponceau reagent (0.1% Ponceau S in 5% acetic acid) prior to immunodetection. Immunodetection of Cdr1p and Cdr2p was performed with anti-Cdrp (1:1,000 dilution), anti-Cdr2p (1:4,000 dilution) (12), or anti-Cdr1p (1: 4,000 dilution) (9) polyclonal antibodies and an ECL chemiluminescence kit (SuperSignal chemiluminescent substrate; Pierce).
Drug susceptibility testing.
Liquid microtiter plate assays were performed as described previously (46). The drug concentrations tested were 0.2, 0.4, 1.5, 3.1, 6.2, 12.5, 25, 50, 100, 150, and 200 μg/ml for FLC; 0.001, 0.002, 0.005, 0.009, 0.019, 0.038, 0.075, 0.15, 0.2, 0.3, 0.4, 0.6, and 1.2 μg/ml for KTC; 0.0004, 0.0008, 0.0016, 0.0031, 0.0063, 0.0125, 0.025, 0.05, 0.1, 0.2, and 0.4 μg/ml for ITC; 0.8, 1.6, 3.1, 6.3, 12.5, 19, 25, 38, 50, 75, and 100 μg/ml for FPZ; 0.032, 0.063, 0.125, 0.25, 1, 2, 4, 8, and 16 μg/ml for R6G; and 0.032, 0.063, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, and 32 μg/ml for AMB. Stock solutions of FLC, FPZ, and R6G were prepared in water at concentrations of 5, 10, and 128 mg/ml, respectively; stock solutions of KTC, ITC, and AMB were prepared in dimethyl sulfoxide at concentrations of 3, 1, and 10 mg/ml, respectively. Cell growth was measured spectrophotometrically by determining the OD620 after 48 h of incubation at 30°C in YPD. The MICs for 50% of the strains tested (MIC50s) were determined as the first concentration of drug capable of reducing growth by 50% compared to that of control cells grown in the absence of the drug. The data are presented as the mean of three independent experiments performed in duplicate. Spot assays were performed as described previously (30). Cells grown overnight were resuspended in YPD to an OD600 of 0.1. Tenfold serial dilutions of each strain were spotted onto YPD plates containing the drugs tested.
RESULTS
Deletion of the CDR1 and CDR2 genes in C. albicans Ar clinical isolate 5674.
To determine the contribution of CDR1 and CDR2 to clinical azole resistance, we used the SAT1 flipper strategy, which allows the dominant selection of transformants with the SAT1 gene, which confers nourseothricin resistance (28), to delete the CDR1 and CDR2 genes, independently or in combination, from the Ar clinical isolate 5674. This strain and As strain 5457 were isolated from the same patient and shown by DNA fingerprinting analysis to be highly related (30). Compared to strain 5457, strain 5674 is resistant to several azole derivatives and overexpresses the CDR1 and CDR2 RNAs at high levels due to a strong gain-of-function mutation in transcription factor Tac1p (N972D) (30, 46). Tac1p appears to be the key determinant of azole resistance in strain 5674, since deletion of the TAC1 gene from that strain causes a complete loss of azole resistance, the resulting tac1Δ/tac1Δ mutant being as susceptible to azole drugs as strain 5457 (46). This proposition is also supported by the demonstration that strain 5674 does not overexpress the MDR1 or ERG11 gene (30), suggesting that no other transcriptional pathways are activated in this strain besides the Tac1p pathway, and does not carry mutations in ERG11 (K. S. Barker and P. D. Rogers, personal communication). Finally, strain 5674, as well as other Ar strains carrying an activated Tac1p protein, overexpresses many genes that encode proteins with predicted functions in lipid metabolism, phospholipid translocation, and protein trafficking which could possibly modulate Cdr1p and Cdr2p activity (16). Therefore, the use of strain 5674 to delete CDR1 and CDR2 allows the analysis of Cdr1p and Cdr2p function in a clinically relevant, well-characterized host.
We constructed a CDR2 deletion cassette consisting of the SAT1-FLP marker flanked by approximately 1 kb of CDR2 upstream and downstream sequences amplified from strain SC5314 (see Fig. S1A in the supplemental material). The correct integration of the deletion cassette at the CDR2 locus was verified by Southern blotting (see Fig. S1C in the supplemental material). Two independent heterozygous mutants with the expected integration profile were chosen to delete the second allele, yielding two independent homozygous cdr2Δ/cdr2Δ mutants, STY7 and STY8 (see Fig. S1C in the supplemental material). Deletion of the CDR1 gene from strain 5674 was performed essentially as described for CDR2, with the exception that two different CDR1 deletion constructs were used to delete the two alleles, generating the two independent homozygous cdr1Δ/cdr1Δ mutants STY19 and STY20 (see Fig. S2 in the supplemental material). CDR1 was also deleted from strain STY7, producing two mutant strains lacking both transporter genes, STY31 and STY32 (cdr1Δ/cdr1Δ cdr2Δ/cdr2Δ) (see Fig. S2 in the supplemental material). Finally, we constructed strain STY47, in which one allele of CDR2 was reintegrated at its original locus in strain STY31 (see Fig. S3 in the supplemental material).
Cdr1p and Cdr2p expression in strains 5457 and 5674 and strain 5674 mutant derivatives.
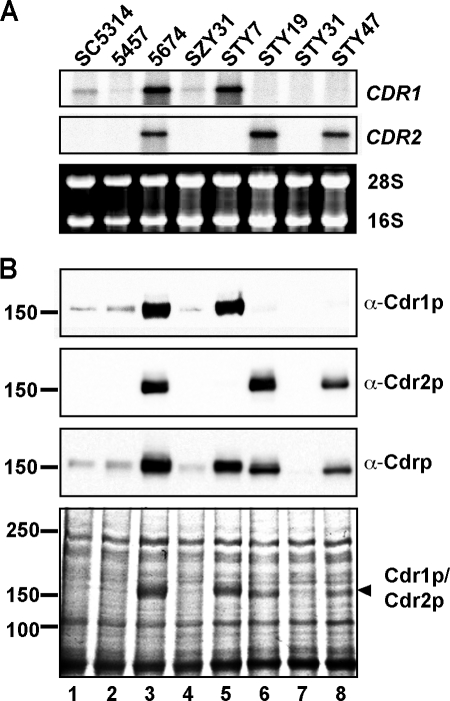
We analyzed the expression of the CDR1 and CDR2 genes in strains SC5314, 5457, and 5674 and different 5674 mutant derivatives by Northern and Western blotting.
For the Northern blot analysis, total RNA was prepared and the mRNA levels of CDR1 and CDR2 were detected with gene-specific probes derived from the first 340 and 294 bp of the CDR1 or CDR2 open reading frame, respectively, a region where the level of sequence homology between the two genes is low (30). Low levels of CDR1 transcript were detected in As strains SC5314 and 5457, and high levels were detected in Ar strain 5674 (Fig. 1A, top, lanes 1 to 3), whereas CDR2 transcripts were detected only in the Ar strain (Fig. 1A, middle, lanes 1 to 3). As previously reported, deleting the TAC1 gene from strain 5674 (SZY31) decreased CDR1 and CDR2 expression to levels similar to those detected in the As strains (Fig. 1A, lane 4) (46). In strain STY7 (cdr2Δ/cdr2Δ), we detected a signal for CDR1 but not for CDR2, confirming the deletion of CDR2 in that strain (Fig. 1A, lane 5). Similarly, the absence of a signal for CDR1 in strain STY19 (cdr1Δ/cdr1Δ) was consistent with the deletion of CDR1 in that strain (Fig. 1A, lane 6). Furthermore, we did not detect any signal for CDR1 or CDR2 in strain STY31 (cdr1Δ/cdr1Δ cdr2Δ/cdrΔ), in line with the deletion of the two genes from that strain (Fig. 1A, lane 7). The CDR1 and CDR2 RNA levels detected in strains STY7 and STY19, respectively, were comparable to those detected for each gene in strain 5674, indicating that the expression of one gene remained unaffected by the deletion of the other one, ruling out the presence of a potential compensatory overexpression effect. Finally, detection of a full-length CDR2 transcript confirmed the expression of CDR2 in CDR2-complemented strain STY47 (Fig. 1A, lane 8).
FIG. 1.
Expression of CDR1 and CDR2 in strains SC5314, 5457, and 5674 and in 5674 mutant derivatives. (A) Northern blot analysis. Total RNA extracts were prepared from the strains indicated at the top and analyzed by Northern blotting with gene-specific probes for CDR1 (top) or CDR2 (middle). rRNAs are shown as loading controls (bottom). (B) Western blot analysis. For the immunodetection of Cdr1p and Cdr2p, total membrane protein extracts were prepared from the strains and analyzed by Western blotting with the anti-Cdr1p, anti-Cdr2p, and anti-Cdrp antibodies. A Coomassie-stained gel of the protein extracts is shown at the bottom, with the positions of the molecular mass standards and the predicted positions of Cdr1p and Cdr2p indicated on the left and right, respectively. The values on the left are molecular sizes in kilodaltons.
Immunodetection of Cdr1p and Cdr2p was performed with three different polyclonal antibodies, two antibodies specific for Cdr1p or Cdr2p that were raised against the NH2-terminal domain of the Cdr1p (anti-Cdr1p) or Cdr2p (anti-Cdr2p) protein, respectively, and one antibody that was raised against a short peptide sequence perfectly conserved between the two proteins and that recognizes both transporters (anti-Cdrp) (9, 12). By using the anti-Cdr1p antibody, we found that the pattern of Cdr1p expression was consistent with the one observed by Northern blotting, namely, low levels in strains SC5314, 5457, and SZY31 and high levels in strain 5674 (Fig. 1B, lanes 1 to 4). The Cdr1p levels in strain STY7 were similar to those in strain 5674, confirming that Cdr1p expression was unaffected by the deletion of CDR2 (Fig. 1B, lanes 3 and 5). Finally, Cdr1p was undetectable in strains STY19, STY31, and STY47, consistent with the deletion of the CDR1 gene from these strains (Fig. 1B, lanes 6 to 8). Western blotting with the anti-Cdr2p antibody also showed that Cdr2p expression in these strains was consistent with the Northern blot results. Cdr2p levels were similar in strains 5674 and STY19, indicating that Cdr2p expression remained unchanged by the deletion of CDR1 (Fig. 1B, lanes 3 and 6). Cdr2p expression was lower in strain STY47 than in strain 5674 or STY19, consistent with the presence of only one allele of CDR2 in STY47 versus two alleles in 5674 and STY19 (Fig. 1B, lane 8). Finally, Western blotting with the generic anti-Cdrp antibody confirmed the results obtained with the anti-Cdr1p and anti-Cdr2p specific antibodies. The absence of a signal in strain STY31 demonstrated that the anti-Cdrp antibody, which was previously characterized with S. cerevisiae strains expressing Cdr1p or Cdr2p (12), does not recognize any other proteins in C. albicans besides Cdr1p and Cdr2p and is therefore specific for these two transporters. The results obtained with the generic antibody also showed that Cdr2p appears to be expressed at slightly (approximately twofold) lower levels than Cdr1p, an observation corroborated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie staining (Fig. 1B, compare lanes 5 and 6). In addition, the Western blot assay with the generic antibody and the Coomassie gel showed that Cdr2p migrates slightly faster than Cdr1p (Fig. 1B, compare lanes 5 and 6). Since the two transporters have very similar predicted lengths (1,501 residues for Cdr1p and 1,499 for Cdr2p), this difference in migration may potentially reflect distinct posttranslational modifications.
Susceptibilities of the cdr mutants to azole drugs and other antifungal agents.
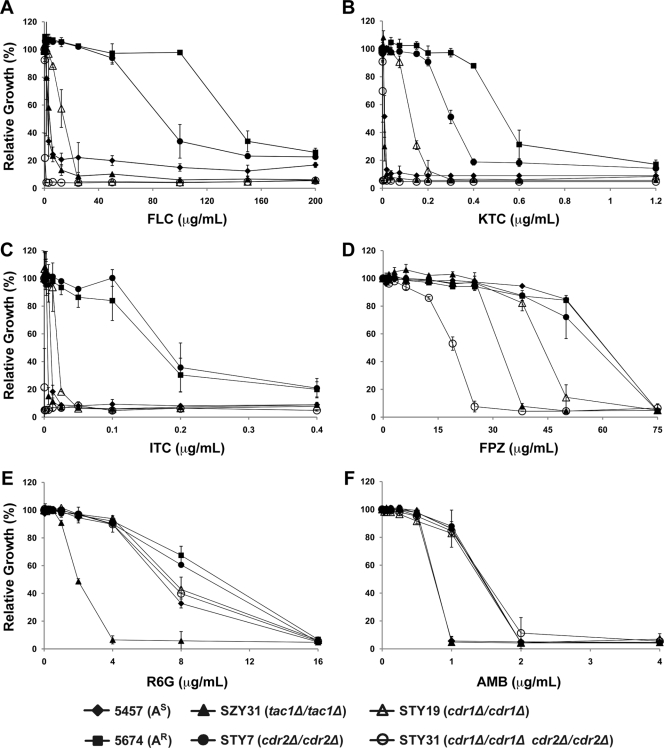
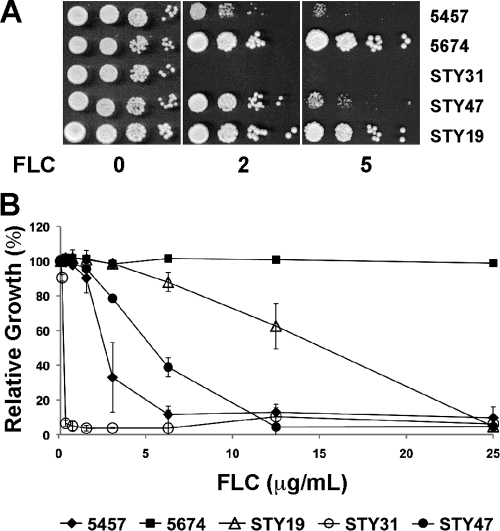
We used MIC assays to determine the functional consequences of deleting CDR1 and/or CDR2 on the azole resistance phenotype of strain 5674 (Fig. 2). All of the experiments were performed with two independently generated mutant strains per knockout construction (Table 1) and yielded similar results; thus, only one set of strains is shown in Fig. 2 for clarity. As expected, strain 5674 was more resistant to the three azoles tested than was strain 5457, whereas deleting TAC1 from strain 5674 (SZY31) decreased the resistance of the cells to levels similar to those observed in strain 5457 (Fig. 2A, B, and C). Deleting CDR1 from strain 5674 had a major effect, reducing resistance to FLC, KTC, and ITC by 6-, 4-, and 8-fold, respectively, while deleting CDR2 had a smaller effect (1.5-, 1.5-, and 1.0-fold) (Fig. 2A, B, and C; Table 2). These results demonstrate that Cdr1p is the major determinant of azole resistance in strain 5674 while Cdr2p plays a more minor role, even when normalized for the slightly lower abundance of Cdr2p than of Cdr1p (Fig. 1B). Interestingly, the deletion of both genes had a drastic effect and caused a reduction of the resistance to FLC, KTC, and ITC by 375-, 300-, and 500-fold, respectively, highlighting a strong synergism between the two transporters (Table 2). Reintroducing one allele of CDR2 under the control of its own promoter at its original locus in strain STY31 (yielding strain STY47) partially restored FLC resistance, causing a 16-fold increase in FLC MIC50s (6.3 μg/ml for strain STY47 versus 0.4 μg/ml for strain STY31; Fig. 3). This experiment confirmed that the strong azole hypersusceptibility of strain STY31 was due to the simultaneous inactivation of the Cdr1p and Cdr2p functions (Fig. 3). The level of FLC resistance of strain STY47 was lower than that of strain STY19, consistent with the lower levels of Cdr2p expression in that strain versus STY19 (Fig. 1).
FIG. 2.
Profiles of resistance of strains 5457 and 5674 and strain 5674-derived tac1 and cdr mutants to azole drugs and different compounds with antifungal activity, as determined by microtiter plate liquid assays. (A) FLC resistance. Cells were incubated for 48 h at 30°C in liquid YPD medium with the indicated concentrations of FLC. The data are presented as the relative growth of cells in FLC-containing medium compared to the growth of the same strain in FLC-free medium, which was set at 100%. The data are the mean of three independent experiments performed in duplicate. (B) KTC resistance. (C) ITC resistance. (D) FPZ resistance. (E) R6G resistance. (F) AMB resistance. For panels B to F, the experiments were performed as described for panel A.
TABLE 2.
Drug susceptibilities of the strains used in this study
| Strain | MIC50 (μg/ml)a
|
|||||
|---|---|---|---|---|---|---|
| FLC | KTC | ITC | FPZ | R6G | AMB | |
| 5457 (As) | 3.1 | 0.018 | 0.0125 | 75 | 8 | 1 |
| 5674 (Ar) | 150 | 0.6 | 0.2 | 75 | 16 | 2 |
| SZY31 (tac1Δ/tac1Δ) | 6.2 | 0.009 | 0.0063 | 38 | 2 | 1 |
| STY7 (cdr2Δ/cdr2Δ) | 100 | 0.4 | 0.2 | 75 | 16 | 2 |
| STY19 (cdr1Δ/cdr1Δ) | 25 | 0.15 | 0.025 | 50 | 8 | 2 |
| STY31 (cdr1Δ/cdr1Δ cdr2Δ/cdr2Δ) | 0.4 | 0.002 | 0.0004 | 25 | 8 | 2 |
Values presented are MIC50s after 48 h of incubation (derived from Fig. 2).
FIG. 3.
Phenotypic analysis of the CDR2 revertant. The FLC susceptibilities of strains 5457, 5674, STY19, STY31, and STY47 were analyzed by spot (A) and MIC (B) assays as described in the legends to Fig. 2 and 4.
We also tested FPZ, a calmodulin inhibitor with antifungal properties previously shown to be a substrate of Cdr1p and Cdr2p and a strong inducer of Tac1p activity (6, 32, 46). We found that strain 5674 was not more resistant to FPZ than was strain 5457 (Fig. 2D and Table 2), possibly because this compound can directly induce Tac1p function. In line with this, we found that strain SZY31 (tac1Δ/tac1Δ) was twofold more susceptible to FPZ than was strain 5674. Deleting CDR1 or CDR2 from strain 5674 had only a marginal or no effect on the resistance of the cells to FPZ (1.5- and 1-fold, respectively). When comparing strains STY7 (cdr2Δ/cdr2Δ; Cdr1p-expressing strain) and STY19 (cdr1Δ/cdr1Δ; Cdr2p-expressing strain) to strain STY31 (cdr1Δ/cdr1Δ cdr2Δ/cdr2Δ; no Cdr1p and Cdr2p), we found that the presence of CDR1 or CDR2 contributed to three- and twofold increased FPZ resistance, respectively. Finally, the decrease in FPZ susceptibility was about 1.5-fold between strains 5674 and STY19 (due to the deletion of CDR1) and 2-fold between strains STY19 and STY31 (due to the deletion of CDR2). Based upon these small differences, it can be concluded that Cdr1p and Cdr2p display similarly low activities toward FPZ. These results also suggest that FPZ is a poorer substrate for Cdr1p and Cdr2p than are azole drugs and/or that it possesses a narrower window of antifungal activity.
We similarly investigated R6G, a fluorescent substrate of many ABC transporters that has been used to study the functions of Cdr1p and Cdr2p in S. cerevisiae (12, 15, 31) and C. albicans (19, 20). Our results showed that strain 5674 was only twofold more resistant to R6G than was strain 5457 (Fig. 2E and Table 2). Deleting CDR1 from strain 5674 reduced the resistance of the cells to R6G by twofold, to levels similar to those observed in strain 5457, while deletion of CDR2 clearly had no effect. These results suggest that R6G is not a good substrate to study Cdr1p and Cdr2p in Ar C. albicans cells. Interestingly, we found that the tac1Δ/tac1Δ mutant is eightfold more susceptible than strain 5674, indicating that Tac1p possesses another, as-yet-unidentified, target affecting cell susceptibility to R6G.
Finally, we also tested AMB, which targets ergosterol. We found that all of the strains had the same profile, with the exception of 5457 and the tac1Δ/tac1Δ mutant, which were slightly hypersusceptible (Fig. 2F and Table 2). These results demonstrate that Tac1p plays a minor role in regulating cell tolerance to AMB but through a target other than Cdr1p and Cdr2p.
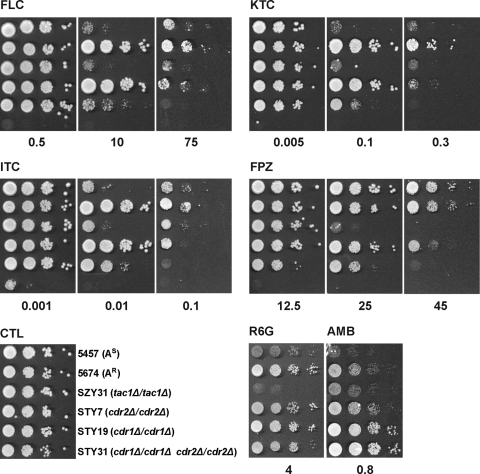
To assess the phenotypic consequences of deleting the CDR genes by an independent method, the strains were also analyzed by spot assay (Fig. 4). The results obtained with this method were consistent with those obtained with the MIC assays, confirming the results described above and indicating that the observed phenotypes are similar on solid and liquid media.
FIG. 4.
Profiles of resistance of strains 5457 and 5674 and strain 5674-derived tac1 and cdr mutants to azole drugs and different compounds with antifungal activity, as determined by spot assays. Serial 10-fold dilutions of cells, starting at an OD600 of 0.1, were spotted onto YPD plates containing FLC, KTC, ITC, FPZ, R6G, or AMB at the indicated concentrations (micrograms per milliliter) or no drug (CTL). The plates were incubated for 48 h at 30°C.
DISCUSSION
Deletion of the CDR1 and CDR2 genes from Ar clinical isolate 5674 with the SAT1 flipper system, coupled to the characterization of the resulting mutants by quantitative MIC assays, allowed us to directly determine the relative contributions of the Cdr1p and Cdr2p transporters to clinical azole resistance. We show that deleting CDR1 reduced resistance levels 4- to 8-fold, depending on the different azoles tested (FLC, KTC, and ITC), while deleting CDR2 only slightly affected FLC and KTC resistance (by 1.5-fold) and did not affect ITC resistance. Thus, Cdr1p plays a major role in FLC, KTC, and ITC resistance whereas Cdr2p plays a more minor role. It is possible that removing Cdr1p or Cdr2p affects azole resistance directly, by diminishing active azole export, and/or indirectly, by altering the activity of other proteins able to modulate intracellular azole accumulation (including Cdr1p or Cdr2p) or the phospholipid composition of the membrane. Assuming that transporter-mediated resistance to an antifungal compound reflects the ability of the transporter to export this compound, as previously proposed (11, 15, 33), our data could be interpreted as meaning that Cdr1p is a better transporter of azoles than is Cdr2p, although it cannot be ruled out that the effect of CDR2 deletion might be masked by Cdr1p activity or by other, as-yet-unknown, compensatory mechanisms.
Our results show that deleting CDR2 from the cdr1Δ/cdr1Δ mutant has a much stronger effect than deleting the gene from strain 5674. Since Cdr1p and Cdr2p have some substrates in common, this could potentially be due to a masking effect of the activity of Cdr2p by Cdr1p, as previously proposed in a similar study of ABC transporters in S. cerevisiae (8). In that study, deleting PDR5 from a wild-type S. cerevisiae strain did not affect oligomycin susceptibility whereas deleting PDR5 from a yor1Δ mutant strain (carrying a deletion of the YOR1 gene, which encodes another ABC transporter) led to pronounced oligomycin sensitivity, stronger than that observed in the yor1Δ mutant strain (8). Similarly, in the case of S. cerevisiae PDR16 and PDR17, which encode two homologous phospholipid transfer proteins, deleting PDR16 from a wild-type strain resulted in increased susceptibility of the cells to KTC and miconazole by 10- and 20-fold, respectively, and deleting PDR17 had no effect, whereas deleting both genes from the same strain led to 40- and 80-fold increased susceptibility (42). In the present study, however, the double deletion of CDR1 and CDR2 was performed in an already resistant strain. We found that deleting both genes not only abolished azole resistance but further reduced it to levels lower than those observed in As strain 5457 and the tac1Δ/tac1Δ mutant (Fig. 2A to C). This could be potentially due, at least in part, to the Tac1p-independent low level of Cdr1p expression in these strains (Fig. 1), which has been eliminated from strain STY31 as a result of the CDR1 deletion. In fact, the deletion of both genes had a striking effect as it reduced azole resistance by 300- to 500-fold (by comparison to 4- to 8-fold for cdr1Δ/cdr1Δ and 1.0- to 1.5-fold for cdr2Δ/cdr2Δ), uncovering a strong synergism between the two transporters. Synergism between transporters has previously been reported for bacterial multidrug resistance pumps (18) and also members of the P-type ATPase and ABC transporter families involved in regulating the phospholipid composition of the membrane of erythrocytes in mammals (7). Cdr1p and Cdr2p have been shown to function as phospholipid floppases, being able to translocate fluorescent phospholipids across the membrane lipid bilayer in an ATP-dependent fashion (36, 39), and it is possible that the abrogation of Cdr1p and Cdr2p activity may cause a major alteration of the asymmetrical distribution of phospholipids in the cell membrane which would exacerbate the cell response to drugs. Since it was not observed with AMB (Fig. 2F and 4), which directly targets ergosterol, this synergism appears to be specific for azoles and possibly involves the accumulation in the already perturbed plasma membrane of toxic ergosterol intermediates as a result of Erg11p inhibition.
R6G is a heterocyclic, lipophilic, and cationic fluorescent compound that is a substrate for many ABC transporters. Because of its antifungal properties and the ease of its detection, R6G has been used extensively to study yeast ABC transporters for their ability to confer R6G resistance and transport, these two parameters being closely correlated (11, 15). In particular, it was shown that expression of Pdr5p, Cdr1p, and Cdr2p in S. cerevisiae confers high levels of R6G resistance and efflux (11, 15, 31; our unpublished observation). In one of these studies, R6G resistance was quantified by MIC assay and shown to be more than 1,000-fold greater than that of the negative control strain (31). Based on these findings, it was expected that deletion of the CDR1 and CDR2 genes from strain 5674 would affect cell susceptibility to R6G. Instead, we found that CDR1 and CDR2 deletion only marginally reduced R6G resistance in strain 5674 (Fig. 2E). A similar situation has been observed in other Ar strains, TIMM3165 and GU5, in which elevated levels of the CDR1 and CDR2 RNAs were accompanied by a 128-fold increase in resistance to FLC but only by a 4-fold increase in resistance to R6G, compared to As strains (3, 19). Interestingly, we found that deletion of TAC1 from strain 5674 significantly decreased the resistance of the cells to R6G (by eightfold), uncovering the existence of another, as-yet-unidentified, Tac1p target that modulates R6G resistance. Our recent analysis of the Tac1p regulon identified a gene, orf19.4531, whose protein product may have this function. This gene encodes a putative ABC transporter of the pleiotropic drug resistance subfamily homologous to Cdr1p and Cdr2p (16). orf19.4531 was found to be upregulated together with CDR1 and CDR2 in a TAC1-dependent manner in four independent Ar clinical strains, including strain 5674 (16). Alternatively, the decreased resistance of the cells to R6G may be due to another Tac1p target or to a combination of other Tac1p targets, since many of these genes are predicted to regulate the lipid or phospholipid composition of the plasma membrane in C. albicans. We are currently investigating the role of these genes in azole and R6G resistance by deleting them from strains 5674 and STY31. This approach may allow us to identify new genes that affect drug resistance either directly or indirectly by regulating Cdr1p or Cdr2p function.
Supplementary Material
Acknowledgments
We thank Sadri Znaidi for critical reading of the manuscript, Sébastien Lemieux for advice, Joachim Morschhäuser for the gift of pSFS2A, Dominique Sanglard for providing the anti-Cdr1p antibody, the Institute for Research in Immunology and Cancer Genomic Platform for DNA sequencing, and the Candida Genome Database for sequence information.
This work was supported by a research grant to M.R. from the Canadian Institutes of Health Research (MT-15679). The Institute for Research in Immunology and Cancer is supported by the Canadian Center of Excellence in Commercialization and Research, the Canadian Foundation for Innovation, and the Fonds de Recherche en Santé du Québec.
Footnotes
Published ahead of print on 17 February 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Akins, R. A. 2005. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med. Mycol. 43:285-318. [DOI] [PubMed] [Google Scholar]
- 2.Balzi, E., M. Wang, S. Leterme, D. L. Van, and A. Goffeau. 1994. PDR5, a novel yeast multidrug resistance conferring transporter controlled by the transcription regulator PDR1. J. Biol. Chem. 269:2206-2214. [PubMed] [Google Scholar]
- 3.Banerjee, D., N. Martin, S. Nandi, S. Shukla, A. Dominguez, G. Mukhopadhyay, and R. Prasad. 2007. A genome-wide steroid response study of the major human fungal pathogen Candida albicans. Mycopathologia 164:1-17. [DOI] [PubMed] [Google Scholar]
- 4.Coste, A., A. Selmecki, A. Forche, D. Diogo, M. E. Bougnoux, C. d'Enfert, J. Berman, and D. Sanglard. 2007. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot. Cell 6:1889-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coste, A., V. Turner, F. Ischer, J. Morschhäuser, A. Forche, A. Selmecki, J. Berman, J. Bille, and D. Sanglard. 2006. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics 172:2139-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coste, A. T., M. Karababa, F. Ischer, J. Bille, and D. Sanglard. 2004. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot. Cell 3:1639-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daleke, D. L. 2008. Regulation of phospholipid asymmetry in the erythrocyte membrane. Curr. Opin. Hematol. 15:191-195. [DOI] [PubMed] [Google Scholar]
- 8.Decottignies, A., A. M. Grant, J. W. Nichols, H. de Wet, D. B. McIntosh, and A. Goffeau. 1998. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J. Biol. Chem. 273:12612-12622. [DOI] [PubMed] [Google Scholar]
- 9.de Micheli, M., J. Bille, C. Schueller, and D. Sanglard. 2002. A common drug-responsive element mediates the upregulation of the Candida albicans ABC transporters CDR1 and CDR2, two genes involved in antifungal drug resistance. Mol. Microbiol. 43:1197-1214. [DOI] [PubMed] [Google Scholar]
- 10.Dunkel, N., T. T. Liu, K. S. Barker, R. Homayouni, J. Morschhäuser, and P. D. Rogers. 2008. A gain-of-function mutation in the transcription factor Upc2p causes upregulation of ergosterol biosynthesis genes and increased fluconazole resistance in a clinical Candida albicans isolate. Eukaryot. Cell 7:1180-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst, R., P. Kueppers, C. M. Klein, T. Schwarzmueller, K. Kuchler, and L. Schmitt. 2008. A mutation of the H-loop selectively affects rhodamine transport by the yeast multidrug ABC transporter Pdr5. Proc. Natl. Acad. Sci. USA 105:5069-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauthier, C., S. Weber, A. M. Alarco, O. Alqawi, R. Daoud, E. Georges, and M. Raymond. 2003. Functional similarities and differences between Candida albicans Cdr1p and Cdr2p transporters. Antimicrob. Agents Chemother. 47:1543-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillum, A. M., E. Y. Tsay, and D. R. Kirsch. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179-182. [DOI] [PubMed] [Google Scholar]
- 14.Jha, S., N. Dabas, N. Karnani, P. Saini, and R. Prasad. 2004. ABC multidrug transporter Cdr1p of Candida albicans has divergent nucleotide-binding domains which display functional asymmetry. FEMS Yeast Res. 5:63-72. [DOI] [PubMed] [Google Scholar]
- 15.Lamping, E., B. C. Monk, K. Niimi, A. R. Holmes, S. Tsao, K. Tanabe, M. Niimi, Y. Uehara, and R. D. Cannon. 2007. Characterization of three classes of membrane proteins involved in fungal azole resistance by functional hyperexpression in Saccharomyces cerevisiae. Eukaryot. Cell 6:1150-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, T. T., S. Znaidi, K. S. Barker, L. Xu, R. Homayouni, S. Saidane, J. Morschhäuser, A. Nantel, M. Raymond, and P. D. Rogers. 2007. Genome-wide expression and location analyses of the Candida albicans Tac1p regulon. Eukaryot. Cell 6:2122-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Löffler, J., H. Einsele, H. Hebart, U. Schumacher, C. Hrastnik, and G. Daum. 2000. Phospholipid and sterol analysis of plasma membranes of azole-resistant Candida albicans strains. FEMS Microbiol. Lett. 185:59-63. [DOI] [PubMed] [Google Scholar]
- 18.Lomovskaya, O. 2002. Interactions among multiple efflux pumps: additive and synergistic effects on antimicrobial resistance, abstr. 1188. In Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 19.Maebashi, K., M. Niimi, M. Kudoh, F. J. Fischer, K. Makimura, K. Niimi, R. J. Piper, K. Uchida, M. Arisawa, R. D. Cannon, and H. Yamaguchi. 2001. Mechanisms of fluconazole resistance in Candida albicans isolates from Japanese AIDS patients. J. Antimicrob. Chemother. 47:527-536. [DOI] [PubMed] [Google Scholar]
- 20.Maesaki, S., P. Marichal, H. Vanden Bossche, D. Sanglard, and S. Kohno. 1999. Rhodamine 6G efflux for the detection of CDR1-overexpressing azole-resistant Candida albicans strains. J. Antimicrob. Chemother. 44:27-31. [DOI] [PubMed] [Google Scholar]
- 21.Mathews, H. L., and L. Witek-Jaunsek. 2002. Host defense against oral, esophageal, and gastrointestinal candidiasis, p. 179-192. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, DC.
- 22.Morschhaäuser, J., K. S. Barker, T. T. Liu, J. Blaβ-Warmuth, R. Homayouni, and P. D. Rogers. 2007. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog. 3:1603-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niimi, M., K. Niimi, Y. Takano, A. R. Holmes, F. J. Fischer, Y. Uehara, and R. D. Cannon. 2004. Regulated overexpression of CDR1 in Candida albicans confers multidrug resistance. J. Antimicrob. Chemother. 54:999-1006. [DOI] [PubMed] [Google Scholar]
- 24.Odds, F. C., A. J. Brown, and N. A. Gow. 2003. Antifungal agents: mechanisms of action. Trends Microbiol. 11:272-279. [DOI] [PubMed] [Google Scholar]
- 25.Prasad, R., P. De Wergifosse, A. Goffeau, and E. Balzi. 1995. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 27:320-329. [DOI] [PubMed] [Google Scholar]
- 26.Rai, V., S. Shukla, S. Jha, S. S. Komath, and R. Prasad. 2005. Functional characterization of N-terminal nucleotide binding domain (NBD-1) of a major ABC drug transporter Cdr1p of Candida albicans: uncommon but conserved Trp326 of Walker B is important for ATP binding. Biochemistry 44:6650-6661. [DOI] [PubMed] [Google Scholar]
- 27.Raychaudhuri, S., and W. A. Prinz. 2006. Uptake and trafficking of exogenous sterols in Saccharomyces cerevisiae. Biochem. Soc. Trans. 34:359-362. [DOI] [PubMed] [Google Scholar]
- 28.Reuss, O., A. Vik, R. Kolter, and J. Morschhäuser. 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341:119-127. [DOI] [PubMed] [Google Scholar]
- 29.Romsicki, Y., and F. J. Sharom. 1999. The membrane lipid environment modulates drug interactions with the P-glycoprotein multidrug transporter. Biochemistry 38:6887-6896. [DOI] [PubMed] [Google Scholar]
- 30.Saidane, S., S. Weber, X. De Deken, G. St-Germain, and M. Raymond. 2006. PDR16-mediated azole resistance in Candida albicans. Mol. Microbiol. 60:1546-1562. [DOI] [PubMed] [Google Scholar]
- 31.Saini, P., N. A. Gaur, and R. Prasad. 2006. Chimeras of the ABC drug transporter Cdr1p reveal functional indispensability of transmembrane domains and nucleotide-binding domains, but transmembrane segment 12 is replaceable with the corresponding homologous region of the non-drug transporter Cdr3p. Microbiology 152:1559-1573. [DOI] [PubMed] [Google Scholar]
- 32.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405-416. [DOI] [PubMed] [Google Scholar]
- 33.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1996. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob. Agents Chemother. 40:2300-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuetzer-Muehlbauer, M., B. Willinger, R. Egner, G. Ecker, and K. Kuchler. 2003. Reversal of antifungal resistance mediated by ABC efflux pumps from Candida albicans functionally expressed in yeast. Int. J. Antimicrob. Agents 22:291-300. [DOI] [PubMed] [Google Scholar]
- 36.Shukla, S., V. Rai, P. Saini, D. Banerjee, A. K. Menon, and R. Prasad. 2007. Candida drug resistance protein 1, a major multidrug ATP binding cassette transporter of Candida albicans, translocates fluorescent phospholipids in a reconstituted system. Biochemistry 46:12081-12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shukla, S., P. Saini, Smriti, S. Jha, S. V. Ambudkar, and R. Prasad. 2003. Functional characterization of Candida albicans ABC transporter Cdr1p. Eukaryot. Cell 2:1361-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sims, C. R., L. Ostrosky-Zeichner, and J. H. Rex. 2005. Invasive candidiasis in immunocompromised hospitalized patients. Arch. Med. Res. 36:660-671. [DOI] [PubMed] [Google Scholar]
- 39.Smriti, S. Krishnamurthy, B. L. Dixit, C. M. Gupta, S. Milewski, and R. Prasad. 2002. ABC transporters Cdr1p, Cdr2p and Cdr3p of a human pathogen Candida albicans are general phospholipid translocators. Yeast 19:303-318. [DOI] [PubMed] [Google Scholar]
- 40.Smriti, S. S. Krishnamurthy, and R. Prasad. 1999. Membrane fluidity affects functions of Cdr1p, a multidrug ABC transporter of Candida albicans. FEMS Microbiol. Lett. 173:475-481. [DOI] [PubMed] [Google Scholar]
- 41.Taglicht, D., and S. Michaelis. 1998. Saccharomyces cerevisiae ABC proteins and their relevance to human health and disease. Methods Enzymol. 292:130-162. [DOI] [PubMed] [Google Scholar]
- 42.van den Hazel, H. B., H. Pichler, M. A. do Valle Matta, E. Leitner, A. Goffeau, and G. Daum. 1999. PDR16 and PDR17, two homologous genes of Saccharomyces cerevisiae, affect lipid biosynthesis and resistance to multiple drugs. J. Biol. Chem. 274:1934-1941. [DOI] [PubMed] [Google Scholar]
- 43.Verrier, P. J., D. Bird, B. Burla, E. Dassa, C. Forestier, M. Geisler, M. Klein, U. Kolukisaoglu, Y. Lee, E. Martinoia, A. Murphy, P. A. Rea, L. Samuels, B. Schulz, E. J. Spalding, K. Yazaki, and F. L. Theodoulou. 2008. Plant ABC proteins—a unified nomenclature and updated inventory. Trends Plant Sci. 13:151-159. [DOI] [PubMed] [Google Scholar]
- 44.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wirsching, S., S. Michel, and J. Morschhäuser. 2000. Targeted gene disruption in Candida albicans wild-type strains: the role of the MDR1 gene in fluconazole resistance of clinical Candida albicans isolates. Mol. Microbiol. 36:856-865. [DOI] [PubMed] [Google Scholar]
- 46.Znaidi, S., X. De Deken, S. Weber, T. Rigby, A. Nantel, and M. Raymond. 2007. The zinc cluster transcription factor Tac1p regulates PDR16 expression in Candida albicans. Mol. Microbiol. 66:440-452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.