Abstract
BALB/c mice immunized intraperitoneally (i.p.) and intravenously (i.v.) with Leishmania donovani promastigote membrane antigens (LAg), either free or encapsulated in liposomes, were protected against challenge infection with L. donovani, whereas mice immunized by the subcutaneous (s.c.) and intramuscular routes were not protected. Protected mice showed strong parasite resistance in both the liver and spleen, along with enhanced immunoglobulin G2a and delayed-type hypersensitivity responses. Again, mice vaccinated through the i.p. and i.v. routes showed high levels of NO production after challenge infection. s.c. vaccination resulted in an increased capacity of the spleen cells to produce prechallenge transforming growth factor β (TGF-β) levels during the in vitro antigen recall response, whereas i.p. immunization induced production of prechallenge gamma interferon, interleukin-12 (IL-12), and IL-4 levels, with a Th1 bias. Exposure to antigen-stimulated splenocyte supernatants of i.p. but not s.c. immunized mice activated macrophages for in vitro parasite killing. As an enhanced level of TGF-β was detected in supernatants from unprotected s.c. immunized mice, neutralization by anti-TGF-β antibody enhanced in vitro macrophage killing activity. The suppressive role of this cytokine was evaluated in vivo by vaccination with liposomal LAg and anti-TGF-β antibody. Upon parasite challenge, these animals showed significant protection in both the liver and spleen. Moreover, the addition of recombinant TGF-β in splenocyte supernatants of i.p. immunized mice in vitro as well as in vivo inhibited the protective ability of the macrophages by the i.p. route. Thus, the induction of high prechallenge TGF-β limits the efficacy of vaccination by routes that are nonprotective.
Leishmaniasis is a group of vector-borne diseases caused by obligate intracellular protozoa belonging to the genus Leishmania. Clinical manifestations range from self-limiting cutaneous leishmaniasis to visceral leishmaniasis (VL), which is a fatal infection if it is left untreated. With an estimated 350 million people at risk for acquiring infection with Leishmania parasites, the worldwide prevalence is estimated at approximately 12 million cases, with 1.5 million new reports of cutaneous leishmaniasis and 500,000 new reports of VL each year. The World Health Organization considers leishmaniasis to be one of the most serious, epidemic-prone parasitic infectious diseases occurring sporadically in poor, rural farming areas (11).
Because of the lack of effective and low-cost treatments and the irreversibility of tissue damage during infection, considerable effort has been devoted to vaccine development. Much recent research on vaccine development against leishmaniasis has been directed toward determining strategies that specifically stimulate protective immune responses in the absence of those that may cause pathology and/or interfere with protection (16). Protective immunity against Leishmania is largely attributed to an IL-12-driven Th1 response and gamma interferon (IFN-γ) production. Although a disease-promoting role for IL-4 and the Th2 response in VL is more difficult to identify, a role for IL-10 and transforming growth factor β (TGF-β) has been documented during murine infection (20). Elevated levels of IL-10 have also been reported in clinical studies of VL (30). TGF-β has potent immunosuppressive properties, enhances disease progression, and may be instrumental in prevention of cure of leishmaniasis (39). IL-10 can be produced by many cell types, including B cells, macrophages, dendritic cells (DCs), and Th2 cells. Recently, a population of Th1 cells was found to be a source of IL-10 in Leishmania infection (27). Macrophages, fibroblasts, and inflammatory cells, such as neutrophils and eosinophils, are sources of TGF-β. Moreover, distinct populations of regulatory T cells (Treg cells) can be the source of IL-10 and TGF-β. Natural Treg cells produce both IL-10 and TGF-β. Inducible regulatory T-cell populations include T regulatory 1 (Tr1) cells, which secrete high levels of IL-10 with or without TGF-β, whereas T helper 3 (Th3) regulatory T cells express high levels of TGF-β (5).
Extensive vaccine studies against Leishmania with animal models have been carried out using strategies ranging from live parasites to nonliving protein- or DNA-based vaccines (16). The success of these vaccines depends upon the expression of immunity favorable for resistance against challenge infection. Of the many variables in vaccination, a major factor is the route of immunization. Vaccination with live parasites by different routes has shown that the subcutaneous (s.c.) route influences cell types recruited to the secondary site of infection and clears parasites more efficiently than the intradermal route (33). The intramuscular (i.m.) route has been the route of choice for DNA vaccination. However, optimization of this vaccine for generation of long-lasting protection revealed that intradermal immunization was more efficient, requiring fivefold less vaccine than both the i.m. and s.c. routes (22). For leishmanial protein antigens, including single proteins, different routes, such as the intraperitoneal (i.p.), intravenous (i.v.), and s.c. routes, have been used for generation of protective immunity (14, 17, 31). While it is agreed that the route of immunization of protein antigens may also influence the development of an immune response, the effects of various routes on the level and type of immune response generated and their subsequent impact on challenge infection have remained largely unknown.
We previously examined the ability of membrane antigens of Leishmania donovani promastigotes (LAg) to induce protective immunity in BALB/c mice. We reported that when the i.p. route of immunization was used, LAg entrapped in positively charged liposomes induced significant levels of protection (1). In the present study, we compared the protective efficacies of LAg, alone or entrapped in these liposomes and administered by four different routes, namely, the i.p., i.v., s.c., and i.m. routes, in BALB/c mice against L. donovani infection to understand the immunological correlates of protective and nonprotective routes. In addition, we investigated the mechanisms underlying the failure of vaccination by the nonprotective routes.
MATERIALS AND METHODS
Animals and parasites.
BALB/c mice, bred in the Indian Institute of Chemical Biology's animal facility (Kolkata, India), were 4 to 6 weeks old at the onset of the experiments. Mice were handled in accordance with institutional guidelines and used with relevant committee approval. L. donovani strain AG83 (MHOM/IN/1983/AG83) promastigotes were grown at 22°C in medium 199 supplemented with penicillin G sodium (100 U/ml), streptomycin sulfate (100 μg/ml), and 10% heat-inactivated fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO) and subcultured in the same medium at an average density of 2 × 106 cells/ml (1).
Preparation of LAg.
LAg were prepared from L. donovani promastigotes as described earlier (1). Briefly, stationary-phase promastigotes were washed in phosphate-buffered saline (PBS), pH 7.2, and were resuspended in 5 mM Tris-HCl buffer, pH 7.6. The suspension was vortexed and centrifuged at 2,310 × g for 10 min. The crude ghost membrane pellet was resuspended in the same buffer and sonicated for 3 min by an ultrasound probe sonicator (Misonix, Farmingdale, NY). The suspension was centrifuged at 5,910 × g for 30 min, and the supernatant containing the LAg was stored at −70°C until use. The amount of protein obtained from a 1.0-g cell pellet was approximately 14 mg (18).
Entrapment of antigens in liposomes.
Liposomes were prepared with egg lecithin (27 μmol), cholesterol (Sigma-Aldrich), and stearylamine (Fluka, Buchs SG) at a molar ratio of 7:2:2 as described previously (1). Empty and LAg-containing liposomes were prepared by the dispersion of lipid film in 1 ml PBS alone or containing 1 mg/ml LAg. The amount of associated LAg per milligram of egg lecithin was 36 μg.
Vaccination and challenge infection.
For vaccination, mice received three doses of 20 μg of LAg, either in PBS or incorporated into liposomes, by four different routes, i.e., the i.p., i.v., s.c., and i.m. routes, in a total volume of 200 μl at 2-week intervals. Alternatively, animals received three s.c. vaccinations of LAg entrapped in liposomes together with 100 μg of either anti-TGF-β (clone 9016.2; R&D Systems, Minneapolis, MN) or control isotype antibody (mouse immunoglobulin G1 [IgG1]; R&D Systems). Again, mice were injected i.p. with three doses of LAg entrapped in liposomes alone or in combination with 5 μg of recombinant TGF-β (rTGF-β; R&D Systems) at 2-week intervals. Control mice received nothing, empty liposomes, or anti-TGF-β antibody through the respective routes. Ten days after the last booster, groups of mice were either sacrificed for immunological assays or infected i.v. with 2.5 × 107 freshly transformed stationary-phase promastigotes in 200 μl PBS.
Evaluation of infection.
After 2 and 4 months of challenge infection, the course of infection was monitored by the microscopic examination of Giemsa-stained impression smears of liver and spleen. The parasite load was expressed in Leishman Donovan units, calculated by the following formula: number of amastigotes per 1,000 cell nuclei × organ weight (milligram) (32).
Antibody responses.
Sera from immunized and infected animals were analyzed by enzyme-linked immunosorbent assay (ELISA) for the presence of LAg-specific antibodies. In brief, 96-well microtiter plates (Nunc, Naperville, IL) were coated with LAg (25 μg/ml) and blocked to prevent nonspecific binding. The plates were then incubated with sera at a 1:1,000 dilution, followed by horseradish peroxidase-conjugated goat anti-mouse IgG (1:5,000) (Sigma-Aldrich), IgG1, and IgG2a (1:1,000) (BD Pharmingen, San Diego, CA). The color reaction was developed, and the absorbance was read in an ELISA plate reader (Thermo, Waltham, MA) at 450 nm (1).
DTH.
After the last vaccination, 2 and 4 months after challenge infection, delayed-type hypersensitivity (DTH) was determined as an index of cell-mediated immunity. The response was evaluated by measuring the difference in footpad swelling 24 h following intradermal inoculation of the test footpad with 50 μl of LAg (800 μg/ml) and the swelling of the control (PBS-injected) footpad with a constant-pressure caliper (Starrett Company, Athol, MA) (1).
Cytokine assays.
Single-cell suspensions were prepared from spleens of immunized, infected (for 2 and 4 months) mice and cultured in RPMI 1640 supplemented with 10% FBS, penicillin G sodium (100 U/ml), streptomycin sulfate (100 μg/ml), and 50 μM β-mercaptoethanol (Sigma-Aldrich) in triplicate in a 96-well flat-bottomed plate (Nunc) at a density of 2 × 105 cells/well in a final volume of 200 μl. Cells were stimulated with LAg (10 μg/ml) for 72 h, and supernatants were collected. IFN-γ, IL-12, IL-4, IL-10, tumor necrosis factor alpha (TNF-α) (BD Pharmingen), and TGF-β (R&D Systems) levels were measured in supernatants by ELISA following the manufacturers’ instructions (19). For TGF-β, supernatants were acidified prior to the assay.
Determination of NO concentration.
NO, quantified by the accumulation of nitrite in the supernatants, was measured according to the method of Ding et al. (12). Briefly, samples were mixed with an equal volume of Griess reagent [1% sulfanilamide and 0.1% N-(1-napthyl)-ethylenediamine dihydrochloride in 2.5% H3PO4] and incubated at room temperature for 10 min. Absorbance was then measured at 540 nm. Sodium nitrite was used as a standard (19).
In vitro growth of L. donovani in macrophages.
Macrophages were collected by peritoneal lavage from naïve mice and cultured on glass coverslips (18 mm2; 106 macrophages/coverslip) in 0.5 ml of RPMI 1640 with 10% FBS (3). Macrophages from naïve mice were pretreated for 12 to 18 h with spleen cell supernatants from immunized mice alone or in combination with rTGF-β (R&D Systems) at 0.5 to 100 ng/ml. rIFN-γ (R&D Systems) at 10 ng/ml was used as a positive control. For neutralization of cytokines, macrophages were pretreated with anti-IL-10 antibody, anti-IL-4 antibody (BD Pharmingen), and anti-TGF-β antibody (R&D Systems) at 10 μg/ml. Appropriate isotype-matched antibodies were used as controls. Macrophages were infected with promastigotes at a ratio of 10 parasites/macrophage. After 3 h of incubation, unphagocytosed parasites were removed by washing with PBS and cultured in the same medium with supernatants, rIFN-γ, antibodies, or rTGF-β at similar concentrations for 72 h. Cells fixed in methanol were stained with Giemsa stain for determination of intracellular parasite numbers. Results are shown as amastigote numbers per 100 macrophages, and at least 200 macrophages were counted per coverslip.
Statistical analysis.
All data were analyzed by one-way analysis of variance, using Graph Pad Prism, version 4.0 (Graph Pad Software, San Diego, CA). Results with P values of <0.05 were considered statistically significant.
RESULTS
Immunization by i.p. and i.v. routes, but not s.c. and i.m. routes, induces protection against L. donovani infection in BALB/c mice.
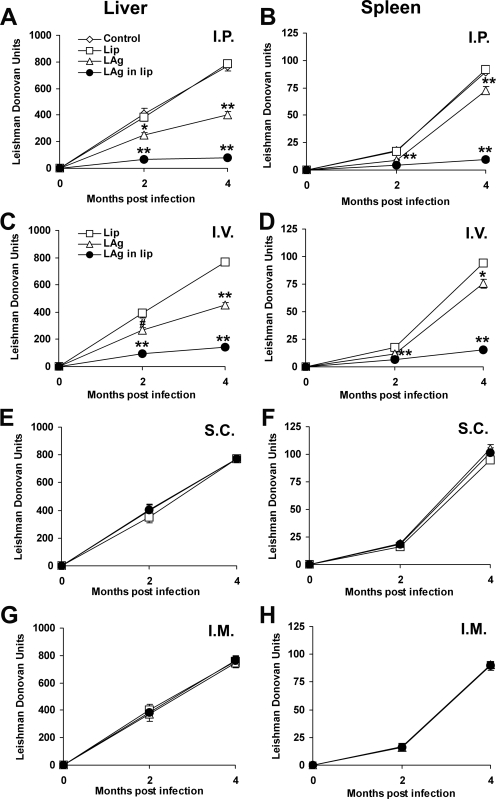
The outcomes of challenge infections in BALB/c mice following vaccination via four different routes with LAg and LAg in liposomes were evaluated. In contrast to self-limiting infection (26, 29), inoculation of BALB/c mice with L. donovani strain AG83 leads to progressive infection in the liver and spleen, corresponding with hepato- and splenomegaly (2, 7, 19, 24). Figure 1A shows that mice vaccinated with LAg through the i.p. route demonstrated partial reductions in liver parasite load at 2 (38%; P < 0.01) and 4 (48%; P < 0.001) months of infection compared to controls. Higher levels of reduction in parasite burden were observed in mice immunized i.p. with LAg in liposomes at 2 (83%) and 4 (90%) months of infection compared to controls (P < 0.001) and mice immunized with free antigen (P < 0.01). These findings strengthen our previous observation that LAg in liposomes could significantly reduce parasite burdens in BALB/c mice (1). Similarly, the splenic parasite burden was reduced with free antigen (48% at 2 months compared to controls; P < 0.001), and this change increased further with liposomal LAg immunization (ranging from 76% at 2 months to 89% at 4 months compared to controls; P < 0.001) (Fig. 1B). Besides the i.p. route, reductions in parasite burden were also observed when mice were immunized through the i.v. route (Fig. 1C and D). Although the levels of reduction were lower, liposomal LAg induced significant protection in both the liver (77% and 83% at 2 and 4 months, respectively) and spleen (64% and 83% at 2 and 4 months, respectively) compared to controls (P < 0.001). Interestingly, immunization by the s.c. or i.m. route with either free or liposomal LAg did not result in any reduction in parasite growth in the liver or spleen (Fig. 1E to H). Mice immunized through the s.c. or i.m. route developed progressive visceral infection, as seen in controls.
FIG. 1.
Clinical outcomes following L. donovani challenge in BALB/c mice immunized via four different routes. Mice were immunized three times with 20 μg of LAg, alone or entrapped in liposomes, at 2-week intervals through the i.p., i.v., s.c., and i.m. routes. Control groups received nothing or empty liposomes (Lip) through the respective routes. Ten days after the last immunization, the mice were challenged intravenously with 2.5 × 107 promastigotes of L. donovani. The parasite loads in the livers and spleens of i.p. (A and B), i.v. (C and D), s.c. (E and F), and i.m. (G and H) immunized animals are expressed in Leishman Donovan units. Data represent mean values ± standard errors (SE) for five mice per group at the designated time points and are representative of two independent experiments with similar results. #, P < 0.05; *, P < 0.01; **, P < 0.001 for comparison to control groups (mice left unimmunized or immunized with empty liposomes by the respective routes).
Immunization through i.p. and i.v. routes induces positive humoral and DTH responses.
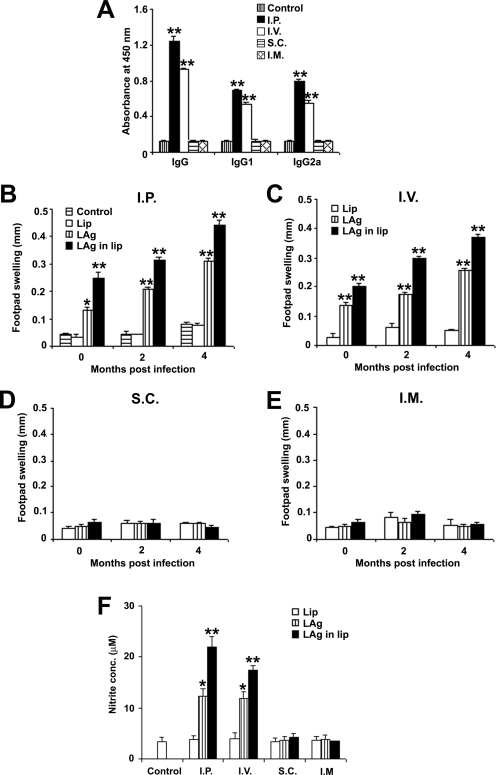
To investigate whether protection in immunized mice correlated with a specific pattern of immunoglobulin production, mouse sera were assayed for LAg-specific IgG as well as IgG1 and IgG2a isotypes, convenient surrogate markers of Th1 and Th2 CD4+ T-cell differentiation (10) (Fig. 2A). After immunization, sera from different groups of mice were examined at various dilutions (1/100, 1/1,000, 1/10,000, and 1/100,000), and the 1/1,000 dilution was found to be nonsaturating (data not shown). Mice immunized with LAg in liposomes via the i.p. and i.v. routes induced significantly higher levels of IgG, IgG1, and IgG2a than those in controls (P < 0.001). DTH, an index of cell-mediated immunity, was evaluated in vaccinated mice to determine the correlation with the observed protection in the different vaccination groups. Mice immunized with free LAg as well as LAg in liposomes through the i.p. and i.v. routes induced significant levels of DTH, which gradually increased after 2 and 4 months of infection in comparison to the control groups (P < 0.001) (Fig. 2B and C). The level of DTH induced by LAg in liposomes was significantly higher than that induced by free LAg (P < 0.001). Mice immunized via the s.c. and i.m. routes did not exhibit any significant level of DTH, with values comparable to those for controls (Fig. 2D and E).
FIG. 2.
LAg-specific immune responses in mice immunized via four different routes. (A) Serum samples were collected from liposomal LAg-immunized mice and assayed for LAg-specific IgG, IgG1, and IgG2a levels by ELISA. Data are presented as the absorbance at 450 nm and are means ± SE for five mice per group in duplicate wells. Data are representative of two independent experiments with similar results. (B to E) Mice were immunized and infected with L. donovani. DTH responses were measured in i.p. (B), i.v. (C), s.c. (D), and i.m. (E) immunized animals. Results are shown as means ± SE for five mice per group at the designated time points and are representative of two independent experiments with similar results. (F) Four months after infection, splenocytes derived from different vaccinated groups were stimulated with LAg (10 μg/ml) for 72 h, and the level of nitrite in the culture supernatants was assessed. Results are means ± SE for five mice per group done in duplicate and are representative of two independent experiments with similar results. *, P < 0.01; **, P < 0.001 for comparison to control groups (mice left unimmunized or immunized with empty liposomes by the respective routes).
Production of nitrite in protected i.p. and i.v. immunized mice.
NO is a critical macrophage-derived effector molecule for the control of Leishmania infections (35). To determine whether protective and nonprotective routes could induce NO production, the level of nitrite was estimated in the antigen-stimulated culture supernatants of splenocytes isolated from mice in different vaccinated groups after 4 months of infection. Significant nitrite production was observed in i.p. and i.v. immunized mice (P < 0.01 compared to controls), whereas in s.c. and i.m. immunized mice the generation of nitrite was inhibited (Fig. 2F). These data reflect the activated state of splenic macrophages specific to leishmanial antigen in i.p. immunized animals, whereas in s.c. immunized animals the macrophages failed to be activated.
Production of antigen-specific TGF-β following vaccination by the nonprotective s.c. route.
To investigate the immunomodulatory responses associated with different routes, LAg-specific recall cytokine responses were measured in splenocyte cultures of mice vaccinated by the protective i.p. and nonprotective s.c. routes. Table 1 shows that following liposomal LAg vaccination, splenocytes from i.p. immunized mice produced significant amounts of IFN-γ, IL-12, and IL-4 in comparison to controls as well as s.c. immunized mice (P < 0.001). The level of IFN-γ was significantly higher in i.p. immunized mice, with a ratio of IFN-γ to IL-4 of 2.62 ± 0.37, showing a skewing toward a Th1 response. A nonsignificant increase in the level of IL-10 was detected in spleen cell supernatants derived from s.c. vaccinated mice compared with controls. The level of TNF-α remained low in both i.p. and s.c. immunized mice. In contrast, the level of TGF-β was elevated eightfold in s.c. immunized mice compared to controls and i.p. immunized mice (P < 0.001), indicating a preferential increase in antigen-specific TGF-β production in response to the nonprotective s.c. route. Progressive disease with L. donovani infection after 4 months showed high levels of IL-4 and IL-10, with reduced levels of the Th1 cytokines IFN-γ and IL-12, as also observed earlier in control animals (7, 19). In addition, control animals demonstrated high levels of TGF-β with a lower level of TNF-α. s.c. immunized animals exhibited similar cytokine profiles to those of controls. In contrast, IFN-γ, IL-12, and TNF-α levels were elevated in i.p. immunized mice, whereas IL-4, IL-10, and TGF-β levels were decreased significantly compared to those in controls as well as s.c. immunized animals following challenge infection (P < 0.001).
TABLE 1.
Cytokine levels in spleen cell supernatants from mice immunized via different routes
| Vaccination group | Cytokine concn (pg/ml)a
|
|||||
|---|---|---|---|---|---|---|
| IFN-γ | IL-12 | IL-4 | IL-10 | TNF-α | TGF-β | |
| Groups after immunization | ||||||
| Control | 21 ± 3 | 22 ± 3 | 15 ± 2 | 16 ± 4 | 11 ± 1 | 93 ± 16 |
| Liposomes i.p. | 22 ± 3 | 24 ± 3 | 14 ± 2 | 20 ± 1 | 13 ± 5 | 105 ± 6 |
| Liposomes s.c. | 23 ± 4 | 22 ± 2 | 13 ± 3 | 18 ± 4 | 12 ± 2 | 102 ± 8 |
| Liposomal LAg i.p. | 120 ± 7b | 95 ± 8b | 48 ± 5b | 22 ± 4 | 19 ± 5 | 114 ± 6 |
| Liposomal LAg s.c. | 28 ± 3 | 25 ± 3 | 21 ± 3 | 31 ± 3 | 14 ± 4 | 924 ± 87b |
| Groups 4 mo after infection | ||||||
| Control | 31 ± 2 | 27 ± 4 | 93 ± 8 | 230 ± 21 | 24 ± 4 | 1,652 ± 81 |
| Liposomes i.p. | 37 ± 2 | 28 ± 3 | 87 ± 8 | 228 ± 14 | 24 ± 3 | 1,624 ± 113 |
| Liposomes s.c. | 32 ± 4 | 30 ± 4 | 86 ± 6 | 220 ± 11 | 29 ± 4 | 1,540 ± 125 |
| Liposomal LAg i.p. | 274 ± 18b | 168 ± 10b | 37 ± 4b | 33 ± 4b | 126 ± 9b | 113 ± 9b |
| Liposomal LAg s.c. | 38 ± 3 | 32 ± 3 | 97 ± 7 | 228 ± 8 | 28 ± 4 | 1,680 ± 77 |
Data are for five mice and are expressed as means ± standard errors. Data are representative of two independent experiments with similar results.
P < 0.001 in comparison to control groups.
Differential macrophage stimulatory activity in spleen cell supernatants from animals immunized by different routes and TGF-β-dependent inhibitory activity of supernatants from the nonprotective s.c. route.
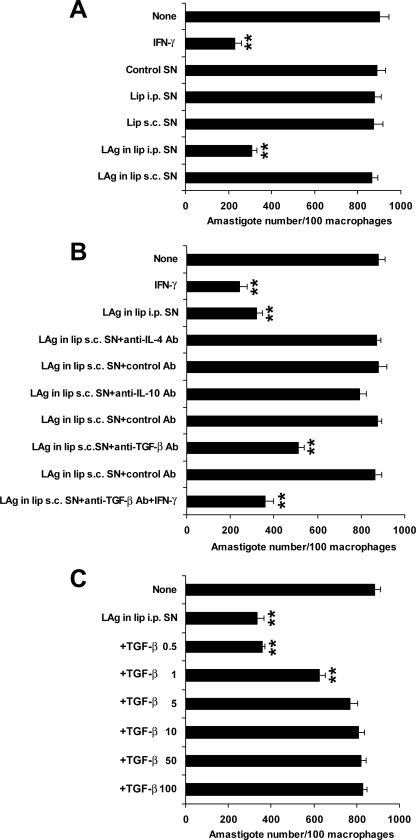
Because cytokine-activated macrophages are thought to play a role in the immune effector mechanism of resistance in vaccine models, the induction of macrophage-activating cytokines for intracellular parasite killing upon vaccination by different routes was examined. Supernatants derived from antigen-stimulated spleen cells from i.p. immunized animals activated macrophages to kill parasites in vitro compared to controls (P < 0.001) (Fig. 3A). In contrast, spleen supernatants from mice immunized by the nonprotective s.c. route were not potent macrophage activators. IL-4, IL-10, and TGF-β can prevent macrophage activation. To test the possible effect of these cytokines in supernatants from nonprotected mice, we added antibodies to these cytokines during the cytotoxicity assay. Neither anti-IL-4 nor anti-IL-10 antibodies significantly increased the level of macrophage activity observed with s.c. supernatants above that seen with control antibodies (Fig. 3B). Interestingly, s.c. supernatants in the presence of anti-TGF-β antibody showed a dramatic increase in macrophage-activating capacity, and the killing activity was further increased in the presence of IFN-γ (P < 0.001) compared to the control level. These data suggest a potential role of TGF-β in the suppression of killing activity via the nonprotective s.c. route. Since TGF-β can inhibit macrophage activation, we then tested the effect of the addition of rTGF-β to i.p. supernatants on macrophage activation of leishmanicidal activity. Inhibition with i.p. supernatants was achieved at levels of rTGF-β of >1 ng/ml (Fig. 3C), indicating that TGF-β can abolish macrophage activity in this system.
FIG. 3.
Effects of splenocyte supernatants (SN) of immunized mice on intracellular parasite growth in peritoneal macrophages in the absence (A) and presence of anticytokine antibodies (Ab) (B) or TGF-β (C). Peritoneal macrophages harvested from naive mice were pretreated for 12 to 18 h with supernatants from s.c. immunized mice either alone or in the presence of 10 μg/ml of each of the anticytokine antibodies and appropriate isotype-matched control antibodies. IFN-γ at 10 ng/ml was used as a positive control. Again, macrophages were pretreated with supernatants from i.p. immunized mice alone or with various concentrations of TGF-β ranging from 0.5 to 100 ng/ml. After 3 h of infection, unphagocytosed parasites were removed by washing with PBS and cultured in the same medium with supernatants, IFN-γ, antibodies, or TGF-β, using similar concentrations. Seventy-two hours later, the number of intracellular amastigotes was determined. Results are means ± SE for five experiments done in triplicate. **, P < 0.001 for comparison to control groups.
Neutralization of TGF-β during vaccination induces protection, whereas the addition of TGF-β causes a failure of protection.
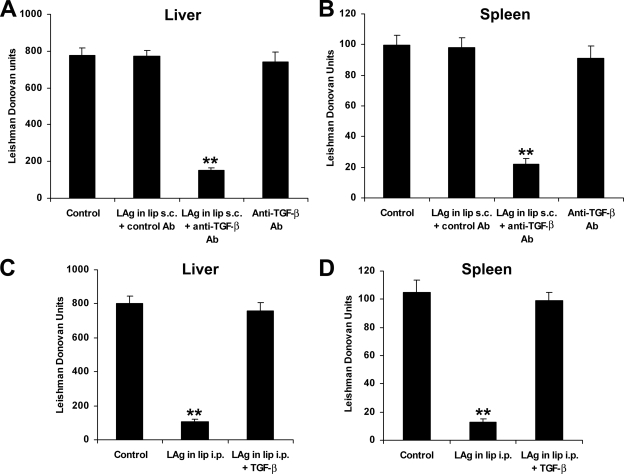
To more directly assess the role of TGF-β in the unprotected s.c. immunized mice, the animals received neutralizing anti-TGF-β antibody together with liposomal LAg vaccination. The neutralization of TGF-β was confirmed by the lower TGF-β levels in the sera of animals receiving the vaccine with anti-TGF-β antibody than in those of animals receiving the vaccine with control antibody (data not shown). We found that after 4 months of infection, animals covaccinated with anti-TGF-β antibody through the s.c. route showed an 80% reduction in liver parasite load (Fig. 4A) and a 78% reduction in spleen parasite load (Fig. 4B) compared to controls (P < 0.001). Since anti-TGF-β antibody was administered much prior to infection challenge, the effect of TGF-β neutralization was at the vaccination stage, not on the infection itself. These results therefore confirm that augmented TGF-β production during vaccination via the s.c. route is responsible for the failure of induction of protective immunity. Additionally, to determine whether TGF-β could alter the resistance against leishmanial infection, mice were injected with liposomal LAg along with rTGF-β during protective i.p. vaccination. rTGF-β treatment led to increases in parasite burden in both the liver and spleen after 4 months of challenge infection, comparable to the case in controls (Fig. 4C and D). Thus, a role of TGF-β in the failure of protection in leishmaniasis was established.
FIG. 4.
In vivo effects of anti-TGF-β antibody (Ab) and TGF-β during vaccination via nonprotective s.c. and protective i.p. routes. Mice were vaccinated three times with liposomal LAg with 100 μg of anti-TGF-β or control antibody at 2-week intervals through the s.c. route. Control groups received nothing or anti-TGF-β antibody. Ten days after the last immunization, the mice were challenged intravenously with 2.5 × 107 promastigotes of L. donovani. After 4 months, parasite loads in the liver (A) and spleen (B) were measured and expressed in Leishman Donovan units. Mice were vaccinated three times with liposomal LAg, alone or in combination with 5 μg TGF-β, at 2-week intervals through the i.p. route and infected with L. donovani. After 4 months of challenge infection, parasite loads in the liver (C) and spleen (D) were measured and expressed in Leishman Donovan units. Data represent mean values ± SE for five mice per group. **, P < 0.001 for comparison to control groups.
DISCUSSION
In this study, we evaluated the protective efficacy of LAg in liposomes administered through different routes and compared the immune responses for protection against L. donovani in a BALB/c mouse model. Previously, we reported that positively charged liposomes enhanced the immunogenicity of the associated LAg and induced significant levels of protection when injected i.p. (1). Here we demonstrated that the route of vaccination influenced the development of immune responses for either protection or failure of protection against L. donovani: i.p. and i.v. routes were protective, whereas s.c. and i.m. routes failed to induce protection. Moreover, experiments performed to understand the immune mechanism revealed that the high prechallenge level of TGF-β production did predict the failure of liposomal LAg vaccination via the s.c. route after challenge infection.
Examination of immune responses of vaccinated mice demonstrated that protected animals immunized via the i.p. as well as i.v. route stimulated the production of IgG2a and elicited profound DTH responses. This result extends our earlier report that protection in mice vaccinated via the i.p. route was associated with increased DTH responses and enhanced IgG2a production (1). Analysis of cytokines from spleen cells revealed that animals vaccinated via the protective i.p. route produced both prechallenge IFN-γ and IL-4, with a skewing toward a Th1 response essential for resistance against L. donovani (7, 19, 20). Interestingly, unprotected mice induced elevated levels of TGF-β, with downregulation of both Th1 and Th2 cytokines, before infection. It has been reported that TNF-α acts as a triggering signal for NO production (13), and NO has a central role in parasite clearance (25). The production of NO and TNF-α in splenocyte culture supernatants from i.p. immunized mice as well as the failure of NO generation in s.c. immunized mice confirms that an NO-mediated macrophage effector mechanism is critical in the control of L. donovani replication in the mouse (25). Splenocyte supernatants from unprotected mice lacked macrophage activation in vitro, and anti-IL-4 and anti-IL-10 antibodies did not substantially increase macrophage leishmanicidal activity. The inhibitory activity of s.c. supernatants was neutralized by anti-TGF-β antibody, and the addition of rTGF-β to i.p. supernatants inhibited macrophage activation in vitro. The observations that neutralization of TGF-β, elicited by the nonprotective s.c. route, by anti-TGF-β antibody in vivo induced protection in both the liver and spleen and that rTGF-β treatment with liposomal LAg vaccination by the protective i.p. route abrogated the protection are powerful evidence in support of the view that TGF-β plays a major role in inhibiting the development of a protective immune effector mechanism against L. donovani infection.
The critical dependence on the route of immunization in obtaining protective immunity using protein antigens has been evaluated for L. major infection. In these studies, i.v. and, to a lesser extent, i.p. immunization was protective, and the s.c. and i.m. routes were ineffective (17). In the present study, we observed that despite the different pattern of disease caused by the species L. donovani, which is phylogenetically distant from L. major (20), i.p. and i.v. routes were protective and s.c. and i.m. routes were nonprotective against VL. To the best of our knowledge, however, none of the previous studies reported the priming of strong TGF-β production following vaccination by the nonprotective routes. TGF-β has multiple suppressive actions on T cells, B cells, and macrophages (28) and can inhibit both Th1 and Th2 differentiation (15). TGF-β has been found to inhibit the DTH response (21) and to suppress macrophage activation and NO production (8, 34). It has been found that the production of TGF-β during Leishmania infection correlates with in vivo susceptibility (4). Moreover, a role of TGF-β has been observed in the failure of protective immunity via specific routes against Schistosoma mansoni (38). Induction of oral tolerance and prevention of Th1 cell-mediated experimental autoimmune encephalomyelitis by feeding of self-antigens were associated with the generation of TGF-β-secreting T cells (9). These T cells, producing large amounts of TGF-β and various amounts of IL-4 and IL-10, have been identified as distinct from Th2 cells and named Th3 cells (9, 23). Th3 cells primarily secrete TGF-β and have suppressive properties for T and other immune cells (36). Characterization of TGF-β-secreting CD4+ clones from animals fed myelin basic protein in experimental autoimmune encephalomyelitis revealed a clone which was TGF-βhigh IL-4low IL-10low. These TGF-βhigh clones produced no IFN-γ or IL-2 (9). In the present study, we found a similar cytokine profile after vaccination via the nonprotective route, suggesting that Th3 cells could be the source of TGF-β during vaccination.
The reason for the production of TGF-β-secreting Th3 cells by the s.c. route or, conversely, the suppression of TGF-β by the i.p. route is not clear at present. Th3 cells are inducible Treg cells, which develop in the periphery from conventional CD4+ T cells after exposure to signals such as regulatory cytokines, immunosuppressive drugs, or antigen-presenting cells (APCs) conditioned by microbial products. Furthermore, some pathogens target sites in which TGF-β is highly produced, such as the gastrointestinal tract, the skin, and the eye (5). The i.p. and s.c. routes of immunization used herein expose the liposomal antigen to different sets of APCs for the primary encounter. When the i.p. route was used, peritoneal macrophages would be the major population of APCs available, whereas DCs would be the main APCs during s.c. immunization. It has been found that induction of the immune response by liposomal delivery of antigen is macrophage dependent, and DCs are considered to be less efficient in phagocytosis than cells of the macrophage lineage (37). This may be enable T cells from i.p. immunized mice to generate a protective response. Conversely, in models of oral tolerance, it has been found that DCs, the most potent APCs in activating resting T cells, possibly present antigens for Th3-cell differentiation under the influence of the gut cytokine milieu (36). Thus, it is interesting to speculate that APCs at the site of vaccination may influence the cytokine profile of the subsequent immune response. To promote protective immune responses, it would be better to select a site of vaccination where Treg cells were not overrepresented, as vaccination itself can generate its own set of Treg cells. The skin contains the highest percentage of natural Treg cells in the body. Moreover, conventional T cells converted into Treg cells in the periphery under subimmunogenic conditions can subsequently be expanded by the delivery of antigen under immunogenic conditions (5, 6). Thus, the optimal conditions of vaccination should include a site that can minimize Treg priming or activation.
In conclusion, we demonstrated that vaccination of BALB/c mice with free LAg or LAg entrapped in liposomes through i.p. and i.v. routes induced protective immunity against infection with L. donovani, whereas s.c. and i.m. routes failed to induce protection with the same antigens. The failure of protection by the s.c. route was manifested by enhanced prechallenge TGF-β production following vaccination. These results clearly illustrate the fact that in designing an effective vaccine against L. donovani or other intracellular pathogens, it is critical to consider the route of immunization that not only leads to production of cytokines directly stimulating protective mechanisms but also can inhibit the production of suppressive cytokines, such as TGF-β.
Acknowledgments
We thank Manjarika De for her help with parasite culture.
This work was supported by grants from the CSIR and the DST, Government of India.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Afrin, F., and N. Ali. 1997. Adjuvanticity and protective immunity elicited by Leishmania donovani antigens encapsulated in positively charged liposomes. Infect. Immun. 652371-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afrin, F., and N. Ali. 1998. Isotype profiles of Leishmania donovani-infected BALB/c mice: preferential stimulation of IgG2a/b by liposome-associated promastigote antigens. J. Parasitol. 84743-748. [PubMed] [Google Scholar]
- 3.Afrin, F., T. Dey, K. Anam, and N. Ali. 2001. Leishmanicidal activity of stearylamine-bearing liposomes in vitro. J. Parasitol. 87188-193. [DOI] [PubMed] [Google Scholar]
- 4.Barral-Netto, M., A. Barral, C. E. Brownell, Y. A. Skeiky, L. R. Ellingsworth, D. R. Twardzik, and S. G. Reed. 1992. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science 257545-548. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid, Y. 2007. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 7875-888. [DOI] [PubMed] [Google Scholar]
- 6.Belkaid, Y. 2008. Role of Foxp3-positive regulatory T cells during infection. Eur. J. Immunol. 38918-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhowmick, S., R. Ravindran, and N. Ali. 2007. Leishmanial antigens in liposomes promote protective immunity and provide immunotherapy against visceral leishmaniasis via polarized Th1 response. Vaccine 256544-6556. [DOI] [PubMed] [Google Scholar]
- 8.Bogdan, C., and C. Nathan. 1993. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann. N. Y. Acad. Sci. 685713-739. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Y., V. K. Kuchroo, J. Inobe, D. A. Hafler, and H. L. Weiner. 1994. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 2651237-1240. [DOI] [PubMed] [Google Scholar]
- 10.Coffman, R. L., D. A. Lebman, and P. Rothman. 1993. Mechanism and regulation of immunoglobulin isotype switching. Adv. Immunol. 54229-270. [DOI] [PubMed] [Google Scholar]
- 11.Desjeux, P. 2004. Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 27305-318. [DOI] [PubMed] [Google Scholar]
- 12.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 1412407-2412. [PubMed] [Google Scholar]
- 13.Fonseca, S. G., P. R. Romao, F. Figueiredo, R. H. Morais, H. C. Lima, S. H. Ferreira, and F. Q. Cunha. 2003. TNF-alpha mediates the induction of nitric oxide synthase in macrophages but not in neutrophils in experimental cutaneous leishmaniasis. Eur. J. Immunol. 332297-2306. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh, A., W. W. Zhang, and G. Matlashewski. 2001. Immunization with A2 protein results in a mixed Th1/Th2 and a humoral response which protects mice against Leishmania donovani infections. Vaccine 2059-66. [DOI] [PubMed] [Google Scholar]
- 15.Gorelik, L., and R. A. Flavell. 2002. Transforming growth factor-beta in T-cell biology. Nat. Rev. Immunol. 246-53. [DOI] [PubMed] [Google Scholar]
- 16.Kedzierski, L., Y. Zhu, and E. Handman. 2006. Leishmania vaccines: progress and problems. Parasitology 133S87-S112. [DOI] [PubMed] [Google Scholar]
- 17.Liew, F. Y., C. Hale, and J. G. Howard. 1985. Prophylactic immunization against experimental leishmaniasis. IV. Subcutaneous immunization prevents the induction of protective immunity against fatal Leishmania major infection. J. Immunol. 1352095-2101. [PubMed] [Google Scholar]
- 18.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193265-275. [PubMed] [Google Scholar]
- 19.Mazumdar, T., K. Anam, and N. Ali. 2004. A mixed Th1/Th2 response elicited by a liposomal formulation of Leishmania vaccine instructs Th1 responses and resistance to Leishmania donovani in susceptible BALB/c mice. Vaccine 221162-1171. [DOI] [PubMed] [Google Scholar]
- 20.McMahon-Pratt, D., and J. Alexander. 2004. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol. Rev. 201206-224. [DOI] [PubMed] [Google Scholar]
- 21.Meade, R., P. W. Askenase, G. P. Geba, K. Neddermann, R. O. Jacoby, and R. D. Pasternak. 1992. Transforming growth factor-beta 1 inhibits murine immediate and delayed type hypersensitivity. J. Immunol. 149521-528. [PubMed] [Google Scholar]
- 22.Mendez, S., Y. Belkaid, R. A. Seder, and D. Sacks. 2002. Optimization of DNA vaccination against cutaneous leishmaniasis. Vaccine 203702-3708. [DOI] [PubMed] [Google Scholar]
- 23.Mills, K. H. 2004. Regulatory T cells: friend or foe in immunity to infection? Nat. Rev. Immunol. 4841-855. [DOI] [PubMed] [Google Scholar]
- 24.Mukhopadhyay, S., S. Bhattacharyya, R. Majhi, T. De, K. Naskar, S. Majumdar, and S. Roy. 2000. Use of an attenuated leishmanial parasite as an immunoprophylactic and immunotherapeutic agent against murine visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 7233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray, H. W., and C. F. Nathan. 1999. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 189741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray, H. W., C. M. Lu, S. Mauze, S. Freeman, A. L. Moreira, G. Kaplan, and R. L. Coffman. 2002. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect. Immun. 706284-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Garra, A., and P. Vieira. 2007. TH1 cells control themselves by producing interleukin-10. Nat. Rev. Immunol. 7425-428. [DOI] [PubMed] [Google Scholar]
- 28.Prud'homme, G. J., and C. A. Piccirillo. 2000. The inhibitory effects of transforming growth factor-beta-1 (TGF-beta1) in autoimmune diseases. J. Autoimmun. 1423-42. [DOI] [PubMed] [Google Scholar]
- 29.Rosas, L. E., H. M. Snider, J. Barbi, A. A. Satoskar, G. Lugo-Villarino, T. Keiser, T. Papenfuss, J. E. Durbin, D. Radzioch, L. H. Glimcher, and A. R. Satoskar. 2006. Cutting edge: STAT1 and T-bet play distinct roles in determining outcome of visceral leishmaniasis caused by Leishmania donovani. J. Immunol. 17722-25. [DOI] [PubMed] [Google Scholar]
- 30.Saha, S., S. Mondal, R. Ravindran, S. Bhowmick, D. Modak, S. Mallick, M. Rahman, S. Kar, R. Goswami, S. K. Guha, N. Pramanik, B. Saha, and N. Ali. 2007. IL-10- and TGF-beta-mediated susceptibility in kala-azar and post-kala-azar dermal leishmaniasis: the significance of amphotericin B in the control of Leishmania donovani infection in India. J. Immunol. 1795592-5603. [DOI] [PubMed] [Google Scholar]
- 31.Stager, S., D. F. Smith, and P. M. Kaye. 2000. Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J. Immunol. 1657064-7071. [DOI] [PubMed] [Google Scholar]
- 32.Stauber, L. A., E. M. Franchino, and J. Grun. 1958. An 8-day method for screening compounds against Leishmania donovani in the golden hamster. J. Protozool. 5269-273. [Google Scholar]
- 33.Tabbara, K. S., N. C. Peters, F. Afrin, S. Mendez, S. Bertholet, Y. Belkaid, and D. L. Sacks. 2005. Conditions influencing the efficacy of vaccination with live organisms against Leishmania major infection. Infect. Immun. 734714-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vodovotz, Y., C. Bogdan, J. Paik, Q. W. Xie, and C. Nathan. 1993. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J. Exp. Med. 178605-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei, X. Q., I. G. Charles, A. Smith, J. Ure, G. J. Feng, F. P. Huang, D. Xu, W. Muller, S. Moncada, and F. Y. Liew. 1995. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375408-411. [DOI] [PubMed] [Google Scholar]
- 36.Weiner, H. L. 2001. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 3947-954. [DOI] [PubMed] [Google Scholar]
- 37.Wijburg, O. L., G. P. van den Dobbelsteen, J. Vadolas, A. Sanders, R. A. Strugnell, and N. van Rooijen. 1998. The role of macrophages in the induction and regulation of immunity elicited by exogenous antigens. Eur. J. Immunol. 28479-487. [DOI] [PubMed] [Google Scholar]
- 38.Williams, M. E., P. Caspar, I. Oswald, H. K. Sharma, O. Pankewycz, A. Sher, and S. L. James. 1995. Vaccination routes that fail to elicit protective immunity against Schistosoma mansoni induce the production of TGF-beta, which down-regulates macrophage antiparasitic activity. J. Immunol. 1544693-4700. [PubMed] [Google Scholar]
- 39.Wilson, M. E., S. M. Jeronimo, and R. D. Pearson. 2005. Immunopathogenesis of infection with the visceralizing Leishmania species. Microb. Pathog. 38147-160. [DOI] [PubMed] [Google Scholar]