Abstract
The levels of regulatory T cells (Treg cells), analyzed by Foxp3 mRNA expression, were determined in lesions from patients with acute cutaneous leishmaniasis (ACL) and chronic cutaneous leishmaniasis (CCL). We demonstrated that Treg cells preferentially accumulate in lesions from ACL patients during the early phase of infection (lesion duration of less than 1 month). In addition, levels of Foxp3 mRNA transcripts were significantly higher in specimens from patients with CCL than in those from patients with ACL, suggesting a critical role of intralesional Treg cells in CCL. Intralesional Treg cells from both ACL and CCL patients were shown to have suppressive functions in vitro, since they inhibited the gamma interferon (IFN-γ) produced by CD4+ CD25− T cells purified from peripheral blood mononuclear cells from the same patient in response to Leishmania guyanensis stimulation. Intralesional 2,3-indoleamine dioxygenase (IDO) mRNA expression was associated with that of Foxp3, suggesting a role for IDO in the suppressive activity of intralesional Treg cells. In addition, a role, albeit minor, of interleukin-10 (IL-10) was also demonstrated, since neutralization of IL-10 produced by intralesional T cells increased IFN-γ production by effector cells in an in vitro suppressive assay. These results confirm the role of intralesional Treg cells in the immunopathogenesis of human Leishmania infection, particularly in CCL patients.
Human leishmaniasis corresponds to a spectrum of clinical forms with different levels of immunopathogenesis. Localized acute cutaneous leishmaniasis (ACL) is described as self-limited, often ulcerated skin lesions in which the growth of parasites is contained. Another common clinical presentation is chronic cutaneous leishmaniasis (CCL), which is characterized by the presence of nodular, hyperkeratotic, or ulcerating lesions lasting for more than 6 months in untreated patients. In this clinical presentation, there are few parasites in lesions. Currently, most of the drugs used in the treatment of ACL are poorly effective in CCL (27).
The description of different populations of CD4+ T cells allowed the characterization of CD4+ T-cell subsets in diverse pathologies. In the murine model of infection with Leishmania major, CD4+ Th1 cells, which produce interleukin-2 (IL-2) and gamma interferon (IFN-γ), and Th2 cells, which produce IL-4, IL-5, and IL-13, confer resistance and susceptibility to infection, respectively (26). For humans, although Th1 and Th2 cytokines have been analyzed extensively in the different forms of leishmaniasis, the exact roles of Th1 and Th2 CD4+ cells and the cytokines they produce have not yet been elucidated.
Another subpopulation of T cells, CD4+ CD25+ T cells, named regulatory T cells (Treg cells), has been described for both mice and humans. Although the role of Treg cells was first described as controlling peripheral reactivity to prevent tissue damage, it is now well admitted that they also control immune responses during many microbial infections (4). Numerous studies have analyzed the role of Treg cells in the murine model of infection with Leishmania. In this model, Treg cells control specific immune responses in both susceptible and resistant mice. Indeed, in resistant C57BL/6 mice, Treg cells control Th1-cell responses by IL-10-dependent and -independent mechanisms and favor parasite persistence (5). In susceptible BALB/c mice, Treg cells control Th2-cell responses (3). In addition, in a model of CCL due to infection with L. major, it has been shown that Treg cells accumulate at the site of infection, rendering the Th1 response induced by infection with the L. major Sd strain ineffective (1). Treg cells were also shown to limit immunopathogenesis caused by L. amazonensis by inhibiting the activities of L. amazonensis-specific effector cells (17). In contrast, in a murine model of infection with L. mexicana, it was recently demonstrated that depletion of CD4+ CD25+ T cells did not alter the course of infection or the immune responses, suggesting that susceptibility to infection with L. mexicana is not controlled by Treg cells (31).
Several mechanisms for Treg cell suppression have been identified either in vitro or in vivo, including direct contact of T cells, production of cytokines, such as IL-10, transforming growth factor beta (TGF-β), and IL-35, and of different T-cell-derived suppressor molecules, such as cyclic AMP and HO-1, and activation of 2,3-indoleamine dioxygenase (IDO) in antigen-presenting cells (APCs) through interaction with CLTA-4. However, the precise mechanisms of Treg cell suppression in specific pathologies have yet to be determined and could be caused by a combination of several of these mechanisms (29).
Limited information is available on the role of Treg cells during human infection with Leishmania. Treg cells with immunosuppressive functions have been identified clearly in skin lesions from patients infected with L. braziliensis, but their exact role in pathogenesis has not been evaluated (10). More recently, Treg cells have also been identified in peripheral blood mononuclear cells (PBMC) from acute and cured localized cutaneous leishmaniasis patients infected with L. braziliensis (28).
We previously demonstrated that CD4+ CD25+ T cells (Treg cells) producing TGF-β generated in response to L. guyanensis are present in PBMC from healthy controls and that these cells express CLA and CCR4, suggesting that they could migrate into the inflamed skin, where Leishmania parasites are located, to exert their functions (18). The fact that we were unable to detect CD4+ CD25+ TGF-β-producing cells in PBMC from localized cutaneous leishmaniasis patients suggested that in these patients, Treg cells could be recruited to the site of infection (18).
To confirm this hypothesis, we determined the presence of Treg cells in PBMC and skin biopsy specimens from patients with ACL due to L. guyanensis and analyzed their role in the pathogenesis of the disease. In addition, we compared the presence of Treg cells in infected skin of ACL and CCL patients infected with L. guyanensis. We demonstrate that Treg cells accumulate at the site of infection, particularly during the early phase of infection in ACL patients and in CCL lesions. Furthermore, we demonstrate that levels of IDO mRNA parallel the levels of Foxp3 mRNA, suggesting that IDO is one of the possible molecules involved in the immunosuppressive function of intralesional Treg cells.
MATERIALS AND METHODS
Patients.
Thirty-eight patients (30 males and 8 females; 15 to 58 years old) with ACL and 11 patients (10 males and 1 female; 23 to 49 years old) with CCL, both due to L. guyanensis, were examined for the precise clinical presentation of the lesions, including localization and number, before treatment with pentamidine isethionate (Pentacarinat; Rhone Poulenc). ACL and CCL patients were defined as patients with lesions for less than and more than 6 months, respectively. Clinical characterization of the patients is given in Table 1. Ten healthy subjects living in the same area of endemicity were included in this study. All subjects were seronegative for human immunodeficiency virus. Informed consent was obtained from the patients, and the human experimentation guidelines of the Centre Hospitalier Andrée Rosemon in Cayenne, French Guyana, were followed in the conduct of this research.
TABLE 1.
Clinical characterization of 38 patients with ACL and 11 patients with CCL
| Characteristic | Value for patients with:
|
|
|---|---|---|
| ACL | CCL | |
| Sex (% male) | 79.9 | 90.9 |
| Duration of lesions (mo) (%) | ||
| <1 | 47.05 | |
| 1-3 | 35.30 | |
| 3-6 | 17.65 | |
| >6 | 100 | |
| No. of lesions | ||
| Median no. (range) | 1 (1-5) | 1 (1-3) |
| Ulcerated lesions (%) | 94.12 | 88 |
| Nodular lesions (%) | 5.88 | 12 |
| Lesion localization (%) | ||
| Arms | 33.35 | 36.4 |
| Legs | 44.45 | 54.5 |
| Trunk | 16.65 | 9.1 |
| Head | 5.55 | |
Parasitological diagnosis was done by collecting serous dermal fluid from the border of the lesion, with May Grunwald Giemsa staining. Parasite isolates were determined to be L. guyanensis on the basis of isoenzyme polymorphism on parasite culture.
RNA extraction from biopsy specimens and real-time RT-PCR.
Biopsies were done with a 2-mm-diameter punch, and total RNA was isolated as already described, using an RNeasy Mini kit (Qiagen) (8). First-strand cDNA synthesis was done on total RNA by using a first-strand cDNA synthesis kit (Superscript III first-strand synthesis system; Invitrogen). Real-time reverse transcription-PCR (RT-PCR) was performed on a 7300 real-time system (Applied Biosystems). Amplification was performed in a total volume of 25 μl for 40 cycles. 6-Carboxyfluoescein-MGB-labeled primer and probe sets for IFN-γ (Hs 00174143-m1), IL-10 (Hs00174086-m1), Foxp3 (Hs 00203958-m1), CTLA-4 (Hs 00175480-m1), IDO (Hs 00158032-m1), and β-actin (Hs 99999903-m1) as an endogenous control were obtained from Applied Biosystems. The relative quantification of products was determined by the comparative cycle threshold method, using the equation 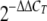 . Each gene of interest was normalized to the β-actin endogenous gene, and results are expressed in arbitrary units or relative to the expression of the same gene in PBMC. For CCL patients, some results are presented as changes in expression of the specific gene in lesions from patients with CCL relative to those from patients with ACL, defined as a reference (equivalent to 1).
. Each gene of interest was normalized to the β-actin endogenous gene, and results are expressed in arbitrary units or relative to the expression of the same gene in PBMC. For CCL patients, some results are presented as changes in expression of the specific gene in lesions from patients with CCL relative to those from patients with ACL, defined as a reference (equivalent to 1).
Flow cytometry.
PBMC were isolated after venipuncture on a Ficoll-Hypaque gradient (d = 1.077). For immunofluorescence staining, cells were washed and stained for 30 min at 4°C with an optimal dilution of fluorescein isothiocyanate-conjugated anti-CD4 (RPA-T4, an immunoglobulin G1 [IgG1]; Pharmingen, San Diego, CA). Cells were washed, fixed, and permeabilized with freshly prepared fixation/permeabilization solution for 30 min at 4°C following the manufacturer's instructions (Pharmingen). Cells were then resuspended in permeabilization buffer with phycoerythrin-conjugated anti-Foxp3 monoclonal antibody (MAb) (PCH101, a rat IgG2a; eBiosciences) or its isotype control. After 30 min of incubation, cells were washed twice with permeabilization buffer and finally suspended in phosphate-buffered saline. A total of 50,000 events were acquired using a FACS Scan machine, and analysis was performed with Cell Quest Pro software.
Cocultures and IFN-γ production.
CD4+ T cells were isolated with anti-CD4 magnetic beads as described by the manufacturer (Dynal). CD4 MAbs were released with Detachbeads as described by the manufacturer (Dynal, Compiègne, France). For CD4+ CD25+ T-cell negative selection, isolated CD4+ T cells were incubated with anti-CD25 MAb (M-1251, an IgG1; Pharmingen) and anti-mouse immunoglobulin magnetic beads. The purity of CD4+ CD25+ T cells was >92%.
Cells from skin lesions were obtained by mechanical disaggregation with a Medimachine (BD Pharmingen) in accordance with the manufacturer's instructions after 1 h at 37°C with 0.28 Wunsch unit/ml of Liberase Blendzyme 2 (Roche Diagnostics). Cell viability was assessed by trypan blue exclusion. In some experiments, CD4+ CD25+ T cells from intralesional T cells were purified as described above.
To analyze the suppressive activity of skin lesion cells, CD4+ CD25− T cells purified from PBMC (106/ml) were cocultured in the presence of cells from biopsy specimens of the same patient (104 cells), with or without 106 live L. guyanensis parasites, in the presence of autologous PBMC treated with mitomycin used as APCs. In some experiments, CD4+ CD25+ T cells purified from skin cells instead of intralesional T cells were added to the cultures. Mouse anti-IL-10 (JES3-9D7, an IgG1; Pharmingen) or the isotype control (107.3, an anti-trinitrophenol IgG1; Pharmingen) was added at 5 μg/ml. The supernatants were recovered after 5 days of culture and stored at −20°C. IFN-γ production was analyzed using a specific IFN-γ enzyme-linked immunosorbent assay (ELISA) (Pharmingen) with a sensitivity of 10 pg/ml. Suppression of IFN-γ production was calculated as follows: (IFN-γ in CD4+ CD25− T cells − IFN-γ in CD4+ CD25− and CD4+ CD25+ cells or total skin cells)/(IFN-γ in CD4+ CD25− T cells) × 100.
Statistical analysis.
We used the Wilcoxon rank sum test to compare levels of cytokines within the different groups of patients.
RESULTS
Foxp3-expressing cells are observed less in PBMC from ACL patients than in control subjects.
We examined the levels of Treg cells in PBMC from both healthy subjects and ACL patients suffering from leishmaniasis due to L. guyanensis without any in vitro stimulation. First, we analyzed the levels of Treg cells in PBMC by mRNA expression of their specific transcription factor, Foxp3. Figure 1A clearly shows that Foxp3 mRNA is expressed at significantly higher levels in PBMC from control subjects than in those from ACL patients (P < 0.04). The percentage of CD4+ T cells expressing the Foxp3 transcription factor was determined for PBMC from both controls and ACL patients, using a specific MAb. As shown in Fig. 1B and in accordance with Foxp3 mRNA expression, the number of cells expressing Foxp3, even if not significantly different, was higher in PBMC from controls than in those from ACL patients.
FIG. 1.
Foxp3-expressing cells are less frequent in PBMC from ACL patients than in those from control subjects. (A) Foxp3 mRNA levels were analyzed ex vivo in PBMC from control subjects (n = 10) and ACL patients (n = 25). The Foxp3 mRNA level was normalized to the β-actin endogenous gene level, and results are expressed as relative mRNA expression. *, P < 0.04 (Wilcoxon rank test). Foxp3 mRNA expression was set equal to 1 in PBMC of ACL patients. (B) Percentage of Foxp3+ CD4+ T cells, analyzed ex vivo in PBMC of control subjects (n = 5) and ACL patients (n = 12). Flow cytometry was performed as described in Materials and Methods. (C) CD4+ CD25+ T cells purified from PBMC of control subjects and patients with ACL were cocultured with autologous CD4+ CD25− T cells isolated from PBMC (ratio, 1:100) in the presence of live L. guyanensis and mitomycin-treated PBMC used as APCs. After 5 days of culture, IFN-γ levels in supernatants of cultures were assessed using a specific IFN-γ ELISA kit. Numbers are percentages of suppression, calculated as described in Materials and Methods. *, P < 0.05 (Wilcoxon rank test) compared to CD4+ CD25− T cells.
To test if Treg cells within PBMC from control patients display suppressive functions in vitro, we performed the classical suppression assay by culturing CD4+ CD25+ cells with CD4+ CD25− T cells (ratio of 104 to 106; 1:100) in the presence of L. guyanensis (6). The IFN-γ produced was measured after 5 days of culture, as previously described (18). As shown in Fig. 1C, in contrast to CD4+ CD25+ T cells isolated from PBMC of ACL patients, CD4+ CD25+ T cells from control subjects were able to significantly suppress the secretion of IFN-γ by CD4+ CD25− T cells. The same results were obtained by analyzing IL-10 production by CD4+ CD25− T cells stimulated with L. guyanensis (data not shown). This was not due to an increase of mortality of cells, since the percentages of viable cells at the end of culture were identical in the presence or absence of CD4+ CD25+ T cells (data not shown). In addition, CD4+ CD25+ T cells also suppressed the IFN-γ produced by CD4+ CD25− T cells in response to anti-CD3 and anti-CD28 MAbs (data not shown). Altogether, these results demonstrate the presence of Treg cells with suppressive activity within PBMC of control subjects but not in those of ACL patients.
Treg cells are recruited to the site of infection with L. guyanensis in ACL patients.
Since we previously demonstrated that CD4+ CD25+ T cells express CLA and CCR4 and consequently might migrate into inflamed skin (18), we analyzed the expression of Foxp3 mRNA within the lesions of ACL patients.
As shown in Fig. 2A, the levels of Foxp3 mRNA were statistically higher in lesions than in PBMC of ACL patients (P < 0.05). Indeed, there was a >5-fold increase of Foxp3 mRNA in lesions compared to PBMC, demonstrating the recruitment of Treg cells at the site of infection.
FIG. 2.
Accumulation of intralesional Treg cells with suppressive activity in lesions of patients with ACL due to L. guyanensis. (A) Foxp3 mRNA levels were analyzed ex vivo in PBMC and biopsy specimens from ACL patients. The Foxp3 mRNA level was normalized to the β-actin endogenous gene level, and results are expressed in arbitrary units. *, P < 0.05 (Wilcoxon rank test). (B) Foxp3 and IFN-γ mRNA levels were analyzed by real-time RT-PCR in lesions from patients with ACL with different times of duration of lesions. Foxp3 and IFN-γ mRΝΑ levels were normalized to the β-actin endogenous gene level, and results are expressed as relative expression of the gene of interest in lesions compared to the level in PBMC. *, P < 0.05 (Wilcoxon rank test) for Foxp3 and IFN-γ mRNA expression in lesions of less than 1 month compared to results obtained for lesions of 1 to 3 months and more than 3 months. Purified total T cells (C) or CD4+ CD25+ T cells (D) in skin lesions from patients with ACL were cocultured with autologous CD4+ CD25− T cells isolated from PBMC (ratio, 1:100) in the presence of live L. guyanensis and mitomycin-treated PBMC used as APCs. After 5 days of culture, IFN-γ levels in supernatants of cultures were assessed using a specific IFN-γ ELISA kit. Numbers are percentages of suppression, calculated as described in Materials and Methods.
The association of the presence of Treg cells in the lesions of ACL patients with clinical data was then analyzed. We did not find any association between the level of Treg cells analyzed by Foxp3 mRNA expression and the number, location, or type of lesions (data not shown). However, we found that levels of Treg cells were associated with the time of duration of lesions. As shown in Fig. 2B, shorter lesion durations were associated with higher levels of Foxp3 mRNA transcripts within the lesions. Indeed, levels of Foxp3 mRNA were statistically higher in lesions with durations of less than 1 month than in lesions with a time of evolution of more than one month (P < 0.05).
Treg cells present within lesions of ACL patients have a suppressive function.
Since Treg cells have been described to inhibit the IFN-γ produced by CD4+ CD25− T cells (6), we determined whether or not Treg cells detected at the site of infection of ACL have suppressive activities on IFN-γ production. First, we analyzed the expression of IFN-γ transcripts within lesions at different times of development. The level of IFN-γ mRNA expression was significantly lower in lesions with a duration of less than 1 month (in which Foxp3 mRNA expression was high) than in lesions with a duration of more than 1 month (in which Foxp3 mRNA expression was low) (P < 0.05) (Fig. 2B). The fact that, in lesions, an increase in IFN-γ mRNA expression is associated with a decrease of expression of Foxp3 mRNA suggests that lesional Treg cells of ACL patients are functionally suppressive.
To establish whether total skin lesion-derived cells are able to actively inhibit the IFN-γ produced by effector T cells, intralesional total cells were isolated and cocultured with CD4+ CD25− T cells obtained from PBMC of the same patient and stimulated with live L. guyanensis in the presence of autologous APCs. For six subjects analyzed, the IFN-γ produced by CD4+ CD25− T cells in response to Leishmania antigens was decreased in the presence of intralesional total cells (suppression spread from 30.7 to 61.3%) (Fig. 2C).
To confirm that the intralesional cells, which mount suppressive activity, were CD4+ CD25+ T cells, intralesional CD4+ CD25+ T cells were purified from two patients, using magnetic bead-activated cell sorting separation. CD4+ CD25+ T cells obtained from lesions were able to reduce IFN-γ production by CD4+ CD25− T cells from PBMC of the same patients, as demonstrated in Fig. 2D (suppression of 41.40 and 50.80% in patients 7 and 8, respectively).
Altogether, these results demonstrate that Treg cells accumulate preferentially in lesions from ACL patients and are able to suppress the local effector immune response.
IDO induction is the main suppressive mechanism used by Treg cells in ACL lesions.
Several molecular mechanisms of Treg cell suppression have been described, including mechanisms dependent on cell-cell contact and/or cytokines, such as IL-10.
The expression of IL-10 was first investigated within lesions of ACL patients. At the site of infection, levels of IL-10 mRNA expression remained constant whatever the duration of development of lesions (Fig. 3A). Furthermore, IL-10 levels secreted by intralesional T cells stimulated with L. guyanensis were quite hard to detect (11 ± 3.5 pg/ml for six patients) (data not shown). However, to determine if the small quantity of IL-10 produced at the site of infection could be responsible for the suppressive activity of Treg cells, intralesional total cells were isolated and cocultured in the presence of CD4+ CD25− T cells obtained from PBMC of the same patient and stimulated with live L. guyanensis in the presence of autologous APCs and neutralizing anti-IL-10 or control MAbs. As shown in Fig. 3B, neutralization of IL-10 only partially restored the IFN-γ produced by CD4+ CD25− T cells in four of the five ACL patients tested. These results demonstrate a moderate role for IL-10 in the suppressive activity of Treg cells and suggest that other mechanisms are involved in the suppressive activity of intralesional Treg cells obtained from lesions of ACL patients.
FIG. 3.
IL-10 and IDO are involved in intralesional Treg cell suppressive activity. (A) IL-10 mRNA levels were analyzed by real-time RT-PCR with lesions from patients with ACL with different times of duration of lesions. IL-10 mRNA levels were normalized to the β-actin endogenous gene level. Results are expressed as relative expression of IL-10 in lesions compared to the IL-10 mRNA level in PBMC. (B) Purified total T cells or CD4+ CD25+ T cells in skin lesions from patients with ACL were cocultured with autologous CD4+ CD25− T cells isolated from PBMC (ratio, 1:100) in the presence of live L. guyanensis, mitomycin-treated PBMC used as APCs, and anti-IL-10 or control MAb. After 5 days of culture, IFN-γ levels in supernatants of cultures were assessed using a specific IFN-γ ELISA kit. Numbers are percentages of suppression, calculated as described in Materials and Methods. *, P < 0.05 (Wilcoxon rank test) compared to CD4+ CD25− T cells. (C) Foxp3, CTLA-4, and IDO mRNA levels were analyzed by real-time RT-PCR with lesions from patients with ACL with different times of duration of lesions. Levels of the gene of interest were normalized to the β-actin endogenous gene level, and results are expressed as relative expression of the gene of interest in lesions compared to the level in PBMC. *, P < 0.05 (Wilcoxon rank test) for mRNA expression in lesions of less than 1 month compared to results obtained for lesions of 1 to 3 months and >3 to <6 months; **, P < 0.05 (Wilcoxon rank test) for mRNA expression in lesions of less than 1 month compared to results obtained for lesions of >3 to <6 months.
Treg cells can induce a response by stimulating APCs, particularly dendritic cells, through CLTA-4 activation and production of IDO, a molecule with demonstrated suppressive activities. Interestingly, as described for Foxp3 mRNA expression, the levels of IDO mRNA expression decreased with increasing times of duration of lesions (P < 0.05). Indeed, Fig. 3C clearly shows that a decrease of IDO mRNA expression in lesions from patients with lesions developing for more than 1 month (1 to 3 months and more than 3 months) is associated with a decrease of Foxp3 mRNA in lesions from the same groups of patients. In contrast, although CTLA-4 mRNA expression seemed to be lower in samples from patients with lesions for less than 1 month, there was no significant difference in samples obtained from patients with lesions lasting more than 1 month. Thus, CTLA-4 mRNA expression was maintained at similar levels, independently of the levels of Foxp3 and IDO mRNA expression (Fig. 3C).
Treg cells accumulate in lesions from patients with CCL.
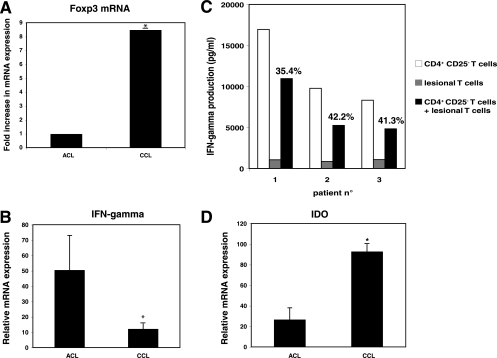
Since in the murine model of chronic infection with L. major it has been shown that Treg cells accumulate at the site of infection (1), we determined the presence of Treg cells in lesions from CCL patients (more than 6 months of lesion development). Levels of Foxp3 mRNA transcripts were significantly higher in specimens from patients with CCL than in those from patients with ACL. Indeed, an 8.5- ± 1.37-fold increase of Foxp3 mRNA expression was measured in lesions from CCL patients compared to mRNA levels in ACL patients (Fig. 4A). These results suggest that Treg cells accumulate preferentially in lesions of CCL patients.
FIG. 4.
High Treg cell levels in lesions from CCL patients. (A) Expression of Foxp3 in lesions of patients with ACL and CCL due to L. guyanensis. The Foxp3 mRNA level was normalized to the β-actin endogenous gene level in ACL and CCL lesion and expressed as the relative expression of Foxp3 mRNA expression. Results are presented as increases of the specific gene in patients with CCL compared to patients with ACL, defined as the reference (onefold change). *, P < 0.05 (Wilcoxon rank test). (B) IFN-γ mRNA levels were analyzed by real-time RT-PCR with lesions from patients with ACL or CCL. The IFN-γ mRNA level was normalized to the β-actin endogenous gene level, and results are expressed as relative expression of IFN-γ mRNA in lesions compared to the level in autologous PBMC. (C) Purified T cells from skin lesions from patients with CCL were cocultured with autologous CD4+ CD25− T cells isolated from PBMC (ratio, 1:100) in the presence of live L. guyanensis and mitomycin-treated PBMC used as APCs. After 5 days of culture, IFN-γ levels in supernatants of cultures were assessed using ELISA. Numbers represent percentages of suppression, calculated as described in Materials and Methods. *, P < 0.05 (Wilcoxon rank test). (D) IDO mRNA levels were analyzed by real-time RT-PCR with lesions from patients with ACL or CCL. IDO mRNA levels were normalized to the β-actin endogenous mRNA expression level, and results are expressed as relative IDO mRNA expression in lesion compared to the level in PBMC. *, P < 0.05 (Wilcoxon rank test).
To determine whether intralesional Treg cells control IFN-γ production by effector T cells in CCL patients, we analyzed the expression of IFN-γ transcripts within lesions. Levels of IFN-γ mRNA expression were significantly lower (fourfold) in chronic leishmaniasis than in acute leishmaniasis (P < 0.04) (Fig. 4B). Thus, to confirm whether total skin lesion-derived cells are able to actively inhibit IFN-γ secretion by effector T cells, skin lesion cells were isolated and cocultured in the presence of CD4+ CD25− T cells obtained from PBMC of the same patient and stimulated with live L. guyanensis in the presence of autologous APCs. For three subjects with CCL, the IFN-γ produced by CD4+ CD25− T cells in response to Leishmania antigens was strongly reduced by cells from skin lesions with suppression of IFN-γ of 35.4%, 42.2%, and 41.3% (for patients 1, 2, and 3, respectively) (Fig. 4C) (P < 0.05).
Since we demonstrated with ACL lesions that both IL-10 and IDO might contribute to the suppressive activity of Treg cells, we similarly analyzed the expression of these molecules within the lesions of CCL patients and compared them to those observed in ACL patients. Levels of IL-10 mRNA expression were not different in CCL and ACL patients (data not shown). However, the levels of IDO mRNA expression were statistically higher (3.54 times) in lesions from CCL patients than in those from ACL patients (Fig. 4D) (P < 0.05).
DISCUSSION
We previously demonstrated the presence in PBMC from healthy subjects of CD4+ CD25+ T cells producing TGF-β1, a phenotype that could correspond to Treg cells. These cells express CCR4 and CLA, suggesting that they could migrate to inflammatory sites within the skin. The fact that we were unable to detect a similar population of cells in PBMC from ACL patients suggested that these cells are effectively recruited to the site of parasite infection (18). In the present work, expression of Foxp3, a Treg cell-specific transcription factor, was analyzed, and we confirmed the presence of a larger number of Treg cells in PBMC from control subjects than in those from ACL patients infected with L. guyanensis. We also demonstrated the recruitment of Treg cells to the site of infection of these ACL patients. Indeed, Foxp3 mRNA expression was increased fivefold in biopsy specimens from ACL patients compared to PBMC of these patients, and flow cytometry analysis showed that the number of CD4+ T cells expressing Foxp3 was higher in PBMC from healthy subjects than in those from ACL patients. Intralesional Treg cells were shown to have suppressive activity, since CD4+ CD25+ T cells purified from biopsy specimens decreased IFN-γ production of CD4+ CD25− T cells stimulated with L. guyanensis. In addition, the inverse association between IFN-γ and Foxp3 mRNA expression within the lesions reinforced a local role in controlling effector immune responses for Treg cells in the lesions.
Sahli et al. previously reported the presence of Foxp3+ T cells in PBMC from patients with ACL due to L. braziliensis. Interestingly, levels of Treg cells were identical in healed subjects, those with active leishmaniasis, and resistant subjects. However, in these experiments, the level of Treg cells was not evaluated in control subjects, and the number of Treg cells within lesions was not determined (28). In another study, the accumulation of Treg cells with suppressive activity was described for lesions of patients suffering from leishmaniasis due to L. braziliensis (10), confirming that Treg cells are present predominantly at the site of infection during infection with Leishmania.
Altogether, these results demonstrate the importance of Treg cells in human infection with Leishmania. This was reinforced by recent results showing that Treg cells are associated with unresponsiveness to treatment in patients suffering from CL due to L. guyanensis (9).
We analyzed the association of Foxp3 mRNA levels with clinical data for ACL patients. We found no correlation except with the time of duration of lesions. Indeed, Foxp3 mRNA expression was statistically higher in lesions with a time of duration of less than 1 month. Interestingly, we have previously shown that a Th2 response precedes a Th1 response in lesions of ACL patients and that the switch from the Th2 to Th1 response occurs at around 1 month of development of lesions (7). Since in our present data the IFN-γ levels were higher in lesions with a duration of more than 1 month than in lesions with a duration of less than 1 month, we might hypothesize that a decrease of Treg cell expression is associated with the Th2/Th1 switch observed in these patients.
Several mechanisms have been described to explain the suppressive activity of Treg cells, including the presence of cytokines such as IL-10 and TGF-β. The fact that we were unable to detect TGF-β production by CD4+ CD25+ T cells isolated from skin lesions from either ACL or CCL patients and stimulated by L. guyanensis (data not show) suggests that TGF-β does not play a role in the suppression induced by Treg cells in lesions. Although IL-10 mRNA expression and IL-10 production by intralesional CD4+ CD25+ T cells were quite low in ACL patients, we analyzed its role in the suppressive activity of Treg cells in vitro. Total skin cells were cocultured in the presence of CD4+ CD25− T cells stimulated with L. guyanensis in the presence of a neutralizing anti-IL-10 MAb. Surprisingly, although the IL-10 produced by intralesional cells was low, its neutralization increased, albeit modestly, the IFN-γ production of CD4+ CD25− T cells, suggesting that some of the suppressive activity of Treg cells involves IL-10. However, since we were unable to analyze the exact role of each subpopulation of cells due to the extremely small numbers of viable CD25+ and CD25− T cells recovered from skin cells, we cannot rule out that the IL-10 produced by intralesional cells comes from intralesional Foxp3− T cells, not from Foxp3+ T cells, as already described for infection with other Leishmania species, either in humans (23) or in mice (2, 22, 23).
IDO, an enzyme involved in the rejection of allogeneic fetuses, has also been identified as a regulator of the immune response. IDO is upregulated at sites of inflammation and in cells exposed to cytokines such as IFN-γ and is further potentiated by IL-10 (15, 20). CTLA-Ig was also found to induce IDO expression through ligation to CD80/CD86 (15). Treg cells constitutively express CTLA-4 (25) and have been demonstrated to induce IDO expression. Interestingly, the IL-10 produced by Treg cells themselves could possibly maintain IDO expression in APCs (19). In the present work, we demonstrated that in lesions from ACL patients, levels of IDO mRNA expression paralleled the levels of Foxp3 mRNA, suggesting that IDO is one of the immunosuppressive molecules induced by Treg cells. The fact that CTLA-4 levels remained identical whatever the levels of Foxp3 and IDO mRNA expression suggests that CTLA-4 activation is not involved in this mechanism. However, CTLA-4 is expressed by a multitude of different cells, and thus the level of CTLA-4 mRNA expression detected in lesions from ACL patients could be due to its expression on different cells within skin and not only to Treg cells. Since IL-10 has been demonstrated to maintain IDO expression (15, 20), it may be speculated that low levels of intralesional IL-10 could maintain IDO expression.
The immunosuppressive role of IDO could be explained by different mechanisms, e.g., the deprivation of tryptophan compromising the function of T cells; the production of tryptophan metabolites, such as 3-OH-kyrunenine and 3-OH-anthralinic acid, which demonstrate some antiproliferative activity (14, 30); and the induction of apoptosis of Th1 cells but not Th2 cells in vitro (13). In this context, apoptosis of IFN-γ-producing cells might explain the low levels of IFN-γ mRNA expression detected in lesions with high levels of IDO and Foxp3 mRNA expression. However, the fact that the percentages of viable cells at the end of the coculture assays were identical in the presence or absence of CD4+ CD25+ T cells does not favor this hypothesis. Finally, IDO was recently shown to promote the maturation of DCs favoring the expansion of CD4+ CD25+ T cells (11, 16), and tryptophan metabolites were shown to enhance the expression of the Foxp3-encoding gene (12). Thus, we cannot exclude that tryptophan metabolites induced by IDO activation could results in Treg cell activation. Consequently, IDO could be the cause—and not the consequence—of Treg cell suppression.
Interestingly, IDO activity can be reversed in mice by the specific inhibitor 1-methyl tryptophan (15), suggesting that IDO could be a target for immunointervention in human infections. Indeed, inhibition of IDO activity might restore a functional immune response and, particularly, the production of an IFN-γ response. Moreover, IDO was first described as an antimicrobial molecule (24) and as an anti-Leishmania component (21).
In the present work, we clearly demonstrate a higher level of Treg cells within lesions from patients with CCL due to L. guyanensis than in those with ACL. The fact that levels of IFN-γ mRNA expression were lower in chronic than in acute lesions suggested that intralesional Treg cells are functionally suppressive. Indeed, cells purified from skin lesions of CCL patients downregulated the IFN-γ produced by CD4+ CD25− cells isolated from PBMC of the same patients in response to L. guyanensis stimulation. Altogether, these results demonstrate that the chronicity of lesions due to L. guyanensis infection is associated with local immunosuppression due to the presence of Treg cells, suggesting that Treg cells could have a physiological role in downregulation of Leishmania-specific immune responses. If this hypothesis is confirmed, the chronicity of diseases might be the consequence of the excessive downregulation of a successful effector response. We also demonstrated that the level of IDO mRNA expression is higher in CCL lesions than in ACL lesions. Since this high level of IDO mRNA expression is associated with high levels of Foxp3 mRNA, it reinforces the hypothesis that IDO might be one of the immunosuppressive molecules induced by Treg cells during human leishmaniasis.
In conclusion, we demonstrated a recruitment of Treg cells with active suppressive activity within the lesions of patients suffering from leishmaniasis due to L. guyanensis, particularly in CCL lesions. The precise mechanisms of suppression are not defined yet, but our present data suggest a role for IL-10 and IDO, indicating that the use of IDO and the metabolites of tryptophan as a target for chemotherapy should be examined carefully for infection with Leishmania.
Acknowledgments
We have no financial conflicts of interest.
This work was supported by Institute Pasteur, the French Ministry of Research, and the Swiss National Foundation (grants 310000-107719 and 310000-120325/1).
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Anderson, C. F., S. Mendez, and D. L. Sacks. 2005. Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. J. Immunol. 1742934-2941. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, C. F., M. Oukka, V. J. Kuchroo, and D. Sacks. 2007. CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 204285-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aseffa, A., A. Gumy, P. Launois, H. R. MacDonald, J. A. Louis, and F. Tacchini-Cottier. 2002. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J. Immunol. 1693232-3241. [DOI] [PubMed] [Google Scholar]
- 4.Belkaid, Y. 2007. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 7875-888. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid, Y., R. B. Blank, and I. Suffia. 2006. Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunol. Rev. 212287-300. [DOI] [PubMed] [Google Scholar]
- 6.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420502-507. [DOI] [PubMed] [Google Scholar]
- 7.Bourreau, E., J. Gardon, R. Pradinaud, H. Pascalis, G. Prevot-Linguet, A. Kariminia, and L. Pascal. 2003. Th2 responses predominate during the early phases of infection in patients with localized cutaneous leishmaniasis and precede the development of Th1 responses. Infect. Immun. 712244-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourreau, E., G. Prevot, J. Gardon, R. Pradinaud, and P. Launois. 2001. High intralesional interleukin-10 messenger RNA expression in localized cutaneous leishmaniasis is associated with unresponsiveness to treatment. J. Infect. Dis. 1841628-1630. [DOI] [PubMed] [Google Scholar]
- 9.Bourreau, E., C. Ronet, E. Darsissac, M. C. Lise, D. Sainte-Marie, E. Clity, F. Tacchini-Cottier, P. Couppie, and P. Launois. 2009. In leishmaniasis due to L. guyanensis, distinct intralesional IL-10 and Foxp3 mRNA expression are associated with unresponsivess to treatment. J. Infect. Dis. 199576-579. [DOI] [PubMed] [Google Scholar]
- 10.Campanelli, A. P., A. M. Roselino, K. A. Cavassani, M. S. Pereira, R. A. Mortara, C. I. Brodskyn, H. S. Goncalves, Y. Belkaid, M. Barral-Netto, A. Barral, and J. S. Silva. 2006. CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J. Infect. Dis. 1931313-1322. [DOI] [PubMed] [Google Scholar]
- 11.Chen, W., X. Liang, A. J. Peterson, D. H. Munn, and B. R. Blazar. 2008. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J. Immunol. 1815396-5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Luca, A., C. Montagnoli, T. Zelante, P. Bonifazi, S. Bozza, S. Moretti, C. D'Angelo, C. Vacca, L. Boon, F. Bistoni, P. Puccetti, F. Fallarino, and L. Romani. 2007. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J. Immunol. 1795999-6008. [DOI] [PubMed] [Google Scholar]
- 13.Fallarino, F., U. Grohmann, C. Vacca, R. Bianchi, C. Orabona, A. Spreca, M. C. Fioretti, and P. Puccetti. 2002. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 91069-1077. [DOI] [PubMed] [Google Scholar]
- 14.Frumento, G., R. Rotondo, M. Tonetti, G. Damonte, U. Benatti, and G. B. Ferrara. 2002. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 196459-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grohmann, U., C. Orabona, F. Fallarino, C. Vacca, F. Calcinaro, A. Falorni, P. Candeloro, M. L. Belladonna, R. Bianchi, M. C. Fioretti, and P. Puccetti. 2002. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat. Immunol. 31097-1101. [DOI] [PubMed] [Google Scholar]
- 16.Hill, M., S. Tanguy-Royer, P. Royer, C. Chauveau, K. Asghar, L. Tesson, F. Lavainne, S. Remy, R. Brion, F. X. Hubert, M. Heslan, M. Rimbert, L. Berthelot, J. R. Moffett, R. Josien, M. Gregoire, and I. Anegon. 2007. IDO expands human CD4+CD25high regulatory T cells by promoting maturation of LPS-treated dendritic cells. Eur. J. Immunol. 373054-3062. [DOI] [PubMed] [Google Scholar]
- 17.Ji, J., J. Masterson, J. Sun, and L. Soong. 2005. CD4+CD25+ regulatory T cells restrain pathogenic responses during Leishmania amazonensis infection. J. Immunol. 1747147-7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kariminia, A., E. Bourreau, H. Pascalis, P. Couppie, D. Sainte-Marie, F. Tacchini-Cottier, and P. Launois. 2005. Transforming growth factor beta 1 production by CD4+ CD25+ regulatory T cells in peripheral blood mononuclear cells from healthy subjects stimulated with Leishmania guyanensis. Infect. Immun. 735908-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellor, A. L., P. Chandler, B. Baban, A. M. Hansen, B. Marshall, J. Pihkala, H. Waldmann, S. Cobbold, E. Adams, and D. H. Munn. 2004. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int. Immunol. 161391-1401. [DOI] [PubMed] [Google Scholar]
- 20.Munn, D. H., M. D. Sharma, J. R. Lee, K. G. Jhaver, T. S. Johnson, D. B. Keskin, B. Marshall, P. Chandler, S. J. Antonia, R. Burgess, C. L. Slingluff, Jr., and A. L. Mellor. 2002. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 2971867-1870. [DOI] [PubMed] [Google Scholar]
- 21.Murray, H. W., A. Szuro-Sudol, D. Wellner, M. J. Oca, A. M. Granger, D. M. Libby, C. D. Rothermel, and B. Y. Rubin. 1989. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect. Immun. 57845-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagase, H., K. M. Jones, C. F. Anderson, and N. Noben-Trauth. 2007. Despite increased CD4+Foxp3+ cells within the infection site, BALB/c IL-4 receptor-deficient mice reveal CD4+Foxp3-negative T cells as a source of IL-10 in Leishmania major susceptibility. J. Immunol. 1792435-2444. [DOI] [PubMed] [Google Scholar]
- 23.Nylen, S., R. Maurya, L. Eidsmo, K. D. Manandhar, S. Sundar, and D. Sacks. 2007. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J. Exp. Med. 204805-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfefferkorn, E. R. 1984. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc. Natl. Acad. Sci. USA 81908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 192295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13151-177. [DOI] [PubMed] [Google Scholar]
- 27.Romero, G. A., M. V. Guerra, M. G. Paes, and V. O. Macedo. 2001. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: therapeutic response to meglumine antimoniate. Am. J. Trop. Med. Hyg. 65456-465. [DOI] [PubMed] [Google Scholar]
- 28.Salhi, A., V. Rodrigues, Jr., F. Santoro, H. Dessein, A. Romano, L. R. Castellano, M. Sertorio, S. Rafati, C. Chevillard, A. Prata, A. Alcais, L. Argiro, and A. Dessein. 2008. Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis. J. Immunol. 1806139-6148. [DOI] [PubMed] [Google Scholar]
- 29.Tang, Q., and J. A. Bluestone. 2008. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol. 9239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terness, P., T. M. Bauer, L. Rose, C. Dufter, A. Watzlik, H. Simon, and G. Opelz. 2002. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J. Exp. Med. 196447-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas, B. N., and L. U. Buxbaum. 2008. FcgammaRIII mediates immunoglobulin G-induced interleukin-10 and is required for chronic Leishmania mexicana lesions. Infect. Immun. 76623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]