Abstract
Heat shock proteins with molecular masses of ∼60 kDa (Hsp60) are widely distributed in nature and are highly conserved immunogenic molecules that can function as molecular chaperones and enhance cellular survival under physiological stress conditions. The fungus Histoplasma capsulatum displays an Hsp60 on its cell surface that is a key target of the cellular immune response during histoplasmosis, and immunization with this protein is protective. However, the role of humoral responses to Hsp60 has not been fully elucidated. We generated immunoglobulin G (IgG) isotype monoclonal antibodies (MAbs) to H. capsulatum Hsp60. IgG1 and IgG2a MAbs significantly prolonged the survival of mice infected with H. capsulatum. An IgG2b MAb was not protective. The protective MAbs reduced intracellular fungal survival and increased phagolysosomal fusion of macrophages in vitro. Histological examination of infected mice showed that protective MAbs reduced the fungal burden and organ damage. Organs of infected animals treated with protective MAbs had significantly increased levels of interleukin-2 (IL-2), IL-12, and tumor necrosis factor alpha and decreased levels of IL-4 and IL-10. Hence, IgG1 and IgG2a MAbs to Hsp60 can modify H. capsulatum pathogenesis in part by altering the intracellular fate of the fungus and inducing the production of Th1-associated cytokines.
The dimorphic fungus Histoplasma capsulatum var. capsulatum is the most prevalent cause of fungal respiratory disease. It is endemic in the midwestern and southeastern regions of the United States (1, 17, 20), infecting approximately 500,000 individuals each year. The spectrum of diseases due to this fungus is broad. The majority of individuals develop a mild, subclinical infection, but symptomatic diseases range from an influenza-like illness to a chronic cavitary pulmonary disease or a highly lethal disseminated infection, particularly in immunosuppressed individuals, such as individuals with AIDS (21, 24) or patients receiving potent immunomodulators, such as tumor necrosis factor alpha (TNF-α) inhibitors (10, 11).
Host protective immunity can be induced via interaction of H. capsulatum var. capsulatum-infected monocytes/macrophages with T cells (5, 41, 42), resulting in T-cell activation and the release of Th1-associated proinflammatory cytokines, such as interleukin-12 (IL-12), gamma interferon (IFN-γ), and TNF-α (7, 27, 42). T-cell activation can also be induced by immunization with several protein-containing antigens and extracts from H. capsulatum var. capsulatum (14, 19, 36). In particular, a heat shock protein with a molecular mass of 62 kDa (Hsp60) is an immunodominant antigen that can induce protective cellular responses, which are strictly dependent on the presence and activation of a specific Vβ8.1/8.2+ subset of CD4+ T lymphocytes (14, 19).
Antibody responses in histoplasmosis have been characterized previously (23-25, 35, 40), but the role of the antibodies in pathogenesis is unclear. Depletion of CD4+ and CD8+ T cells in B-lymphocyte knockout mice results in fungal burdens in organs after infection with H. capsulatum var. capsulatum that are markedly higher than those in wild-type animals (3), suggesting that an antibody may collaborate with T cells in combating histoplasmosis. Consistent with the conflicting results of previous experiments with polyclonal sera for fungal infections such as cryptococcosis (8, 9, 29), administration of polyclonal serum from mice immune to H. capsulatum var. capsulatum did not protect naïve mice from histoplasmosis (39). Interestingly, in vivo experiments with specific monoclonal antibodies (MAbs) to the capsular polysaccharide of the fungus Cryptococcus neoformans have revealed the existence of protective, nonprotective, and disease-enhancing MAbs, suggesting that the divergent results obtained with polyclonal preparations may be a result of the relative proportions of protective and nonprotective antibodies in immune sera (9, 16).
We recently described MAbs that modify the pathogenesis of experimental histoplasmosis. Administration of immunoglobulin M (IgM) isotype MAbs that bind a histone 2B-like protein (H2B) on the surface of H. capsulatum var. capsulatum yeast cells reduces the fungal burden, decreases pulmonary inflammation, and prolongs survival in a murine model of infection (32, 33). These protective MAbs have been associated with enhanced levels of IL-4, IL-6, and IFN-γ in the lungs of infected mice. Additionally, the IgM MAbs increase phagocytosis of the yeast by J774.16 cells through a CR3-dependent process and inhibit yeast cell growth in macrophages, altering the intracellular fate of the fungus (32, 33, 37).
However, the penetration of IgM MAbs into the lung is problematic, and the protective results obtained with the MAbs to H2B were improved when the fungus was opsonized with the MAbs prior to infection. Our current work sought to ascertain whether the administration of IgG isotype MAbs was more effective in modifying histoplasmosis. Therefore, we generated a panel of IgG MAbs against Hsp60 from H. capsulatum var. capsulatum and found that the IgG1 and IgG2a MAbs dramatically altered H. capsulatum var. capsulatum pathogenesis. Interestingly, an IgG2b MAb was not protective, indicating that isotype may impact efficacy.
(The data in this paper are from a thesis to be submitted by A. J. Guimarães in partial fulfillment of the requirements for a Ph.D. degree from the Sue Golding Graduate Division of Medical Science, Albert Einstein College of Medicine, Yeshiva University, Bronx, NY.)
MATERIALS AND METHODS
Fungal strains and growth conditions.
The reference strain H. capsulatum var. capsulatum G217B was obtained from the American Type Culture Collection (Rockville, MD). H. capsulatum var. capsulatum yeast cells were grown in Ham's F-12 medium at 37°C with rotary shaking as described previously (4).
Generation of MAbs against rHsp60.
Animal experiments were performed according to the guidelines of the Institute for Animal Studies of the Albert Einstein College of Medicine. Escherichia coli containing the pET21 plasmid harboring the H. capsulatum var. capsulatum Hsp60 gene was a gift from G. Deepe (University of Cincinnati, Cincinnati, OH). Recombinant Hsp60 (rHsp60) was purified as described previously (14). Two female BALB/c mice (6 to 8 weeks old; Jackson Immunoresearch Labs, West Grove, PA) were immunized intraperitoneally with 50 μg of rHsp60 suspended in a 1:1 (vol/vol) emulsion of complete Freund's adjuvant (Sigma-Aldrich) and phosphate-buffered saline (PBS). Additional doses were administered 2 and 4 weeks after the first immunization in 1:1 (vol/vol) emulsions of incomplete Freund's adjuvant (Sigma-Aldrich). Sera were obtained before each immunization and 2 weeks after the last immunization and analyzed for the presence of antibodies to rHsp60 from H. capsulatum var. capsulatum using an indirect enzyme-linked immunosorbent assay (ELISA) that we developed previously (25, 33), with slight modifications. Briefly, plates were coated overnight at 4°C with 0.5 μg/well of purified rHsp60 (18) diluted in 50 μl of coating buffer (5.292 g/liter of NaHCO3, 6.6678 g/liter of Na2CO3, pH 9.6). Plates were washed three times with TBS-T (10 mM Tris-HCl [pH 7.2], 150 mM NaCl, 0.1% Tween 20) using an automated ELISA washer (BioTek Instruments, Winooski, VT). Plates were blocked with 2% bovine serum albumin (ICN Biomedicals Inc., Aurora, OH) in TBS-T (blocking solution) for 1 h at 37°C and washed three times with TBS-T. Mouse sera were serially diluted 1:2 in blocking solution in a 50-μl mixture and incubated for 1 h at 37°C. After subsequent washes, plates were incubated for 1 h at 37°C with a 1:1,000 dilution of alkaline phosphatase-conjugated goat anti-mouse IgG, IgA, and IgM (Southern Biotechnology Associates Inc., Birmingham, AL). After washing, antibody binding was detected by incubation with p-nitrophenyl phosphate (Sigma-Aldrich, St. Louis, MO) diluted in substrate buffer (50 mM Na2CO3 [pH 9.8], 1 mM MgCl2), and absorbance at 405 nm was measured using a microplate reader (μQuant microplate spectrophotometer; BioTek Instruments, Winooski, VT).
The mouse with the highest antibody titer to Hsp60 after the three injections of rHsp60 was boosted by intravenous injection of 15 μg of rHsp60 2 weeks after the last immunization, and the splenocytes were harvested to produce hybridomas as described previously (30). Hybridomas producing antibodies recognizing rHsp60 but negative with blocking solution were subjected to three rounds of soft agar cloning.
Characterization of MAbs.
The MAbs were purified using protein G agarose beads (Pierce Biotechnology) according to the manufacturer's instructions. The indirect rHsp60 ELISA was used to quantitatively evaluate MAb binding to Hsp60. MAbs were serially diluted 1:2, starting with a 25-μg/ml dilution in 50 μl of blocking buffer, and incubated for 1 h at 37°C. Individual MAbs were analyzed in triplicate. Binding was determined as described previously. The MAbs were also subjected to our whole-yeast-cell ELISA (33).
Binding of MAbs to Hsp60 was also assessed by immunoblotting, as described previously (35). After transfer, membranes containing the rHsp60 were blocked with a 5% skim milk solution in TBS-T. Membranes were cut and washed three times with TBS-T. The strips obtained were probed separately with 5 μg/ml of each MAb and an IgG control (Southern Biotechnology Associates Inc.) diluted in blocking solution for 1 h at 37°C. rHsp60 hyperimmune mouse serum was used as a positive control (28). Strips were washed three times with TBS-T and incubated with a 1:2,000 dilution of an alkaline phosphatase-conjugated goat anti-mouse IgG (Southern Biotechnology Associates Inc.) in blocking solution for 1 h at 37°C. After washing, the strips were developed with an ECL Western blotting substrate (Pierce Biotechnology) by following the manufacturer's directions.
Fluorescent and immunogold microscopy.
Since Hsp60 of H. capsulatum var. capsulatum is present on the yeast surface (28), an immunofluorescence analysis was performed by coincubating the MAbs with H. capsulatum var. capsulatum yeast cells obtained from the logarithmic growth phase in order to assess the pattern of MAb binding. Yeast cells grown for 72 h at 37°C were centrifuged at 1,100 × g for 10 min, and the pellet washed three times with PBS. The yeast cells were counted with a hemacytometer, and aliquots containing 106 yeast cells were centrifuged and suspended in SuperBlock (Pierce Chemical Co., Rockford, IL), incubated for 1 h at 37°C, and washed three times with PBS. The MAbs and an isotype-matched control were diluted to 10 μg/ml in SuperBlock and incubated with the yeast cells for 1 h at 37°C. The cells were washed and suspended in 100 μl of goat anti-mouse IgG conjugated with fluorescein isothiocyanate (FITC) (Southern Biotechnology Associates Inc.) at a 1:100 dilution in SuperBlock and then incubated for 1 h at 37°C. After three washes, cells were suspended in 50 μl of a mounting solution containing 0.01 M of N-propylgallate diluted in PBS-glycerol (1:1, vol/vol). Ten microliters of the suspension was applied to a microscope slide and examined with an Olympus AX70 fluorescence microscope (Olympus America Inc.) using a 495-nm filter and a magnification of ×1,000.
Immunogold transmission electron microscopy was used to evaluate the binding of MAbs to surface Hsp60. Sections of G217B yeast cells were prepared for microscopy as described previously (33). Grids were washed with water and incubated with SuperBlock overnight at 4°C. Grids were incubated with 5 μg/ml of MAb 12D3, an IgG isotype control, or PBS. Grids were washed four times for 5 min with 1% bovine serum albumin diluted in PBS. Immunogold-labeled (10 nm) goat anti-mouse IgG (Goldmark Biologicals, Phillipsburg, NJ) was diluted 1:100 in SuperBlock, added to the grids, and incubated for 2 h at room temperature. After washing, grids were fixed with a solution containing 2.5% glutaraldehyde in PBS for 5 min and washed three times with PBS. The grids were viewed with a JEOL (Tokyo, Japan) 100 CX transmission electron microscope.
Epitope mapping with MAbs to Hsp60.
To map the domains of rHsp60 recognized by the MAbs, several Hsp60 gene segments were cloned and expressed in E. coli as described above, including fragment 1 (amino acids [aa] 40 to 328), fragment 2 (aa 131 to 394), fragment 3 (aa 214 to 484), and fragment 4 (aa 373 to 590) (15). Hsp60-derived MAbs were tested for the capacity to bind to different fragments of Hsp60 by immunoblotting as described above. After initial mapping, we cloned and expressed more specific fragments, including fragments that consisted of aa 196 to 247, aa 297 to 326, aa 327 to 352, and aa 353 to 413. These regions were also identified on a hypothetical model obtained by structural homology with the GroEL protein (EMBL accession number DQ517526) using the SwissPDB viewer (22, 23). MAbs recognizing the same fragment were used in a competition experiment performed with the rHsp60 ELISA. For comparison of MAbs of different isotypes, a constant concentration of one MAb was incubated with increasing amounts of an MAb of a different isotype for 1 h at 37°C. After washing, an alkaline phosphatase-labeled antibody specific for the constant MAb (Southern Biotechnology Associates Inc.) was used, and absorbance at 405 nm was recorded after the reaction was developed with p-nitrophenyl phosphate. For competition ELISAs with MAbs of the same isotype, one MAb was biotinylated with an EZ-Link sulfo-N-hydroxysulfosuccinimide-biotin kit (Pierce Chemical Co.) used according to the instructions of the manufacturer. A constant concentration of the biotinylated MAb was incubated with various amounts of a different nonbiotinylated MAb for 1 h at 37°C. After washing, avidin conjugated with alkaline phosphatase (Sigma-Aldrich) was added, and the preparation was incubated for 1 h at 37°C. Binding of the biotinylated MAb was detected as described above.
Phagocytosis assays.
Phagocytosis assays were performed as described previously (31, 33). Briefly, macrophage-like J774.16 cells were grown in a medium containing 10% heat-inactivated fetal calf serum, 10% NCTC-109 medium (Sigma-Aldrich), 1% Pen-Strep, and 1% nonessential amino acids (Gibco BRL, Grand Island, NY). J774.16 cells were plated at a concentration of 105 cells per well in 96-well cell culture polystyrene plates and grown overnight at 37°C in the presence of 5% CO2. H. capsulatum var. capsulatum yeast cells were collected after 3 days of growth, washed three times with PBS, and heat inactivated. Yeast cells were labeled with 0.1 mg/ml Oregon Green 488 isothiocyanate (Molecular Probes Inc., Eugene, OR) for 30 min at 25°C and washed three times with PBS. The labeled yeast cells were incubated with MAbs, an IgG isotype control, or PBS for 1 h at 37°C. After washing, the yeast cells were added to the macrophages at a ratio of H. capsulatum var. capsulatum cells to macrophages of 2:1, and the plates were incubated for 2 h at 37°C in the presence of 5% CO2. Samples were prepared in triplicate. Quenching of the fluorescence of uningested organisms was performed by addition of trypan blue (1 mg/ml in PBS) and incubation for 15 min at 25°C. Wells were washed with PBS and fixed with a 40% methanol solution. The numbers of macrophages and yeast cells were recorded for each field, and at least 200 macrophages were counted. The phagocytosis index was defined as the ratio of the number of intracellular yeast cells to the number of macrophages counted (33). Additional phagocytosis experiments were performed using macrophages preincubated with anti-mouse CD11b (integrin α-M chain, Mac-1 α-chain, clone M1/70; Southern Biotechnology Associates Inc.) or Fc receptor blocking antibodies (rat anti-mouse CD16/32, clone 93; Southern Biotechnology Associates Inc.).
Macrophage effector functions.
The growth of intracellular yeast in the presence of MAbs was evaluated by preincubating H. capsulatum var. capsulatum yeast cells with MAbs to Hsp60, an isotype-matched control, or PBS prior to coculture with macrophages. Washed yeast cells were added to wells containing J774.16 macrophage-like cells at a ratio of yeast cells to macrophages of 2:1 and incubated for 24 h. The cultures were washed with cold PBS, and the macrophages were lysed by adding sterile water. Aliquots were plated onto brain heart infusion blood agar plates (50 ml/liter of sheep red blood cells, 10 g/liter glucose, 0.1 g/liter cysteine, 1% Pen-Strep) and incubated at 37°C. The percentage of growth was determined by comparison of the number of CFU for H. capsulatum var. capsulatum grown with macrophages and MAbs to the number of CFU for the yeast grown in medium alone.
Assay for phagolysosome formation.
To further explore whether the MAbs affected the intracellular fate of the fungus in macrophages, fusion of phagosomes and lysosomes was evaluated as described previously (37). Monolayers of J774.16 cells were incubated in fresh non-phenol-red medium with 0.5 mg/ml FITC-dextran for 4 h at 37°C in the presence of 5% CO2. Cells were washed three times with PBS and incubated overnight in medium alone. H. capsulatum var. capsulatum yeast cells were collected, washed, and incubated with 40 μg/ml N-hydroxysulfosuccinimide-rhodamine at 4°C for 1 h. The cells were washed and incubated with 100 μg/ml of each MAb. The cells were washed, suspended in Dulbecco modified Eagle medium, and added to the culture of J774.16 cells at a yeast cell/macrophage ratio of 5:1. The plates were then incubated for 1 h at 37°C in the presence of 5% CO2. Uningested yeast cells were removed by washing the preparation with PBS. The cells were fixed in 3.75% paraformaldehyde for 20 min at room temperature. Cells were observed by phase-contrast and fluorescence microscopy at a magnification of ×400. In J774.16 macrophages, the number of rhodamine-labeled H. capsulatum var. capsulatum yeast cells with colocalization of FITC-dextran and the total number of intracellular labeled yeast cells for each condition were counted to determine the percentage of phagosomes fused with lysosomes, which were characterized by red fluorescently labeled yeast cells surrounded by a green fluorescent dextran ring.
CFU and survival studies.
Two hours prior to infection, 6- to 8-week-old C57BL/6 mice were inoculated intraperitoneally with 250 or 500 μg of MAb to H. capsulatum var. capsulatum Hsp60, an IgG isotype-matched control, or PBS. All reagents were screened to ensure the absence of endotoxin with the Limulus amebocyte assay (BioWhittaker Inc., Walkersville, MD). For CFU studies, six mice per group were anesthetized and infected by intranasal injection of 5 × 106 H. capsulatum var. capsulatum yeast cells. Three animals per group were euthanized at days 7 and 14 postinfection. For each mouse, the left upper lobe of lung and pieces of the spleen and liver were fixed in a formalin solution and stained with hematoxylin and eosin for histological examination. The remaining lung, spleen, and liver tissues were weighed and homogenized separately in PBS. The organ homogenates were diluted and plated onto brain heart infusion blood agar and incubated at 37°C, and the numbers of CFU were determined.
Two methods were used for the survival studies. Mice were inoculated intraperitoneally with 250 or 500 μg of an MAb to H. capsulatum var. capsulatum rHsp60, an isotype-matched control MAb, or PBS, which was followed 2 h later by intranasal inoculation of 1.25 × 107 H. capsulatum var. capsulatum yeast cells. Alternatively, to maximize the interaction of the MAb and H. capsulatum var. capsulatum, mice were infected intranasally with 1.25 × 107 yeast cells that had been preincubated with 100 μg of either an MAb to H. capsulatum var. capsulatum rHsp60 or an isotype-matched control MAb, or they received an equivalent volume of PBS.
Cytokine determinations.
Protease inhibitors (Complete Mini; Boehringer Ingelhein Pharmaceuticals Inc., Ridgefield, CT) were added to lung, spleen, and liver homogenates, which were then centrifuged at 6,000 × g for 10 min to remove cell debris. The supernatants were collected and frozen at −80°C until they were assayed for cytokines. The supernatants were tested for IL-2, IL-4, IL-10, IL-12p70, IFN-γ, and TNF-β (Becton Dickinson Biosciences Pharmingen, San Diego, CA) according to the manufacturer's instructions.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego CA). Unless otherwise noted, a one-way analysis of variance using a Kruskall-Wallis nonparametrical test was used to compare the differences between groups, and individual comparisons of groups were done using a Bonferoni posttest. A 95% confidence interval was determined in all experiments. Survival results were analyzed by a Kaplan-Meyer test to determine the differences between groups. A t test was used to compare the number of CFU and cytokines for different groups.
RESULTS
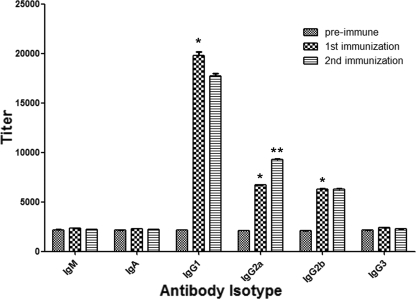
Immunization of mice with rHsp60 induces IgG isotype MAb production.
Immunization with rHsp60 successfully elicited the generation of IgG isotype MAbs (Fig. 1). In contrast, immunization did not induce production of increased concentrations of IgG3, IgA, or IgM isotypes. After fusion and screening of hybridomas for IgGs, the highest ELISA reactivity was found with two IgG1 (11D1 and 6B7), three IgG2a (4E12, 12D3, and 13B7), and one IgG2b (7B6) MAbs. To screen for binding to mammalian Hsp60, MAbs were used in a Western blot containing a macrophage extract, and no reactivity was observed (data not shown).
FIG. 1.
Serological responses to immunizations of mice with rHsp60 selected to generate hybridomas. The results were similar for second immunized mice. A comparison of the titers of preimmune serum and sera obtained 2 weeks after each immunization is shown. The bars are the averages of three independent ELISA experiments, and the error bars indicate standard deviations. *, P < 0.01 for comparisons of preimmune serum and serum obtained after the first immunization; **, P < 0.01 for a comparison of sera obtained after the first and second immunizations, calculated by analysis of variance and adjusted by using the Bonferroni correction.
Binding of MAbs to H. capsulatum var. capsulatum.
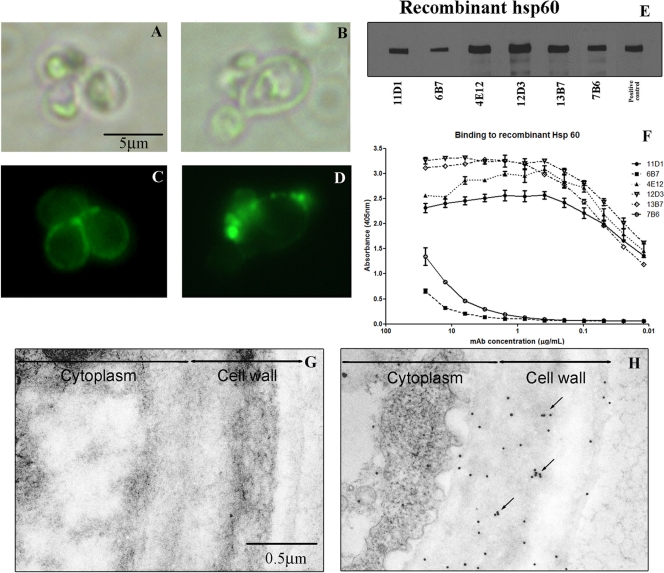
Immunofluorescence microscopy revealed that there were two patterns of MAb binding. MAbs 11D1, 6B7, 4E12, and 7B6 bound diffusely, with increased intensity at the bud necks of replicating yeast cells (Fig. 2A and C), whereas MAbs 12D3 and 13B7 displayed punctuate binding on the yeast cell surface (Fig. 2B and D). Immunogold labeling with MAb 12D3 also shows the distribution of the protein on the surface of H. capsulatum var. capsulatum cells (Fig. 2H), and the clustering of gold balls is consistent with the vesicular transport of Hsp60 (2). To confirm the specificity of the MAbs to Hsp60, yeast cell extract immunoblotting was performed. The MAbs reacted with a protein in the extract having a molecular weight equivalent to that of Hsp60 (Fig. 2E).
FIG. 2.
MAb-labeled Hsp60 on the cell surface of H. capsulatum var. capsulatum: immunofluorescence and bright-field microscopy showing labeling of H. capsulatum var. capsulatum by MAbs to Hsp60. (A and C) Representative images of diffuse binding with MAb 11D1. (B and D) Punctate binding with MAb 12D3. (E) Immunoblot showing binding of MAbs to rHsp60 protein at 60 kDa. rHsp60 hyperimmune mouse serum was used as a positive control. (F) Representative curves for MAb binding to rHsp60 of yeast cells as determined by indirect ELISA. (G and H) Representative immunogold-labeled transmission electron micrographs for control MAb (G) and MAb 12D3 (H), showing the distribution of Hsp60 on the cell wall and surface of the yeast. Clusters of gold balls labeling Hsp60 consistent with vesicular transport are indicated by arrows.
In addition, an indirect ELISA was used to quantitatively evaluate MAb binding to Hsp60. There were variations in binding by the different MAb isotypes, and the relative order of reactivity was IgG2a > IgG1 > IgG2b (Fig. 2F). Although there were differences in binding of IgG2a subclass MAbs to Hsp60, the variations were relatively small and could potentially be explained by differences in affinity because of recognition of different epitopes. However, significantly larger differences in Hsp60 binding were detected with the IgG1 subclass MAbs. As shown below, the MAbs with the lowest reactivity interact with the same epitope region. There were no significant differences in the reactivities of the MAbs to rHsp60 and heat-killed yeast cells (data not shown) as determined by ELISA, suggesting that the MAbs recognize epitopes exposed on the native structure of Hsp60 on the yeast cell surface.
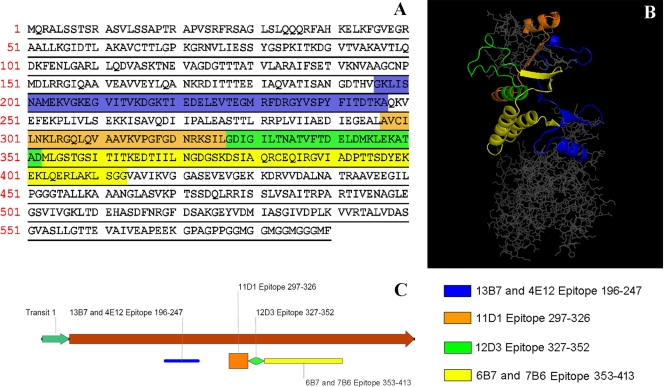
Epitope mapping of MAbs against Hsp60.
Fragments of Hsp60 of H. capsulatum var. capsulatum were synthesized and used to map the binding sites of the MAbs (Fig. 3A and B). For IgG2a isotypes, MAb 12D3 recognized epitopes in the polypeptide from aa 327 to 352, while MAbs 4E12 and 13B7 recognized epitopes in the sequence from aa 196 to 247. Competition ELISAs were performed with MAbs 4E12 and 13B7, and no competition was observed (data not shown), suggesting that these MAbs recognized different epitopes in the mapped sequence. MAb 11D1 (IgG1) recognized an epitope in the sequence from aa 297 to 326 (Fig. 3C). The binding regions of MAbs 6B7 (IgG1) and 7B6 (IgG2b) were mapped between aa 353 to 413, and these two MAbs interacted competitively, as determined by a competition ELISA (data not shown).
FIG. 3.
Representation of H. capsulatum var. capsulatum Hsp60 with associated binding sites for the IgG isotype MAbs. (A) Sequence showing the mapped peptide residues. The different colors correspond to the different binding regions. (B) Localization of the epitopes in the three-dimensional structure of H. capsulatum var. capsulatum Hsp60 generated by homology modeling using GroEL as a template. Binding sites of MAbs 11D1 (orange), 6B7 (yellow), and 7B6 (yellow) are localized in the protein cleft, whereas the recognition sites of the other MAbs, MAbs 4E12 (blue), 13B7 (blue), and 12D3 (green), are on the external surface of the protein. (C) Epitope distribution in the linear model.
MAbs to H. capsulatum var. capsulatum Hsp60 alter effector functions of macrophages in vitro.
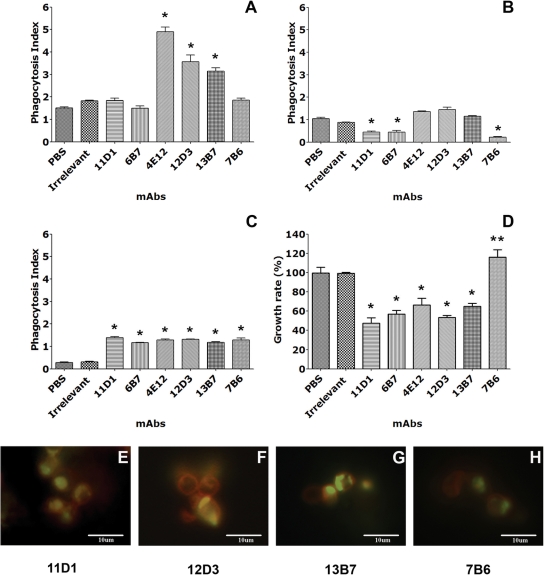
We analyzed the effects of H. capsulatum var. capsulatum Hsp60-specific MAbs on phagocytosis by J774.16 macrophage-like cells. IgG2a MAbs 4E12, 12D3, and 13B7 significantly enhanced phagocytosis of yeast cells compared to PBS and an isotype-matched IgG control MAb (P < 0.05) (Fig. 4A). The IgG1 (11D1 and 6B7) and IgG2b (7B6) MAbs did not alter the overall level of phagocytosis (P > 0.05).
FIG. 4.
MAbs can modify the intracellular fate of H. capsulatum var. capsulatum. (A) Effect of MAbs to Hsp60 on H. capsulatum var. capsulatum phagocytosis by J774.16 cells. (B) Blockage of Fc receptors reduced phagocytosis. (C) Blockage of the CD11 receptor decreased phagocytosis in control groups and in the IgG2a groups, although phagocytosis was significantly greater in all the MAb groups than in the controls. (D) Effect of MAbs to Hsp60 on H. capsulatum var. capsulatum killing by J774.16 cells. In panels A to D, the values are the averages of three independent experiments, and the error bars indicate standard deviations. Each experiment was done in quadruplicate. *, P < 0.001 for comparisons between MAbs and controls; **, P < 0.001 for a comparison between MAb 7B6 and the other MAbs to Hsp60. (E to H) Colocalization of H. capsulatum var. capsulatum yeast cells in phagosomes as determined using the FITC-dextran contents of lysosomes. MAbs 11D1 (E), 12D3 (F), and 13B7 (G) induced phagolysosomal fusion within J774.16 cells. In contrast, the rate of fusion with MAb 7B6 (H) was much lower.
To investigate the dependence of phagocytosis on Fc and CR3 receptors pathways, specific MAbs were utilized to block the interaction of these pathways with Hsp60 MAbs. Blockage of Fc and CD11b receptors using specific MAbs resulted in reductions in the phagocytosis of H. capsulatum var. capsulatum opsonized by IgG2a MAbs 4E12, 12D3, and 13B7 to levels similar to control levels (Fig. 4B). With Fc receptor blockage, phagocytosis in the presence of IgG1 and IgG3b isotype MAbs was reduced compared to the phagocytosis observed for controls (Fig. 4B). In contrast, blockage of CD11b resulted in similar rates of phagocytosis for the different isotypes of MAbs to Hsp60, although the rates were higher than those for the controls (Fig. 4C).
The effect of H. capsulatum var. capsulatum opsonization with MAbs to Hsp60 on fungal intracellular survival in macrophages was assessed by using killing assays. The IgG1 and IgG2a MAbs significantly enhanced killing by macrophages (50 to 70%) compared to PBS or an isotype-matched control MAb (Fig. 4D). Conversely, IgG2b MAb 7B6 reduced the ability of J774.16 cells to kill the fungus, resulting in a significantly increased number of CFU compared to the controls.
We used immunofluorescence microscopy to detect colocalization of FITC-dextran with H. capsulatum var. capsulatum yeast cells within phagosomes to quantify phagolysosomal fusion within macrophages. Phagolysosomal fusion occurred with significantly greater frequency in macrophages containing H. capsulatum var. capsulatum opsonized with IgG1 (range, 69 to 100%) and IgG2a (range, 77 to 100%) MAbs (Fig. 4E, F, and G). The ranges were generated from the results of duplicate assays that were repeated on two separate occasions. In contrast, IgG2b MAb 7B6 (range, 0 to 40%) showed minimal colocalization in the majority of macrophages evaluated (Fig. 4H). Furthermore, minimal phagolysosomal fusion occurred with medium alone (range, 0 to 5%) and an irrelevant control MAb (range, 0 to 11%) (data not shown).
Survival studies using MAbs to Hsp60.
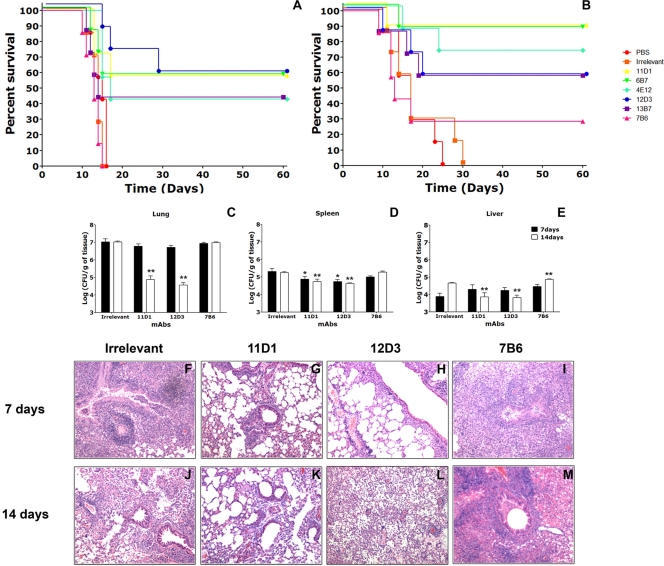
To determine the effect of Hsp60-binding MAbs in histoplasmosis, groups of Hsp60-specific MAb-inoculated and control C57BL/6 mice were infected with 1.25 × 107 H. capsulatum var. capsulatum cells (lethal challenge), and disease and survival were monitored. Mice treated with 500 μg of the IgG1 or IgG2a MAbs had significantly prolonged survival compared to control mice receiving either an irrelevant MAb or PBS (Fig. 5A) (P < 0.05). Mice inoculated with MAb 11D1, 6B7, or 12D3 had a survival rate of 60%. Mice treated with MAb 13B7 or 4E12 had a survival rate of 40%. Surviving mice were euthanized at 60 days postinfection, and their organs were processed for CFU analysis. At 60 days postinfection, no detectable yeast cells were found in the lungs, livers, or spleens. In contrast, MAb 7B6, an IgG2b isotype MAb, was not protective. In fact, mice that received MAb 7B6 started to die 1 to 2 days before the controls started to die, but no statistical significance was observed in a comparison with controls. Survival experiments were also performed with mice inoculated with 250 μg of the Hsp60-binding MAbs, but, with the exception of IgG2a MAb 13B7 (P < 0.05), these MAbs were not protective (data not shown).
FIG. 5.
MAbs to Hsp60 affect the pathogenesis of histoplasmosis. (A) Intraperitoneal injection of 500 μg of IgG1 (11D1 and 6B7) or IgG2a (4E12, 12D3, and 13B7) MAbs 2 h prior to infection significantly prolonged survival (P < 0.05 for a comparison to controls), but injection of IgG2b MAb 7B6 did not prolong survival. (B) IgG1 and IgG2a MAbs were protective when they were preincubated with H. capsulatum var. capsulatum yeast cells prior to infection (P < 0.05), and the IgG2b MAb was not protective. Each panel shows survival results representative of three similar experiments. (C to E) Numbers of CFU in (C) lungs, (D) spleens, and (E) livers at 7 and 14 days after sublethal intranasal challenge with 5 × 106 H. capsulatum var. capsulatum yeast cells for mice treated intraperitoneally with selected MAbs to Hsp60 or an irrelevant MAb. *, P < 0.001 at 7 days postinfection; **, P < 0.001 at 14 days postinfection. (F to M) IgG1 and IgG2a MAbs to Hsp60 decrease inflammation and the fungal burden in tissues. (F to I) Lung sections obtained after 7 days of infection; (J to M) lung sections obtained after 14 days of infection. The lungs of mice treated with protective MAbs 11D1 and 12D3 exhibited peribronchiolar inflammation at day 7 (G and H, respectively) and resolving inflammation at day 14 (K and L, respectively). In contrast, sections of lungs from the control group and MAb 7B6-treated animals exhibited diffuse, dense inflammation with increased tissue infiltration and early granuloma formation at 7 days (I). Although in the lungs of control mice there was evidence of reduced inflammation at day 14 (J), mice treated with MAb 7B6 had progressive necrotizing pneumonia (M).
In experiments testing coincubation of H. capsulatum var. capsulatum yeast cells with MAbs prior to infection in order to maximize opsonization, we found that the average survival time was further extended with the IgG1 and IgG2a MAbs compared to the survival of the control groups (P < 0.05) (Fig. 5B). The IgG1 MAbs were most effective, resulting in a survival rate of 90%. For IgG2a MAbs, MAb 4E12 resulted in a survival rate of 75%, while 60% of mice treated with MAbs 12D3 and 13B7 survived. In contrast, only 30% of mice given MAb 7B6 survived, which was not significantly different from the control value. Again, MAb 7B6-treated mice started to die before control animals did.
Fungal burden.
To further study MAb-mediated protection, we examined lung, spleen, and liver fungal burdens at 7 and 14 days after infection using selected MAbs of each isotype. In mice that received MAb 11D1 or 12D3 there was a significant reduction in the number of pulmonary and splenic CFU compared with the number in mice given an isotype-matched MAb at day 7 (P < 0.05) (Fig. 5C and D), whereas the results for the group that received MAb 7B6 were not significantly different from the results for the control group (P > 0.05). However, there were no significant differences among liver fungal burdens (P > 0.05) (Fig. 5C and E). At day 14, the numbers of CFU in the lungs of mice treated with MAb 11D1 and in the lungs of mice treated with MAb 12D3 showed decreases of 2 and 2.5 log units, respectively, compared to the control group (P < 0.05) (Fig. 5C). The value for the MAb 7B6-treated group was similar to the value for the control group (P > 0.05). The reductions in the numbers of CFU in spleens were even greater at day 14 for animals treated with MAbs 11D1 and 12D3 compared to the controls, whereas the splenic fungal burden did not change in mice treated with MAb 7B6 (Fig. 5D). In liver at day 14, there was a significant reduction in the number of CFU in mice treated with MAb 11D1 or 12D3 (Fig. 5E). In contrast, the fungal burden increased in control and MAb 7B6-treated animals. Hence, MAbs that prolonged survival also reduced the fungal burden, whereas mice treated with the nonprotective MAb 7B6 contained numbers of CFU similar to or higher than the numbers of CFU in the controls.
Less inflammation was present in organs of infected animals treated with protective MAb 11D1 or 12D3. (i) Lungs.
MAb 11D1- or MAb 12D3-treated mice had significantly less inflammation than control mice or animals that received MAb 7B6 (Fig. 5F to M). At day 7, control mice had dense, diffuse pneumonias involving 40 to 60% of the lung. Numerous granulomas were observed, and inflammation extended from the bronchioles to the lung parenchyma, with intraalveolar airspace congestion (Fig. 5F). There was some improvement at day 14 (Fig. 5J). The lungs of mice treated with MAb 7B6 (Fig. 5I) displayed severe pneumonitis with destruction of most of the pulmonary architecture. Cellular infiltration was increased compared to that in control mice and consisted predominantly of mononuclear phagocytes and neutrophils. Lymphocyte infiltration extended throughout the tissue architecture, affecting 60 to 75% of the organ by day 7 and nearly the entire organ by day 14 (Fig. 5M). In contrast, the lungs of MAb 11D1- or MAb 12D3-treated mice had significantly less inflammation than the lungs of control mice at both day 7 (Fig. 5G and H) and day 14 (Fig. 5K and L), as well as more organized granulomas.
(ii) Spleens.
Histopathological examination of infected mice given MAbs or an isotype control MAb revealed more severe pathology in the control group and in mice that received MAb 7B6. Isotype control MAb-treated mice had massive infiltration of macrophages in the red pulp of the spleen and marginal zones. The granulomatous lesions within spleens of mice given MAb 7B6 were even more pronounced, with more extensive disruption and less organization. In mice given MAb 11D1 or 12D3, the red pulps were more organized, without infiltration by inflammatory cells or granulomas. The white pulps had intact B follicles (see Fig. S1 in the supplemental material).
(iii) Livers.
Histological examination of livers from control infected mice showed that there was mild cellular inflammation, with macrophage extravasation, focal aggregation of leukocytes, mild vasculitis, and some aggregates of granulomatous inflammatory cells at day 7 (see Fig. S2 in the supplemental material). Granuloma formation persisted to day 14, but it was less prominent. Mice treated with MAb 7B6 had more intense cellular infiltration and more granulomas that were also larger at days 7 and 14 compared to controls. Livers of animals treated with MAb 11D1 or 12D3 had few granulomas, less macrophage infiltration, and conserved tissue architecture at day 7. Minimal alterations were present at day 14.
Cytokine production.
Cytokine levels were measured in mice infected with H. capsulatum var. capsulatum and treated with an isotype control MAb or with selected MAbs to Hsp60 (Table 1). Evaluations of pulmonary, splenic, and hepatic cytokine levels showed that there were organ-specific differences, especially for IL-12, TNF-α, IL-4, and IL-10. The nonprotective MAb 7B6-treated and infected mice had significantly lower lung levels of IFN-γ and IL-4 after 7 days of infection than control animals. The spleens of MAb 7B6-treated mice had lower levels of IL-12 and IL-4 and higher levels of TNF-β. After 14 days of infection, the IFN-γ and IL-10 levels were increased in the spleens and livers. Also, the TNF-β levels were increased and the IL-4 levels were decreased in the lungs and spleens of infected animals.
TABLE 1.
Cytokine levels in organs of treated animals
| MAb | Organ | Cytokine levels (pg/mg) (avg ± SD)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-2
|
IL-12
|
IFN-γ
|
TNF-β
|
IL-4
|
IL-10
|
||||||||
| 7 days | 14 days | 7 days | 14 days | 7 days | 14 days | 7 days | 14 days | 7 days | 14 days | 7 days | 14 days | ||
| IgG control | Lung | 12.42 ± 3.26 | 10.88 ± 1.66 | 6.56 ± 2.42 | 26.96 ± 7.36 | 18.04 ± 1.05 | 3.28 ± 0.75 | 8.17 ± 3.55 | 0.62 ± 0.36 | 2.26 ± 0.081 | 1.03 ± 0.32 | 0.93 ± 0.19 | 0.64 ± 0.093 |
| Spleen | 3.56 ± 1.65 | 14.38 ± 1.99 | 5.56 ± 2.56 | 31.40 ± 15.14 | 2.18 ± 0.78 | 1.01 ± 0.37 | NDa | 0.10 ± 0 | 2.82 ± 0.68 | 0.65 ± 0.072 | 2.87 ± 0.88 | 0.89 ± 0.38 | |
| Liver | 0.26 ± 0.12 | 0.35 ± 0.20 | 107 ± 16.17 | 45.93 ± 13.87 | 0.86 ± 0.22 | 0.66 ± 0.062 | 481 ± 188 | 1.46 ± 0.18 | 3.04 ± 1.11 | 0.84 ± 0.047 | 0.26 ± 0.054 | 0.62 ± 0.13 | |
| 11D1 | Lung | 16.78 ± 2.07 | 14.00 ± 0.88 | 8.21 ± 6.62 | 6.16 ± 3.58 | 20.65 ± 3.04 | 4.20 ± 0.13 | 2.26 ± 0.79 | 0.46 ± 0.19 | 0.89 ± 0.18b | 0.345 ± 0.018 | 0.80 ± 0.52 | 0.76 ± 0.25 |
| Spleen | 12.28 ± 3.08 | 15.66 ± 2.03 | 36.06 ± 18.22c | 75.26 ± 37.65 | 3.45 ± 0.66 | 4.70 ± 1.77 | 1.52 ± 0c | 0.13 ± 0.12 | 0.65 ± 0.12b | 0.28 ± 0.17 | 1.08 ± 0.26 | 1.94 ± 0.69 | |
| Liver | 1.71 ± 0.44c | 1.13 ± 0.31c | 125.3 ± 35.71 | 108.4 ± 41.63c | 1.33 ± 0.20 | 0.93 ± 0.33 | 367 ± 94 | 16.00 ± 3.22c | 2.14 ± 0.48 | 0.85 ± 0.047 | 0.70 ± 0.41 | 0.50 ± 0.13 | |
| 12D3 | Lung | 15.44 ± 2.06 | 11.70 ± 5.41 | 37.19 ± 21.17c | 8.13 ± 7.13 | 19.04 ± 2.31 | 3.17 ± 0.66 | 37.83 ± 2.85c | 0.35 ± 0.27 | 1.00 ± 0.12b | 0.13 ± 0.048 | 0.51 ± 0.21 | 0.34 ± 0.055 |
| Spleen | 14.85 ± 5.07 | 17.12 ± 4.13 | 48.29 ± 25.65c | 57.79 ± 34.43 | 4.23 ± 0.63 | 3.12 ± 0.62c | 1.07 ± 0.38c | 0.18 ± 0.17 | 0.94 ± 0.28 | 0.63 ± 0.38 | 2.87 ± 0.41 | 2.51 ± 0.27c | |
| Liver | 1.43 ± 0.61c | 0.64 ± 0.48 | 97.77 ± 38.59 | 120 ± 88.01c | 1.10 ± 0.20 | 2.22 ± 0.53c | 223 ± 78.77 | 3.86 ± 1.37 | 2.09 ± 0.068 | 0.87 ± 0.29 | 0.59 ± 0.17 | 0.61 ± 0.34 | |
| 7B6 | Lung | 12.68 ± 4.12 | 8.44 ± 1.89 | 4.4 ± 1.49 | 26.54 ± 7.83 | 13.28 ± 3.34b | 3.17 ± 1.06 | 45.65 ± 14.88 | 2.66 ± 1.59c | 0.89 ± 0.22b | 0.10 ± 0.026b | 0.52 ± 0.089 | 0.65 ± 0.18 |
| Spleen | 13.62 ± 10.41 | 27.87 ± 4.66 | 2.22 ± 1.94b | 66.78 ± 46.44 | 3.78 ± 0.69 | 4.98 ± 0.40c | 1.15 ± 0c | 0.34 ± 0.075c | 0.60 ± 0.35b | 0.31 ± 0.024b | 3.74 ± 0.60 | 8.54 ± 1.46c | |
| Liver | 0.68 ± 0.43 | 0.17 ± 4.66 | 19.41 ± 4.77 | 60.36 ± 42.99 | 0.87 ± 0.21 | 2.34 ± 0.56c | 310 ± 129 | 10.94 ± 2.62c | 0.92 ± 0.17 | 0.55 ± 0.097 | 0.48 ± 0.13 | 1.47 ± 0.26c | |
ND, not determined.
Value significantly less than the value for control mice (P < 0.05).
Value significantly greater than the value for control mice (P < 0.05).
Notably, for mice treated with the two protective MAbs, an IgG1 MAb and an IgG2a MAb, the patterns of cytokine expression were similar. However, some differences in cytokine levels were observed for these two MAbs. Infected mice treated with MAb 12D3 had increased pulmonary and splenic IL-12 and TNF-β levels at day 7, which returned to basal levels by day 14. The pulmonary IL-4 levels were also reduced. After 14 days of infection, the splenic IFN-γ and IL-10 levels were increased. In livers, the IL-2 levels were higher at day 7 and decreased to basal levels at day 14. The IL-12 and IFN-γ liver levels were higher at day 14. MAb 11D1-treated mice had increased levels of IL-12 and TNF-β in their spleens by day 7 after infection. The IL-4 levels were reduced in the lungs and spleens of these animals after 7 days. For the livers there were increases in the IL-2, IL-12, and TNF-β levels only after 14 days of infection.
DISCUSSION
MAbs to H. capsulatum var. capsulatum Hsp60 can modify the pathogenesis of murine histoplasmosis, and their efficacy appears to correspond with both the Ig subclass and the epitope. The importance of IgG antibodies has been described for other systemic mycoses. For instance, in C. neoformans murine models of infection, the protective efficacy of IgG MAbs was a function of Ig dose and fungal inoculum. Prozone-like effects were observed with high doses of MAbs, and IgG1 and IgG2a MAbs prolonged survival only when they were administered before infection with large inocula (38). We previously showed that IgM MAbs to an H2B-like protein located on the surface of the H. capsulatum var. capsulatum yeast cells could mediate protection in histoplasmosis (32, 33). These MAbs increased phagocytosis and killing of H. capsulatum var. capsulatum yeast cells by macrophages, reduced pulmonary inflammation, and prolonged the survival of lethally infected animals. Lower concentrations of MAbs to H2B also acted synergistically with amphotericin B in protection against a lethal infection. However, these MAbs were most effective when they were preincubated with yeast cells prior to infection, due to the poor pulmonary distribution of intraperitoneally injected IgMs.
Our current work describes the generation and use of IgG MAbs against surface immunodominant Hsp60 of H. capsulatum var. capsulatum. Hsp60 is one of the most-characterized molecules on the surface of H. capsulatum var. capsulatum and interacts specifically with CD11b/CD18 (CR3) on macrophages, facilitating the uptake of yeast cells by these phagocytes (28). Although we previously showed that IgM MAbs to H. capsulatum var. capsulatum H2B facilitated phagocytosis via CR3 (32, 33), H. capsulatum var. capsulatum-specific IgG opsonizing MAbs further enable macrophages to engage the fungus via IgG Fc receptors. Interestingly, the IgG1 and the IgG2b MAbs mapped to residues within the chaperonin structural cleft on Hsp60 that are associated with H. capsulatum var. capsulatum's interaction with macrophage CR3 (26). Phagocytosis results showed that these MAbs could interfere with the interaction of Hsp60 and CR3, driving phagocytosis via Fc receptor-mediated processes. IgG1 and IgG2a protective MAbs significantly enhanced phagosomal maturation, which may have facilitated the killing of intracellular yeasts with MAb. Enhanced killing and processing of H. capsulatum var. capsulatum antigen can result in increased T-cell activation (37). Interestingly, IgG2b MAb 7B6 did not enhance phagolysosomal formation, and the intracellular growth of H. capsulatum var. capsulatum in the presence of this MAb was greater than the growth in the controls.
IgG1 and IgG2a MAbs to H. capsulatum var. capsulatum Hsp60 prolonged the survival of mice, reduced the organ fungal burden, and reduced inflammation in part by polarizing toward a host Th-1 response. In contrast, mice that received MAb 7B6 had increased tissue inflammation and increased tissue levels of IL-4 and IL-10, corresponding to inhibition of cellular responses necessary for clearance of the infection. There were no changes in the fungal burdens in the lungs of animals in this group, but higher fungal burdens were observed in the spleens and livers of these animals at 14 days after infection. Control of histoplasmosis depends largely on the presence of activated T-helper CD4 lymphocytes and induction of cellular immune responses (7, 14, 27). In our studies, MAbs that were protective induced higher levels of IL-2 and IL-12 and a trend toward higher levels of IFN-γ by day 7, all of which subsequently decreased as the disease resolved. These MAbs also reduced the levels of IL-4 and IL-10, which correlates with previous findings that decreases in IL-4 and IL-10 levels, along with increases in IFN-γ and TNF-α levels, are essential for controlling infection (12, 34). The increased number of CFU and pronounced Th-2 cytokine expression are consistent with the accelerated deaths of animals compared to controls observed in survival experiments.
Bronchoalveolar macrophages and an early inflammatory response in the lungs are the first line of defense against natural infection by H. capsulatum var. capsulatum. Activated bronchoalveolar macrophages and neutrophils can kill H. capsulatum var. capsulatum by mechanisms dependent on hydrogen peroxide and products of the nitric oxide synthase pathway, whereas fungistasis depends largely on products of the nitric oxide synthase pathway (6). Different MAbs can change the skew in the subpopulations of cells that are infected and the distribution of the fungus, as well as the induction of different effector cell populations, resulting in different outcomes of inflammation and reduction of the fungal burden in infected organs (13). Hence, despite their protective effect, the IgG1 and IgG2a MAbs did not prevent disseminated disease but modified the host response to the fungus in pleotropic ways.
MAbs to cell surface antigens of pathogens can modify the complex dynamics that occur during the interplay between a host and a pathogen. Our data suggest that MAbs to Hsp60 alone or in combination with other MAbs to cell surface antigens could improve the treatment of patients with histoplasmosis. Furthermore, MAbs could be administered prophylactically in outbreaks, especially to high-risk individuals, such as individuals with human immunodeficiency virus infection or patients receiving TNF-α inhibitors.
Supplementary Material
Acknowledgments
A.J.G. was supported in part by an Interhemispheric Research Training Grant in Infectious Diseases, Fogarty International Center (grant NIH D43-TW007129). A.J.G. and J.D.N. were supported in part by grants NIH AI52733 and AI056070-01A2 and by the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (grant NIH AI-51519). R.M.Z.O. was supported in part by CNPq grant 306288/2006-0.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 29 January 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Ajello, L. 1971. Coccidioidomycosis and histoplasmosis. A review of their epidemiology and geographical distribution. Mycopathol. Mycol. Appl. 45221-230. [DOI] [PubMed] [Google Scholar]
- 2.Albuquerque, P. C., E. S. Nakayasu, M. L. Rodrigues, S. Frases, A. Casadevall, R. M. Zancope-Oliveira, I. C. Almeida, and J. D. Nosanchuk. 2008. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell. Microbiol. 101695-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, H. L., and G. S. Deepe, Jr. 2006. B cells and CD4−CD8− T cells are key regulators of the severity of reactivation histoplasmosis. J. Immunol. 1771763-1771. [DOI] [PubMed] [Google Scholar]
- 4.Allendoerfer, R., G. P. Biovin, and G. S. Deepe, Jr. 1997. Modulation of immune responses in murine pulmonary histoplasmosis. J. Infect. Dis. 175905-914. [DOI] [PubMed] [Google Scholar]
- 5.Allendorfer, R., G. D. Brunner, and G. S. Deepe, Jr. 1999. Complex requirements for nascent and memory immunity in pulmonary histoplasmosis. J. Immunol. 1627389-7396. [PubMed] [Google Scholar]
- 6.Brummer, E., and D. A. Stevens. 1995. Antifungal mechanisms of activated murine bronchoalveolar or peritoneal macrophages for Histoplasma capsulatum. Clin. Exp. Immunol. 10265-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cain, J. A., and G. S. Deepe, Jr. 1998. Evolution of the primary immune response to Histoplasma capsulatum in murine lung. Infect. Immun. 661473-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadevall, A. 1998. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 6102-107. [DOI] [PubMed] [Google Scholar]
- 9.Casadevall, A., J. Mukherjee, S. J. Devi, R. Schneerson, J. B. Robbins, and M. D. Scharff. 1992. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J. Infect. Dis. 1651086-1093. [DOI] [PubMed] [Google Scholar]
- 10.Deepe, G. S., Jr. 2005. Modulation of infection with Histoplasma capsulatum by inhibition of tumor necrosis factor-alpha activity. Clin. Infect. Dis. 41(Suppl. 3)S204-S207. [DOI] [PubMed] [Google Scholar]
- 11.Deepe, G. S., Jr. 2007. Tumor necrosis factor-alpha and host resistance to the pathogenic fungus, Histoplasma capsulatum. J. Investig. Dermatol. Symp. Proc. 1234-37. [DOI] [PubMed] [Google Scholar]
- 12.Deepe, G. S., Jr., and R. S. Gibbons. 2003. Protective and memory immunity to Histoplasma capsulatum in the absence of IL-10. J. Immunol. 1715353-5362. [DOI] [PubMed] [Google Scholar]
- 13.Deepe, G. S., Jr., R. S. Gibbons, and A. G. Smulian. 2008. Histoplasma capsulatum manifests preferential invasion of phagocytic subpopulations in murine lungs. J. Leukoc. Biol. 84669-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deepe, G. S., Jr., and R. S. Gibbons. 2002. Cellular and molecular regulation of vaccination with heat shock protein 60 from Histoplasma capsulatum. Infect. Immun. 703759-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deepe, G. S. J., R. Gibbons, G. D. Brunner, and F. J. Gómez. 1996. A protective domain of heat-shock protein 60 from Histoplasma capsulatum. J. Infect. Dis. 174828-834. [DOI] [PubMed] [Google Scholar]
- 16.Dromer, F., J. Charreire, A. Contrepois, C. Carbon, and P. Yeni. 1987. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect. Immun. 55749-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durkin, M. M., P. A. Connolly, and L. J. Wheat. 1997. Comparison of radioimmunoassay and enzyme-linked immunoassay methods for detection of Histoplasma capsulatum var. capsulatum antigen. J. Clin. Microbiol. 352252-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez, F. J., R. Allendoerfer, and G. S. Deepe, Jr. 1995. Vaccination with recombinant heat-shock protein 60 from Histoplasma capsulatum. Infect. Immun. 632587-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez, F. J., A. M. Gomez, and G. S. Deepe, Jr. 1991. Protective efficacy of a 62-kilodalton antigen, HIS-62, from the cell wall and cell membrane of Histoplasma capsulatum yeast cells. Infect. Immun. 594459-4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin, R. A., Jr., and R. M. Des Prez. 1978. State of the art: histoplasmosis. Am. Rev. Respir. Dis. 117929-956. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin, R. A., J. E. Loyd, and R. M. Des Prez. 1981. Histoplasmosis in normal hosts. Medicine (Baltimore) 60231-266. [DOI] [PubMed] [Google Scholar]
- 22.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 182714-2723. [DOI] [PubMed] [Google Scholar]
- 23.Guimaraes, A. J., A. J. Hamilton, H. L. De Matos Guedes, J. D. Nosanchuk, and R. M. Zancope-Oliveira. 2008. Biological function and molecular mapping of M antigen in yeast phase of Histoplasma capsulatum. PLoS ONE 3e3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guimarães, A. J., J. D. Nosanchuk, and R. M. Zancope-Oliveira. 2006. Diagnosis of histoplasmosis. Braz. J. Microbiol. 371-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guimarães, A. J., C. V. Pizzini, H. L. De Matos Guedes, P. C. Albuquerque, J. M. Peralta, A. J. Hamilton, and R. M. Zancope-Oliveira. 2004. ELISA for early diagnosis of histoplasmosis. J. Med. Microbiol. 53509-514. [DOI] [PubMed] [Google Scholar]
- 26.Habich, C., K. Kempe, F. J. Gomez, M. Lillicrap, H. Gaston, R. van der Zee, H. Kolb, and V. Burkart. 2006. Heat shock protein 60: identification of specific epitopes for binding to primary macrophages. FEBS Lett. 580115-120. [DOI] [PubMed] [Google Scholar]
- 27.Lazar-Molnar, E., A. Gacser, G. J. Freeman, S. C. Almo, S. G. Nathenson, and J. D. Nosanchuk. 2008. The PD-1/PD-L costimulatory pathway critically affects host resistance to the pathogenic fungus Histoplasma capsulatum. Proc. Natl. Acad. Sci. USA 1052658-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long, K. H., F. J. Gomez, R. E. Morris, and S. L. Newman. 2003. Identification of heat shock protein 60 as the ligand on Histoplasma capsulatum that mediates binding to CD18 receptors on human macrophages. J. Immunol. 170487-494. [DOI] [PubMed] [Google Scholar]
- 29.Louria, D. B., and T. Kaminski. 1965. Passively-acquired immunity in experimental cryptococcosis. Sabouraudia 480-84. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 604534-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman, S. L., C. Bucher, J. Rhodes, and W. E. Bullock. 1990. Phagocytosis of Histoplasma capsulatum yeasts and microconidia by human cultured macrophages and alveolar macrophages. Cellular cytoskeleton requirement for attachment and ingestion. J. Clin. Investig. 85223-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nosanchuk, J. D. 2005. Protective antibodies and endemic dimorphic fungi. Curr. Mol. Med. 5435-442. [DOI] [PubMed] [Google Scholar]
- 33.Nosanchuk, J. D., J. N. Steenbergen, L. Shi, G. S. J. Deepe, and A. Casadevall. 2003. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J. Clin. Investig. 1121164-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng, J. K., J. S. Lin, J. T. Kung, F. D. Finkelman, and B. A. Wu-Hsieh. 2005. The combined effect of IL-4 and IL-10 suppresses the generation of, but does not change the polarity of, type-1 T cells in Histoplasma infection. Int. Immunol. 17193-205. [DOI] [PubMed] [Google Scholar]
- 35.Pizzini, C. V., R. M. Zancope-Oliveira, E. Reiss, R. Hajjeh, L. Kaufman, and J. M. Peralta. 1999. Evaluation of a Western blot test in an outbreak of acute pulmonary histoplasmosis. Clin. Diagn. Lab. Immunol. 620-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sa-Nunes, A., A. I. Medeiros, R. Nicolete, F. G. Frantz, A. Panunto-Castelo, C. L. Silva, and L. H. Faccioli. 2005. Efficacy of cell-free antigens in evaluating cell immunity and inducing protection in a murine model of histoplasmosis. Microbes Infect. 7584-592. [DOI] [PubMed] [Google Scholar]
- 37.Shi, L., P. C. Albuquerque, E. Lazar-Molnar, X. Wang, L. Santambrogio, A. Gacser, and J. D. Nosanchuk. 2008. A monoclonal antibody to Histoplasma capsulatum alters the intracellular fate of the fungus in murine macrophages. Eukaryot. Cell 71109-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taborda, C. P., J. Rivera, O. Zaragoza, and A. Casadevall. 2003. More is not necessarily better: prozone-like effects in passive immunization with IgG. J. Immunol. 1703621-3630. [DOI] [PubMed] [Google Scholar]
- 39.Tewari, R. P., D. Sharma, M. Solotorovsky, R. Lafemina, and J. Balint. 1977. Adoptive transfer of immunity from mice immunized with ribosomes or live yeast cells of Histoplasma capsulatum. Infect. Immun. 15789-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wheat, L. J., and C. A. Kauffman. 2003. Histoplasmosis. Infect. Dis. Clin. N. Am. 171-19. [DOI] [PubMed] [Google Scholar]
- 41.Wu-Hsieh, B., and D. H. Howard. 1984. Inhibition of growth of Histoplasma capsulatum by lymphokine-stimulated macrophages. J. Immunol. 1322593-2597. [PubMed] [Google Scholar]
- 42.Zhou, P., M. C. Sieve, J. Bennett, K. J. Kwon-Chung, R. P. Tewari, R. T. Gazzinelli, A. Sher, and R. A. Seder. 1995. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-gamma. J. Immunol. 155785-795. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.