Abstract
Toll-like receptor 2 (TLR2) is an essential mediator of corneal inflammation induced by the filarial nematode Onchocerca volvulus, which harbors endosymbiotic Wolbachia bacteria. TLR2 is also required for dendritic cell activation, gamma interferon (IFN-γ) production, and neutrophil recruitment to the cornea. To examine the role of IFN-γ in O. volvulus keratitis, C57BL/6 and IFN-γ−/− mice were immunized subcutaneously, and a soluble antigen extract from O. volvulus adult worms (OvAg) was injected into the corneal stroma of each animal. We found that, in the absence of IFN-γ, neutrophil recruitment to the cornea was significantly impaired, whereas there was no effect on eosinophil infiltration. Since the cornea contains resident macrophages and fibroblasts and our previous studies showed that CXC chemokines mediate neutrophil recruitment, we examined the role of recombinant IFN-γ (rIFN-γ) on each cell type. We found no effect of rIFN-γ on CXC chemokine production by macrophages or corneal fibroblasts, either alone or with filarial extracts; in contrast, rIFN-γ was found to enhance OvAg-induced tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), IL-1α, and IL-1β in macrophages. Furthermore, we found that rTNF-α, rIL-1α, or rIL-1β induced CXC chemokine production by corneal fibroblasts but not by macrophages. To determine the relative contributions of endogenous cytokines, we injected OvAg into the corneal stroma of C57BL/6, IL-1 receptor 1−/− (IL-1R1−/−), and TNF-αR1/2−/− mice and examined neutrophil recruitment. We found that neutrophil infiltration was impaired in IL-1R1−/− mice but not in TNF-αR1/2−/− mice. IFN-γ therefore appears to regulate neutrophil recruitment to the corneal stroma by enhancing TLR2 expression and OvAg-induced IL-1α and IL-1β production by macrophages in the cornea, which then induce IL-1R1-dependent production of CXC chemokine by resident corneal fibroblasts.
Ocular onchocerciaisis (river blindness) is caused by the filarial nematode Onchocerca volvulus. Adult male and female worms in subcutaneous nodules release live first-stage larvae (microfilariae), which migrate through the skin and can penetrate the eye. Microfilariae do not appear to cause disease while alive; however, when the worms die either by natural attrition or after chemotherapy, they stimulate an inflammatory response that leads to the loss of corneal function and eventual blindness. Chronically infected individuals also develop an adaptive immunity characterized by the production of Th1- and Th2-associated cytokines and T-regulatory-cell production (26, 27).
Our studies on the immunopathogenesis of this disease have utilized a murine model of Onchocerca keratitis in which mice are immunized with O. volvulus antigen (OvAg) subcutaneously, followed by the injection of OvAg into the corneal stroma. Our findings indicate that neutrophils, eosinophils, and macrophages infiltrate the corneal stroma, in a manner similar to those in O. volvulus skin disease (11, 12, 22). In addition, neutrophils are the predominant cell type underlying corneal inflammation, since the blockade of neutrophil recruitment to the corneal stroma inhibits corneal disease (13, 14, 24). We recently demonstrated a critical role for TLR2 in the development of corneal disease, showing that TLR2 is required for dendritic cell activation, gamma interferon (IFN-γ) production, and neutrophil infiltration to the cornea but TLR2 has no role in OvAg-induced interleukin-5 (IL-5) or eosinophil production (7). In the present study, we examined the role of TLR2 and IFN-γ in O. volvulus keratitis and found that IFN-γ regulates neutrophil recruitment to the cornea by synergizing with OvAg to increase TLR2 expression and proinflammatory cytokine production.
MATERIALS AND METHODS
Preparation of O. volvulus extract.
A soluble extract of O. volvulus adult worms containing Wolbachia was prepared as described previously (7, 9, 21), and the extract was adjusted to 1 mg/ml. The presence of Wolbachia was ascertained by quantitative PCR using primers against Wolbachia surface protein. The O. volvulus extract containing Wolbachia is referred to here as OvAg.
Murine model of corneal inflammation.
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). IFN-γ−/− mice were kindly provided by Tom McCormick (Case Western Reserve University) or purchased from Jackson Laboratories. TNF-αR1/2−/− mice were kindly provided by Claire M. Doerschuk (Case Western Reserve University, Cleveland, OH). IL-1R1−/− mice were provided by Susanne Mohr (Case Western Reserve University, Cleveland, OH). All knockout mice were on a C57BL/6 background.
Mice were immunized subcutaneously at the base of the tail three times with 10 μg of insoluble OvAg in a 1:1 ratio with squalene-based adjuvant at weekly intervals. Mice were then injected intrastromally with 4 μg of soluble OvAg in 4 μl of saline and euthanized after 24 h.
Quantification of neutrophils in the cornea.
Eyes were embedded in OCT (Sakura Finetek U.S.A., Torrance, CA) and snap-frozen in liquid nitrogen. Then, 5-μm sections were dried, fixed in 4% formaldehyde for 30 min, washed with phosphate-buffered saline (PBS; pH 7.6), and blocked for 30 min at room temperature in PBS-1% fetal calf serum (FCS). To detect neutrophils, slides were incubated with NIMP-R14 rat anti-murine neutrophils (provided by Achim Hoerauf, Rheinische Friedrich Wilhelms University Bonn, Germany); for eosinophils, slides were incubated with rabbit anti-murine major basic protein (anti-MBP; provided by James Lee, Mayo Clinic Arizona). After a second washing step in PBS, slides were stained for 45 min at room temperature with Alexa Fluor 488-tagged anti-rat (Invitrogen) or fluorescein isothiocyanate (FITC)-tagged anti-rabbit antibody (Molecular Probes) for eosinophil staining in PBS-1% FCS. After the final wash, slides were mounted in antifade medium (Vector Laboratories, Burlingame, CA). The number of neutrophils was determined by counting the total number of FITC positive cells from representative 5-μm corneal sections from every mouse in each experiment.
In vitro splenocyte stimulation.
Single cell suspensions were prepared by grinding spleens and filtering the cells through a 40-μm-pore-size mash filter under sterile conditions using Dulbecco modified Eagle medium, 10% FCS, and 1% penicillin-streptomycin. A total of 106 cells in 200 μl were incubated with soluble OvAg at 2 μg/ml. Medium alone was used as a negative control, and α-CD3 at 2 μg/ml was used as a positive stimulus (2C11; eBioscience, San Diego, CA). Cultures were incubated for 72 h at 37°C in 5% CO2. Supernatants were collected and analyzed for IL-5 and IFN-γ by enzyme-linked immunosorbent assay (ELISA).
In vitro macrophage stimulation.
C57BL/6 mice were injected intraperitoneally with 2 ml of aged 4% thioglycolate solution 3 to 4 days prior to peritoneal lavage. Harvested cells were washed and red blood cells were lysed using red blood cell lysis buffer (eBioscience). Macrophages were plated at 105 per well in a 96-well flat bottom plate. Cells were allowed to rest overnight before being stimulated with 5 ng of rIFN-γ (Peprotech)/ml; 0.4, 2, or 10 μg of OvAg/ml; or 10, 100, or 1,000 ng of Pam3CSK (Invivogen)/ml. Cell supernatants were analyzed by ELISA for macrophage inflammatory protein 2 (MIP-2), keratinocyte-derived cytokine (KC), TNF-α, IL-6, IL-1α, and IL-1β production (R&D Systems).
For flow cytometric analysis, 106 cells were stimulated with medium of rIFN-γ for 6 h, harvested, and then washed in fluorescence-activated cell sorting buffer. After 15 min of incubation in Fc block, cells were stained with 0.1 μg/μl of FITC-TLR2 and phycoerythrin-MHC II (both eBioscience) for 30 min. Unbound antibody was removed by washing, and 105 cells were acquired on a BD LSRI flow cytometer using CellQuest (BD Biosciences). The data were analyzed by using WinList (Verity Software House).
Western blot analysis.
Macrophages were harvested and placed in standard lysis buffer containing sodium dodecyl sulfate and β-mercaptoethanol (Sigma). After sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 10% polyacrylamide (Bio-Rad), the proteins were transferred to nitrocellulose, and Western blots was prepared using anti-TLR2 (Santa Cruz) and visualized by using an enhanced chemiluminescence development kit (ECL+Plus; Amersham, Buckinghamshire, England) according to the manufacturer's directions.
In vitro MK/T1 cell stimulation.
The mouse corneal fibroblast cell line MK/T1 was maintained by the tissue culture core facility of the Department of Ophthalmology at Case Western Reserve University. MK/T1 cells were plated at 6,000 cells per well on a 96-well plate for 48 h. At this time, adherent cells were at 80% confluence and were then stimulated with 5 ng of rIFN-γ/ml, 5 ng of rTNF-α/ml (R&D Systems), 5 ng of rIL-1α (Peprotech)/ml, 5 ng of rIL-1β (Peprotech)/ml, 5 ng of rIL-6 (Peprotech)/ml, 2 or 10 μg of OvAg/ml, or 1 μg of Pam3CSK/ml for 18 h. Supernatants were analyzed for KC and lipopolysaccharide (LPS)-induced cytokine (LIX) production by ELISA.
Detection of cytokines in the cornea.
Corneas were excised by using a 2-mm trephine, and corneal tissue was homogenized with a Mixer Mill MM300 (Retsch, Newtown, PA) for 4 min at 30 pulses/s. Cytokine levels in serum samples, homogenized corneas, and tissue culture supernatants were evaluated by sandwich ELISA according to the manufacturer's directions (R&D Systems). Absorption was determined at 450 nm on the VersaMax microplate reader using SoftMaxPro software 4.7.1 (Molecular Devices).
Statistical analysis.
A Student t test was performed for statistical analysis using Prism (GraphPad Software, La Jolla, CA), and data are shown as means ± the standard errors of the mean (SEM), which were calculated by dividing the standard deviation by the square root of the sample size. P values of <0.05 were considered significant.
RESULTS
Neutrophil recruitment to the corneal stroma is impaired in IFN-γ−/− mice.
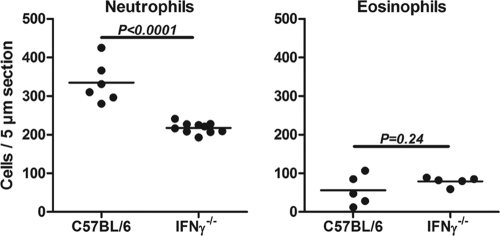
Our recent study showed impaired production of IFN-γ but not IL-4 or IL-5 in TLR2−/− mice immunized with O. volvulus extract containing Wolbachia (7). To determine the role of IFN-γ in neutrophil and eosinophil migration to the cornea, C57BL/6 and IFN-γ−/− mice were immunized subcutaneously, and corneas were injected with OvAg as described previously (7, 9, 21). Mice were euthanized after 24 h; 5-μm corneal sections were immunostained with NIMP-R14, which clearly identifies murine neutrophils (9, 10); and the number of neutrophils per corneal section was determined by direct counting as described above.
As shown in Fig. 1, the number of neutrophils in the cornea was significantly lower in IFN-γ−/− mice compared to C57BL/6 mice, indicating that IFN-γ regulates OvAg-induced neutrophil recruitment to the corneal stroma. In contrast, we found no significant difference in the numbers of corneal eosinophils between C57BL/6 and IFN-γ−/− mice, indicating that IFN-γ selectively regulates neutrophil recruitment to the cornea. Neutrophils were not detected in corneas injected with saline alone (9, 10).
FIG. 1.
Neutrophil and eosinophil recruitment to the corneas of IFN-γ−/− mice. C57BL/6 and IFN-γ−/− mice were immunized three times subcutaneously with OvAg, and OvAg was injected into the corneal stroma of each animal. After 24 h, 5-μm corneal sections were immunostained for neutrophils or eosinophils, and the number of positive cells in the corneal stroma was assessed by fluorescence microscopy. Each data point represents an individual cornea, and the experiment was repeated three times using four to five animals per group with similar results.
IFN-γ increases TLR2 expression on macrophages.
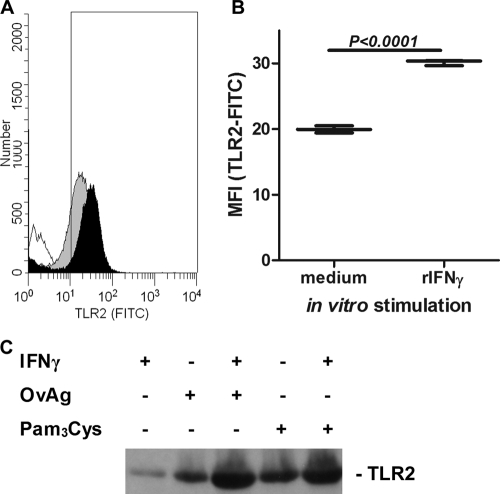
Since macrophages and dendritic cells are present in the normal corneal stroma (1, 4, 5, 17, 18), we next examined whether IFN-γ regulates TLR2 expression by macrophages. Macrophages isolated from the peritoneal cavity were incubated for 6 h with OvAg, IFN-γ, or both, and the surface expression of TLR2 was analyzed by flow cytometry. In addition, total TLR2 expression was examined by Western blot analysis. As shown in Fig. 2, TLR2 surface expression was elevated after stimulation with rIFN-γ (Fig. 2A and B). Costimulation with rIFN-γ, together with either OvAg or the TLR ligand Pam3Cys, further increased TLR2 expression above that of either ligand or IFN-γ alone. These observations indicate that IFN-γ synergizes with OvAg to elevate the expression of TLR2 on macrophages.
FIG. 2.
Effect of IFN-γ on TLR2 expression on macrophages. Peritoneal macrophages were incubated in triplicate with IFN-γ, and the TLR2 surface expression was determined by flow cytometry. (A) The TLR2 expression levels on macrophages incubated in medium alone (gray peak) or in the presence of IFN-γ (black peak) were determined. Unstained cells were also used as a control (unshaded peak). (B) Mean plus highest and lowest values for normalized mean fluorescence intensity (MFI) showing elevated TLR2 expression after incubation with IFN-γ. (C) Western blot showing elevated TLR2 expression in macrophages after incubation with OvAg or Pam3Cys in the presence or absence of IFN-γ.
Synergistic effect of IFN-γ and OvAg on proinflammatory cytokine production.
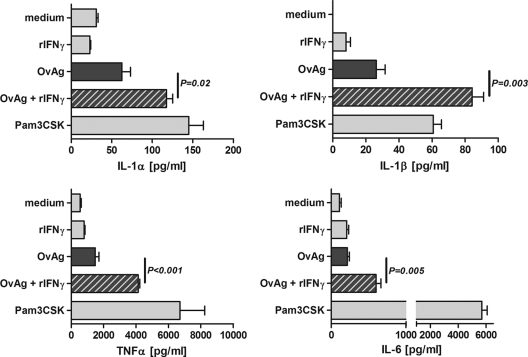
To examine whether IFN-γ has a synergistic effect with OvAg on cytokine production, macrophages isolated from the peritoneal cavity were stimulated for 18 h with either OvAg, rIFN-γ alone, or both, and IL-1α, IL-1β, TNF-α, and IL-6 levels were measured by ELISA. We found a dose-dependent increase of cytokine production (data not shown) and chose 2 μg of OvAg/ml (half-maximal cytokine production) as an optimal dose.
As shown in Fig. 3, macrophages incubated with rIFN-γ alone did not induce cytokine production, whereas OvAg induced ∼2-fold more IL-1α and IL-1 β compared to medium alone but did not stimulate the production of TNF-α or IL-6. In contrast, rIFN-γ together with OvAg stimulated significantly elevated levels of all four cytokines (Fig. 3). The synergistic effect of IFN-γ and OvAg was further increased if macrophages were incubated with rIFN-γ prior to stimulation with OvAg (data not shown).
FIG. 3.
Effect of IFN-γ on proinflammatory cytokine production by macrophages. C57BL/6 macrophages were isolated from the peritoneal cavity and stimulated with IFN-γ, OvAg, or both for 18 h, and IL-1α, IL-1β, TNF-α, and IL-6 production was determined by ELISA. The data are means ± the SEM and are representative of two repeat experiments.
rIFN-γ does not enhance CXC chemokine production.
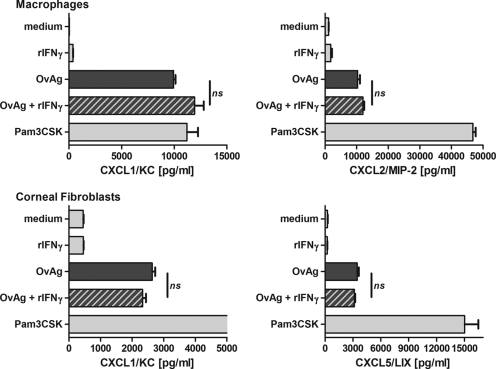
Since CXC chemokines are important for the recruitment of neutrophils to the corneal stroma, with macrophages producing CXCL1/KC and CXCL2/MIP-2 and corneal fibroblasts producing CXCL2/MIP-2 and CXCL5/LIX (14, 28), we next examined whether there is a synergistic effect of IFN-γ on the OvAg-induced production of these chemokines.
Peritoneal macrophages or the MK/T1 murine corneal fibroblasts were incubated with OvAg, rIFN-γ, or both, and the CXCL1/KC, CXCL2/MIP-2, and CXCL5/LIX levels were measured by ELISA. As shown in Fig. 4, OvAg stimulated chemokine production by macrophages and corneal fibroblasts; however, IFN-γ had no effect on chemokine production by either cell type. These data therefore indicate that IFN-γ has a selective effect on the macrophage production of proinflammatory cytokines.
FIG. 4.
Effect of IFN-γ on CXC chemokine production by macrophages and corneal fibroblasts. (Upper panels) Macrophages were isolated from the peritoneal cavity of C57BL/6 mice and, after an overnight resting phase, stimulated for 18 h with either IFN-γ, 2 μg of OvAg/μl, or both IFN-γ and 2 μg of OvAg/μl. The production of CXCL1/KC and CXCL2/MIP-2 was determined by ELISA. (Lower panels) MK-T1 corneal fibroblasts were stimulated in a similar manner, and CXCL1/KC and CXCL5/LIX were quantified by ELISA. The data are means ± the SEM and are representative of two repeat experiments for each cell type. ns, no significant difference.
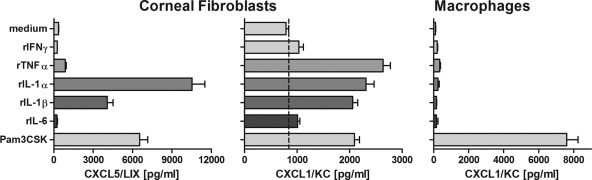
Proinflammatory cytokines induce CXC chemokine production by corneal fibroblasts but not by macrophages.
Since IFN-γ synergizes with OvAg to induce the production of proinflammatory cytokines (Fig. 3), we next examined if these cytokines can directly stimulate CXC chemokine production. Peritoneal macrophages and corneal fibroblasts were isolated as described above and incubated with rTNF-α, rIL-1α, rIL-1β, or rIL-6. Our previous studies showed that corneal fibroblasts secrete high levels of CXCL1/KC and CXCL5/LIX in response to LPS (28); we therefore examined KC and LIX production by ELISA. As shown in Fig. 5, corneal fibroblasts produced KC and LIX in response to rTNF-α, rIL-1α, and rIL-1β, whereas macrophages incubated under the same conditions did not produce CXCL1/KC. rIFN-γ or rIL-6 at the same concentration did not induce cytokine production by either cell type.
FIG. 5.
Role of proinflammatory cytokines on CXC chemokine production by corneal fibroblasts and macrophages. Corneal fibroblasts or peritoneal macrophages were stimulated with IFN-γ, TNF-α, IL-1α, IL-1β, or IL-6 for 18 h, and the production of the CXC chemokines KC and LIX was determined by ELISA. Note that corneal fibroblasts, but not macrophages, produce CXC chemokines in response to TNF-α, IL-1α, and IL-1β. The data are representative of three repeat experiments.
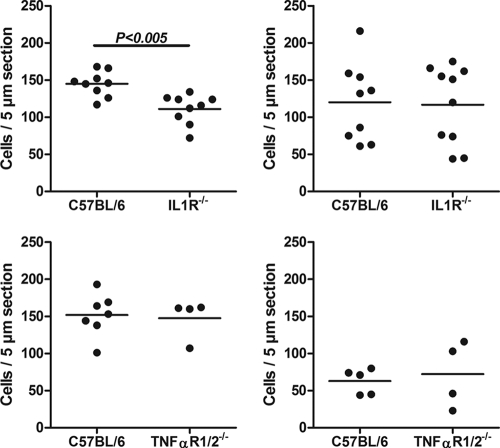
Neutrophil recruitment to the corneal stroma is dependent on IL-1R1 but not on TNF-αR1/2.
Since TNF-α, IL-1α, and IL-1β stimulate CXC chemokine production by corneal fibroblasts, we next examined the role of these proinflammatory cytokines in OvAg-induced neutrophil recruitment to the cornea. Mice deficient in both TNFR1 and TNFR2 (i.e., TNF-αR1/2−/− mice) or IL-1R1−/− mice were immunized subcutaneously and injected intrastromally with OvAg as described above, and the number of neutrophils and eosinophils in the corneal stroma was examined by immunohistochemistry.
Neutrophil recruitment to IL-1R1−/− corneas was significantly lower than with C57BL/6 corneas, whereas there was no difference in eosinophil migration (Fig. 6, upper panels). In contrast, there was no difference in neutrophils or eosinophils in TNF-αR1/2−/− corneas (Fig. 6, lower panels). These findings indicate that IL-1R1 regulates neutrophil recruitment to the cornea.
FIG. 6.
Effect of IL-1R1 and TNF-αR1/2 on neutrophil and eosinophil recruitment to the corneal stroma. C57BL/6, TNF-αR1/2−/−, and IL-1R1−/− mice were immunized with OvAg subcutaneously and then injected into the corneal stroma with OvAg. The number of neutrophils and eosinophils per 5-μm section was determined by immunohistochemistry. The data are means ± the SEM with five mice per group and are representative of three repeat experiments. Note the decreased neutrophil numbers in IL-1R1−/− mice compared to C57BL/6 mice.
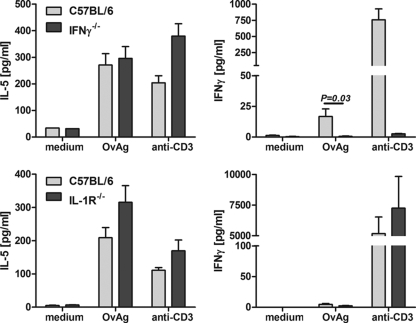
T-cell-associated cytokine production in IFN-γ−/− and IL-1R1−/− mice.
To determine if there is an effect of IFN-γ or IL-1R1 deficiency on IL-5 and IFN-γ production after OvAg immunization, splenocytes were isolated from OvAg-immunized C57BL/6, IFN-γ−/−, and IL-1R1−/− mice and stimulated with OvAg or with immunostimulatory antibody to CD3, and cytokines were measured by ELISA. As shown in Fig. 7, IL-5 production was not significantly different between C57BL/6 splenocytes and IFN-γ−/− or IL-1R1−/− splenocytes incubated with OvAg or anti-CD3. There was also no significant difference in IFN-γ production between C57BL/6 mice and IL-1R1−/− mice. These findings indicate that T-cell activation by OvAg immunization is not inhibited in the absence of IFN-γ or IL-1R1.
FIG. 7.
OvAg-induced cytokine production in IFN-γ−/− and IL-1R1−/− mice. C57BL/6 IFN-γ−/− and IL-1R1−/− mice were immunized subcutaneously with OvAg three times over 3 weeks, and splenocytes were stimulated with medium, OvAg, or with immunostimulatory anti-CD3. IL-5 and IFN-γ production in supernatants were measured by ELISA. The data are means ± the SEM of five mice per group and are representative of three repeat experiments.
DISCUSSION
We recently reported that soluble filarial extract containing Wolbachia induced dendritic cell activation and that IFN-γ production, but not IL-5 production, was dependent on the expression of TLR2 (7). We further showed in that study that CXC chemokine production in the corneal stroma and neutrophil recruitment to the corneal stroma are dependent on TLR2 expression, even in the presence of an adaptive immune response (7, 9). In the present study, we extended these observations to show that IFN-γ regulates this response in immunized mice by increasing CXC chemokine production and proinflammatory cytokine production. We also found differential responses by two resident cell types in the corneal stroma: macrophages and corneal fibroblasts. Even though previous studies showed a role for CXC chemokines in neutrophil infiltration into the corneal stroma in the mouse model of river blindness (14), we did not find differences in CXC chemokine production between C57BL/6 and IFN-γ−/− mice. This is most likely due to the low levels of TLR2 expression and chemokine production in the cornea, resulting in a partial reduction in neutrophil infiltration in the absence of IFN-γ. Our previous studies showed that neutrophil infiltration was completely abrogated in the absence of TLR2 (7, 9).
However, our current finding that IFN-γ regulates TLR2 expression on macrophages is consistent with earlier studies showing that TLR2 is upregulated after stimulation with IFN-γ in a model for biliary epithelial cells (19) and also with human rheumatoid synovial tissue, where IL-12- and IL-18-induced TLR2 expression was found to be IFN-γ dependent (31). Our findings on a role for IL-1R1 are consistent with earlier studies demonstrating a role for IL-1β and caspase-1 in CXC chemokine production and neutrophil recruitment to the cornea in Pseudomonas aeruginosa keratitis (33, 37).
Together, our results indicate that IFN-γ elevates expression of TLR2 on corneal macrophages and that OvAg stimulation of TLR2 induces production of IL-1α and IL-1β. These cytokines likely activate IL-1R1 on corneal fibroblasts to produce CXC chemokines, which mediate recruitment of neutrophils to the corneal stroma.
The transparent nature of the cornea is maintained by tight junctions of the corneal epithelium and by the corneal endothelium, which regulates the hydration level of the cornea. Disruption of the normal function of the cornea leads to loss of transparency initially, and continued inflammatory responses or chronic infection results in corneal opacification and loss of vision. Macrophages and dendritic cells are present in the normal corneal stroma (1, 5, 18, 29, 34) and respond to TLR ligands (3a). In contrast, keratocytes form a network of cells in the corneal stroma and produce collagen and proteoglycans that form the corneal matrix. These cells differentiate into fibroblasts and myofibroblasts. The MK/T1 mouse corneal fibroblast cell line produces CXC chemokines in response to LPS, filarial extracts, or isolated Wolbachia (8, 9, 28). However, we show here that corneal fibroblasts do not respond to IFN-γ; rather, IFN-γ enhances proinflammatory cytokine production by macrophages. Conversely, the proinflammatory cytokines IL-1α, IL-1β, and TNF-α induce CXC chemokine production by corneal fibroblasts, but not macrophages, indicating an indirect role for IFN-γ in CXC chemokine production. Furthermore, we found that neutrophil recruitment was impaired in IL-1R1 gene knockout mice but not in TNFR gene knockout mice, indicating that IL-1α and IL-1β, rather than TNF-α, are important in neutrophil recruitment. IFN-γ signaling involves binding of JAK1 and JAK2 to the IFN-γR1 and consequent STAT1 activation, leading to dimerization of STAT1, translocation to the nucleus and binding to promoter sequences of γ-activated genes (32). Furthermore, IFN-γ synergizes with TLRs to induce an inflammatory response, either by suppressing TLR-induced feedback inhibition (23) or by suppressing MyD88-dependent RNA stability (35) in macrophages, but not in corneal fibroblasts.
IFN-γ was also shown to mediate neutrophil recruitment to the cornea in herpes simplex keratitis by upregulating PECAM-1 expression on vascular endothelial cells (36). However, even though PECAM-1 is upregulated in O. volvulus keratitis (25), we found no change in PECAM-1 expression in IFN-γ−/− mice (data not shown). IFN-γ also did not enhance eosinophil migration to the cornea or direct production of CXC chemokines by macrophages, fibroblasts, or neutrophils. We have yet to determine whether the source of IFN-γ is from serum or is produced by CD4+ T cells or natural killer cells in the corneal stroma, since CD4+ cells are present in the cornea in O. volvulus keratitis (2, 3, 30) and natural killer cells are present in the cornea in P. aeruginosa keratitis (20).
Although we demonstrate a role for adaptive immunity in neutrophil recruitment in the present study and our earlier studies showed a role for B cells and Fc gamma receptors in neutrophil and eosinophil recruitment to the cornea in O. volvulus keratitis (15, 16), Th2-associated responses, especially elevated IL-5 and eosinophilia, are hallmarks of chronic filarial infection. However, more recent studies indicate a role for T regulatory cells in suppressing the host immune response, since FOXP3-positive cells are increased in infected individuals treated with doxycycline (27). In addition, repeated exposure to filarial extracts containing Wolbachia induces TLR2/MyD88-dependent tolerance to synthetic TLR ligands and to CD40 activation (38).
In conclusion, it appears that the role of adaptive immunity in infected individuals (and immunized animals) is primarily to downregulate proinflammatory responses and minimize tissue damage, whereas dead and degenerating worms release Wolbachia, which induces a proinflammatory response that is associated with tissue damage in nodules, in the skin, and in the cornea. Tissue damage appears to be mediated by innate immune responses, directed through TLR2/TLR6, MyD88, and Mal (21) and, as shown here, is also exacerbated by IFN-γ. IFN-γ is associated with clinical disease in filariasis and onchocerciasis patients (6, 26), and we identify here one mechanism by which IFN-γ may contribute.
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Eugenia Diaconu, Carynne M. Fox, Michael J. Harling, and Nicholas Szugye. We also thank Claire Doerschuk, Tom McCormick, and Susanne Mohr for gene knockout mice and Achim Hoerauf and James Lee for the anti-neutrophil and anti-MBP antibodies, respectively.
This study was supported by NIH grants EY10320 and EY11373 (E.P.), grant DA1024/1-1 from the German Research Foundation (K.G.), and by the Research to Prevent Blindness Foundation and the Ohio Lions Eye Research Foundation.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Brissette-Storkus, C. S., S. M. Reynolds, A. J. Lepisto, and R. L. Hendricks. 2002. Identification of a novel macrophage population in the normal mouse corneal stroma. Investig. Ophthalmol. Vis. Sci. 432264-2271. [PMC free article] [PubMed] [Google Scholar]
- 2.Chakravarti, B., T. A. Herring, J. H. Lass, J. S. Parker, R. P. Bucy, E. Diaconu, J. Tseng, D. R. Whitfield, B. M. Greene, and D. N. Chakravarti. 1994. Infiltration of CD4+ T cells into cornea during development of Onchocerca volvulus-induced experimental sclerosing keratitis in mice. Cell. Immunol. 159306-314. [DOI] [PubMed] [Google Scholar]
- 3.Chakravarti, B., S. Lagoo-Deenadayalan, J. S. Parker, D. R. Whitfield, A. Lagoo, and D. N. Chakravarti. 1996. In vivo molecular analysis of cytokines in a murine model of ocular onchocerciasis. I. Upregulation of IL-4 and IL-5 mRNAs and not IL-2 and IFN gamma mRNAs in the cornea due to experimental interstitial keratitis. Immunol. Lett. 5459-64. [DOI] [PubMed] [Google Scholar]
- 3a.Chinnery, H. R., E. C. Carlson, Y. Sun, M. Lin, S. H. Burnett, V. L. Perez, P. G. McMenamin, and E. Pearlman. 2009. Bone marrow chimeras and c-fms conditional ablation (Mafia) mice reveal an essential role for resident myeloid cells in LPS/TLR4-induced corneal inflammation. J. Immunol. 1822738-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinnery, H. R., E. Pearlman, and P. G. McMenamin. 2008. Cutting edge: membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J. Immunol. 1805779-5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinnery, H. R., M. J. Ruitenberg, G. W. Plant, E. Pearlman, S. Jung, and P. G. McMenamin. 2007. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Investig. Ophthalmol. Vis. Sci. 481568-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper, P. J., T. Mancero, M. Espinel, C. Sandoval, R. Lovato, R. H. Guderian, and T. B. Nutman. 2001. Early human infection with Onchocerca volvulus is associated with an enhanced parasite-specific cellular immune response. J. Infect. Dis. 1831662-1668. [DOI] [PubMed] [Google Scholar]
- 7.Daehnel, K., I. Gillette-Ferguson, A. G. Hise, E. Diaconu, M. J. Harling, F. P. Heinzel, and E. Pearlman. 2007. Filaria/Wolbachia activation of dendritic cells and development of Th1-associated responses is dependent on Toll-like receptor 2 in a mouse model of ocular onchocerciasis (river blindness). Parasite Immunol. 29455-465. [DOI] [PubMed] [Google Scholar]
- 8.Gendron, R. L., C. Y. Liu, H. Paradis, L. C. Adams, and W. W. Kao. 2001. MK/T-1, an immortalized fibroblast cell line derived using cultures of mouse corneal stroma. Mol. Vis. 7107-113. [PubMed] [Google Scholar]
- 9.Gillette-Ferguson, I., K. Daehnel, A. G. Hise, Y. Sun, E. Carlson, E. Diaconu, H. F. McGarry, M. J. Taylor, and E. Pearlman. 2007. Toll-like receptor 2 regulates CXC chemokine production and neutrophil recruitment to the cornea in Onchocerca volvulus/Wolbachia-induced keratitis. Infect. Immun. 755908-5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillette-Ferguson, I., A. G. Hise, Y. Sun, E. Diaconu, H. F. McGarry, M. J. Taylor, and E. Pearlman. 2006. Wolbachia- and Onchocerca volvulus-induced keratitis (river blindness) is dependent on myeloid differentiation factor 88. Infect. Immun. 742442-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez-Pena, E. J., J. Knab, and D. W. Buttner. 1998. Immunoelectron microscopic evidence for release of eosinophil granule matrix protein onto microfilariae of Onchocerca volvulus in the skin after exposure to amocarzine. Parasitol. Res. 84607-615. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez-Pena, E. J., J. Knab, and D. W. Buttner. 1996. Neutrophil granule proteins: evidence for the participation in the host reaction to skin microfilariae of Onchocerca volvulus after diethylcarbamazine administration. Parasitology 113(Pt. 4)403-414. [DOI] [PubMed] [Google Scholar]
- 13.Hall, L. R., R. B. Berger, E. Diaconu, and E. Pearlman. 2002. Onchocerca volvulus keratitis (river blindness) is exacerbated in BALB/c IL-4 gene knockout mice. Cell. Immunol. 2161-5. [DOI] [PubMed] [Google Scholar]
- 14.Hall, L. R., E. Diaconu, R. Patel, and E. Pearlman. 2001. CXC chemokine receptor 2 but not C-C chemokine receptor 1 expression is essential for neutrophil recruitment to the cornea in helminth-mediated keratitis (river blindness). J. Immunol. 1664035-4041. [DOI] [PubMed] [Google Scholar]
- 15.Hall, L. R., E. Diaconu, and E. Pearlman. 2001. A dominant role for Fc gamma receptors in antibody-dependent corneal inflammation. J. Immunol. 167919-925. [DOI] [PubMed] [Google Scholar]
- 16.Hall, L. R., J. H. Lass, E. Diaconu, E. R. Strine, and E. Pearlman. 1999. An essential role for antibody in neutrophil and eosinophil recruitment to the cornea: B cell-deficient (microMT) mice fail to develop Th2-dependent, helminth-mediated keratitis. J. Immunol. 1634970-4975. [PubMed] [Google Scholar]
- 17.Hamrah, P., and M. R. Dana. 2007. Corneal antigen-presenting cells. Chem. Immunol. Allergy 9258-70. [DOI] [PubMed] [Google Scholar]
- 18.Hamrah, P., Y. Liu, Q. Zhang, and M. R. Dana. 2003. The corneal stroma is endowed with a significant number of resident dendritic cells. Investig. Ophthalmol. Vis. Sci. 44581-589. [DOI] [PubMed] [Google Scholar]
- 19.Harada, K., K. Isse, and Y. Nakanuma. 2006. Interferon gamma accelerates NF-κB activation of biliary epithelial cells induced by Toll-like receptor and ligand interaction. J. Clin. Pathol. 59184-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazlett, L. D., Q. Li, J. Liu, S. McClellan, W. Du, and R. P. Barrett. 2007. NKT cells are critical to initiate an inflammatory response after Pseudomonas aeruginosa ocular infection in susceptible mice. J. Immunol. 1791138-1146. [DOI] [PubMed] [Google Scholar]
- 21.Hise, A. G., K. Daehnel, I. Gillette-Ferguson, E. Cho, H. F. McGarry, M. J. Taylor, D. T. Golenbock, K. A. Fitzgerald, J. W. Kazura, and E. Pearlman. 2007. Innate immune responses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and Mal, but not TLR4, TRIF, or TRAM. J. Immunol. 1781068-1076. [DOI] [PubMed] [Google Scholar]
- 22.Hoerauf, A., D. W. Buttner, O. Adjei, and E. Pearlman. 2003. Onchocerciasis. BMJ 326207-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu, X., J. Chen, L. Wang, and L. B. Ivashkiv. 2007. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J. Leukoc. Biol. 82237-243. [DOI] [PubMed] [Google Scholar]
- 24.Kaifi, J. T., E. Diaconu, and E. Pearlman. 2001. Distinct roles for PECAM-1, ICAM-1, and VCAM-1 in recruitment of neutrophils and eosinophils to the cornea in ocular onchocerciasis (river blindness). J. Immunol. 1666795-6801. [DOI] [PubMed] [Google Scholar]
- 25.Khatri, S., J. H. Lass, F. P. Heinzel, W. M. Petroll, J. Gomez, E. Diaconu, C. M. Kalsow, and E. Pearlman. 2002. Regulation of endotoxin-induced keratitis by PECAM-1, MIP-2, and Toll-like receptor 4. Investig. Ophthalmol. Vis. Sci. 432278-2284. [PubMed] [Google Scholar]
- 26.King, C. L., S. Mahanty, V. Kumaraswami, J. S. Abrams, J. Regunathan, K. Jayaraman, E. A. Ottesen, and T. B. Nutman. 1993. Cytokine control of parasite-specific anergy in human lymphatic filariasis: preferential induction of a regulatory T helper type 2 lymphocyte subset. J. Clin. Investig. 921667-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korten, S., M. Badusche, D. W. Buttner, A. Hoerauf, N. Brattig, and B. Fleischer. 2008. Natural death of adult Onchocerca volvulus and filaricidal effects of doxycycline induce local FOXP3+/CD4+ regulatory T cells and granzyme expression. Microbes Infect. 10313-324. [DOI] [PubMed] [Google Scholar]
- 28.Lin, M., E. Carlson, E. Diaconu, and E. Pearlman. 2007. CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J. Leukoc. Biol. 81786-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura, T., F. Ishikawa, K. H. Sonoda, T. Hisatomi, H. Qiao, J. Yamada, M. Fukata, T. Ishibashi, M. Harada, and S. Kinoshita. 2005. Characterization and distribution of bone marrow-derived cells in mouse cornea. Investig. Ophthalmol. Vis. Sci. 46497-503. [DOI] [PubMed] [Google Scholar]
- 30.Pearlman, E., J. H. Lass, D. S. Bardenstein, M. Kopf, F. E. Hazlett, Jr., E. Diaconu, and J. W. Kazura. 1995. Interleukin 4 and T helper type 2 cells are required for development of experimental onchocercal keratitis (river blindness). J. Exp. Med. 182931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radstake, T. R., M. F. Roelofs, Y. M. Jenniskens, B. Oppers-Walgreen, P. L. van Riel, P. Barrera, L. A. Joosten, and W. B. van den Berg. 2004. Expression of Toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis Rheum. 503856-3865. [DOI] [PubMed] [Google Scholar]
- 32.Ramana, C. V., M. P. Gil, R. D. Schreiber, and G. R. Stark. 2002. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 2396-101. [DOI] [PubMed] [Google Scholar]
- 33.Rudner, X. L., K. A. Kernacki, R. P. Barrett, and L. D. Hazlett. 2000. Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J. Immunol. 1646576-6582. [DOI] [PubMed] [Google Scholar]
- 34.Sosnova, M., M. Bradl, and J. V. Forrester. 2005. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells 23507-515. [DOI] [PubMed] [Google Scholar]
- 35.Sun, D., and A. Ding. 2006. MyD88-mediated stabilization of interferon-gamma-induced cytokine and chemokine mRNA. Nat. Immunol. 7375-381. [DOI] [PubMed] [Google Scholar]
- 36.Tang, Q., and R. L. Hendricks. 1996. Interferon gamma regulates platelet endothelial cell adhesion molecule 1 expression and neutrophil infiltration into herpes simplex virus-infected mouse corneas. J. Exp. Med. 1841435-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thakur, A., R. P. Barrett, S. McClellan, and L. D. Hazlett. 2004. Regulation of Pseudomonas aeruginosa corneal infection in IL-1 beta converting enzyme (ICE, caspase-1)-deficient mice. Curr. Eye Res. 29225-233. [DOI] [PubMed] [Google Scholar]
- 38.Turner, J. D., R. S. Langley, K. L. Johnston, G. Egerton, S. Wanji, and M. J. Taylor. 2006. Wolbachia endosymbiotic bacteria of Brugia malayi mediate macrophage tolerance to TLR- and CD40-specific stimuli in a MyD88/TLR2-dependent manner. J. Immunol. 1771240-1249. [DOI] [PubMed] [Google Scholar]