Abstract
Paracoccidioidomycosis (PCM) is a systemic granulomatous disease caused by the dimorphic fungus Paracoccidioides brasiliensis. Anti-PCM vaccine formulations based on the secreted fungal cell wall protein (gp43) or the derived P10 sequence containing a CD4+ T-cell-specific epitope have shown promising results. In the present study, we evaluated new anti-PCM vaccine formulations based on the intranasal administration of P. brasiliensis gp43 or the P10 peptide in combination with the Salmonella enterica FliC flagellin, an innate immunity agonist binding specifically to the Toll-like receptor 5, in a murine model. BALB/c mice immunized with gp43 developed high-specific-serum immunoglobulin G1 responses and enhanced interleukin-4 (IL-4) and IL-10 levels. On the other hand, mice immunized with recombinant purified flagellins genetically fused with P10 at the central hypervariable domain, either flanked or not by two lysine residues, or the synthetic P10 peptide admixed with purified FliC elicited a prevailing Th1-type immune response based on lung cell-secreted type 1 cytokines. Mice immunized with gp43 and FliC and intratracheally challenged with P. brasiliensis yeast cells had increased fungal proliferation and lung tissue damage. In contrast, mice immunized with the chimeric flagellins and particularly those immunized with P10 admixed with FliC reduced P. brasiliensis growth and lung damage. Altogether, these results indicate that S. enterica FliC flagellin modulates the immune response to P. brasiliensis P10 antigen and represents a promising alternative for the generation of anti-PCM vaccines.
Paracoccidioidomycosis (PCM) is a granulomatous disease caused by the thermodimorphic fungus Paracoccidioides brasiliensis, which is prevalent in Brazil and other Latin American countries (5, 6, 33). Some individuals develop one of the two main clinical forms of PCM. The acute form is characterized by impaired cellular immunity, negative delayed-type hypersensitivity reactions, increased systemic proliferation of the fungus, and high mortality rate. The chronic form, ranging from mild to severe chronic disease, shows exacerbated host cellular immune responses and formation of granulomas containing fungal cells and may evolve to develop extensive sequelae, including fibrotic lesions and impairment of lung function (33, 39).
Vaccines against PCM are still not available for human use, but promising formulations have been experimentally tested during the last few years. Irradiated P. brasiliensis or cellular antigens fractionated by anion-exchange chromatography conferred partial protection against fungal proliferation in the murine model (11). The extracellular gp43 glycoprotein, the major diagnostic antigen of P. brasiliensis, is the most intensively studied component aimed at a vaccine for PCM control. Previous reports have shown that mice immunized with the purified protein, DNA, or anti-idiotypic monoclonal antibody were partially protected against challenges by P. brasiliensis (28, 36, 37, 40). A 15-amino-acid peptide (QTLIAIHTLAIRYAN), designated P10, contains the gp43 immunodominant CD4+ T-cell-specific epitope presented by major histocompatibility complex class II molecules from three different mouse haplotypes (37) and most human HLA-DR alleles (17, 18). Indeed, parenteral immunization with P10 in complete Freund adjuvant (CFA), or in the form of a truncated multiple-antigen peptide (MAP) complex, induced protective Th1 cellular immune responses in mice against intratracheal (i.t.) challenge with a virulent P. brasiliensis isolate (37, 38, 41).
The rational use of vaccines has been significantly improved after elucidation of innate immune mechanisms in mammalian cells. The recognition of distinct pathogen-associated molecular patterns by members of the Toll-like receptor (TLR) family initiates a signaling cascade mediated by adaptor proteins, including MyD88 and interleukin-1 (IL-1) receptor-associated kinase, that culminates in the production of proinflammatory cytokines, such as tumor necrosis factor alpha and IL-12, and increased expression of cell surface molecules involved in epitope presentation by antigen-presenting cells (APC) (1, 19). Proper APC activation by TLR agonists represents a key step for an effective adaptive immune response induced by pathogens or vaccines and explains, at least in part, the marked adjuvant effects of several bacterial molecules, including lipopolysaccharides, lipoproteins, peptidoglycan fragments, and flagellins (2).
Flagellin, the structural subunit of bacterial flagellum, is a highly conserved protein that induces TLR5-dependent inflammatory responses and exerts strong adjuvant effects on both antibody and cellular immune responses (12, 13, 30). Flagellin has been successfully used as a vaccine adjuvant to generate antigen-specific antibodies and T cells either when administered to mice as the native purified protein (4, 14, 20, 27) or as a hybrid protein genetically fused to the target antigen (10, 15, 16). Additionally, in contrast to other vaccine adjuvants, such as CFA, flagellin may exert strong adjuvant effects following administration through mucosal routes (14, 27).
In the present study, we have evaluated the adjuvant effects and protective efficacy of intranasal (i.n.) anti-PCM vaccine formulations based on Salmonella enterica serovar Dublin FliC flagellin and purified recombinant P. brasiliensis gp43 or the synthetic P10 peptide. In addition, recombinant chimeric flagellins genetically fused to P10 were also tested as potential anti-PCM vaccine antigens. The results demonstrate that S. enterica FliC flagellin modulates the murine immune system favoring either the generation of antibodies (gp43 plus FliC) or activation of cellular immune responses. In accordance with the administered vaccine formulation, mice challenged with P. brasiliensis were differentially protected against exacerbated fungal proliferation. The present results indicate that Salmonella FliC has an important role in the generation of mucus-delivered anti-P. brasiliensis peptide-based vaccine formulations. Furthermore, flagellin-based adjuvants may contribute to the understanding of immune mechanisms involved in PCM development.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are described in Table 1. The strain Salmonella enterica pv. Dublin SL5928 is a nonflagellated aroA attenuated vaccine strain (34). Salmonella pv. Dublin SL5930 is a SL5928 derivative transformed with the plasmid pLS408 encoding the gene fliCd, originally derived from Salmonella enterica serovar München, with a 48-bp deletion generated after removal of a EcoRV-EcoRV fragment from the central hypervariable domain leading to a single EcoRV site used for in-frame insertion of nucleotide sequences encoding heterologous epitopes. All S. Dublin and Escherichia coli strains were routinely cultivated at 37°C with shaking (200 rpm) in Luria-Bertani (LB) broth or on LB agar plates supplemented with ampicillin (100 μg/ml).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| Salmonella Dublin | ||
| SL5928 | aroA his fliC::Tn10 | 24 |
| SL5930 | SL5928 with pLS408; mobile | 24 |
| SLP10 | SL5928 with pLSP10 | This study |
| SLP10L | SL5928 with pLSP10L | This study |
| P. brasiliensis | ||
| Pb18 | Highly virulent human isolate of P. brasiliensis | 35 |
| Plasmids | ||
| pLS408 | pUC19 derivative carrying the fliCd gene from S. Müenchen with 48-bp deletion in the hypervariable region of the FliC gene bla | 24 |
| pLSP10 | pLS408 derivative with a 45-bp insert encoding the P10 epitope | This study |
| pLSP10L | pLS408 derivative with a 57-bp insert encoding the P10 epitope with two flanking lysines | This study |
Fungal strain.
The virulent P. brasiliensis Pb18 strain was maintained by weekly passages on solid Sabouraud medium at 36°C. Before experimental infection, cultures were grown in modified McVeigh-Morton medium at 36°C for 5 to 7 days (31). The fungal cells were washed in phosphate-buffered saline (PBS; pH 7.2) and counted in a hemocytometer. The upper part of cell suspensions that contained isolated or single budding cells was used in the infection experiments and for cell counting after decanting the clusters of yeast cells. The viability of fungal suspensions was determined by staining with trypan blue (Sigma, St. Louis, MO) and was always higher than 90%. The virulence of the Pb18 strain was checked in each experiment by infecting BALB/c mice i.t. and recovering the yeast cells from the infected organs.
Generation of hybrid flagellins genetically fused to P10.
Chimeric Salmonella flagellins were generated after cloning the complementary oligonucleotides into the single EcoRV site of the pLS408-clonded FliC-encoding gene (24). Complementary 45-base oligonucleotides P10fw (5′-GAA ACC CTG ATT GCG ATT CAT ACC CTG GCG ATT CGC TAT GCG AAC-3′) and P10rv (5′-CTT TGG GAC TAA CGC TAA GTA TGG GAC CGC TAA GCG ATA CGC TTG-3′), encoding P10 (QTLIAIHTLAIRYAN), were melted at 65°C, annealed by slowly cooling at room temperature, and blunt end ligated with T4 DNA ligase to the EcoRV-cleaved pLS408. The same procedure was repeated with P10KKfw and P10KKrv oligonucleotides that, in addition to the sequence encoding the P10 peptide, carried on both 5′ and 3′ ends the sequence “AAA AAA,” encoding two additional lysine residues flanking the heterologous epitope (KKQTLIAIHTLAIRYANKK) genetically fused to FliC flagellin, in order to improve the proteolytic processing by cathepsin B and enhance epitope processing and presentation by APC (42). The resulting plasmids (pLSP10, encoding the recombinant FliC genetically fused to P10, and pLSP10L, in which the P10 peptide is flanked by two lysine residues) were introduced by electroporation (using 0.2-cm electroporation cuvettes at 600 Ω, 25 μF, and 1.75 kV; Gene-Pulser [Bio-Rad, Hercules, CA]) into E. coli strain DH5α, and transformants were selected on LB plates containing ampicillin. Recombinant plasmids with the right inserts were screened by EcoRV digestion and sequenced with the BigDye Terminator DNA sequencing kit (PerkinElmer Applied Biosystems, Waltham, MA) using a 15-mer primer (5′-CCA GGT GCC TAC ACC CCG-3′) corresponding to a sequence located 50 bp downstream of the EcoRV insertion site in pLS408. The recombinant plasmids encoding fliCd genetically fused to P10 or the sequence flanked by two additional lysines were named pLSP10 and pLSP10L, respectively. Finally, the plasmids pLSP10 and pLSP10L were introduced into the flagellin-negative S. Dublin SL5928 strain by electroporation and the recombinant vaccine strains named SLP10 and SLP10L, respectively.
Purification of Salmonella flagellins.
Salmonella flagellins, comprising FliC, FliCd-P10, and FliCd-P10L, were harvested from the respective S. Dublin SL5930, SLP10, and SLP10L strains cultivated in LB broth, according to a previously described procedure (4). Briefly, flagellins were obtained after centrifugation of cells, suspended in PBS (pH 7.4), and sheared in a bench mixer at maximal speed (a 1-min treatment repeated three times), followed by another centrifugation step to remove the bacterial cells. Broken flagellar fragments were precipitated with acetone, suspended in PBS, and finally, submitted to heat treatment (65°C for 30 min) to dissociate the flagellin monomers. The protein concentration was determined using the bicinchoninic acid assay (Pierce, Rockford, IL), and the purity of the protein preparations was monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Removal of contaminating lipopolysaccharide was accomplished with Detoxi-Gel columns (Pierce, Rockford, IL) according to the manufacturer's instructions. Endotoxin levels, determined with the chromogenic Limulus amebocyte lysate assay (Cambrex Bio Science, Walkersville, MD), were always below 3.0 endotoxin units/μg of protein.
Purification of gp43 antigen.
P. brasiliensis Pb18 was cultivated in yeast Sabouraud liquid medium for 7 days at 36°C with shaking. The culture was inactivated by adding 0.2 g of thimerosal (Merthiolate)/liter filtered through filter paper, concentrated in a vacuum at 40°C, and dialyzed against distilled water. Purification of gp43 was obtained by affinity chromatography on Affi-Gel (Bio-Rad, Hercules, CA) bound to anti-gp43 monoclonal antibody as previously described (29). Elution was carried out with 50 mM citrate buffer, pH 2.8. The eluate was concentrated in Amicon 10K cells, and the antigen preparation was monitored by SDS-PAGE revealed with silver staining. The protein content was determined by the Bradford method (3).
SDS-PAGE.
SDS-PAGE was performed following standard procedures using a Mini-Protean II vertical electrophoresis unit (Bio-Rad, Hercules, CA). Proteins sorted in 12% polyacrylamide gels were stained with Coomassie blue.
Immunization regimens.
Isogenic pathogen-free 8- to 12-week-old male BALB/c (H-2d) mice were supplied by the Isogenic Mouse Breeding Facility of the Department of Parasitology, Institute of Biomedical Sciences (ICB), University of São Paulo (USP). Animal handling was carried out in accordance with the Brazilian code for use of laboratory animals and was approved by the Ethics Committee of ICB, USP. Groups of 8 to 10 animals were immunized via the i.n. route with 15 μg of each fusion protein (FliCd-P10 or FliCd-P10L) or with a mixture of 20 μg P10 peptide or 25 μg gp43 protein with 5 μg FliC flagellin. Control groups were immunized with sterile PBS, 25 μg of gp43, 5 μg FliC flagellin, or 20 μg of P10 peptide in Freund adjuvant 1:1 (vol/vol). The formulations were instilled into the nostrils (5 μl/nostril) with a micropipette on days 0, 21, and 28. The control group immunized with P10 in Freund adjuvant received 1 dose (20 μg of the peptide plus CFA in a total volume of 50 μl) via the subcutaneous route (rear footpads) and 3 doses (20 μg of the peptide in incomplete Freund adjuvant; a total volume of 200 μl) administered intraperitoneally at 1-week intervals. Immune responses were evaluated 7 days after immunization and 60 days after fungal challenge.
Challenge with P. brasiliensis yeast cells.
Eighty days after the last immunization, mice were inoculated i.t. with 3 × 105 yeast cells/animal of virulent P. brasiliensis Pb18 grown on Sabouraud agar and suspended in sterile saline (0.85% NaCl). A maximum of 50 μl was inoculated per mouse. Briefly, mice were anesthetized intraperitoneally with 200 μl of a solution containing 80 mg of ketamine/kg of body weight and 10 mg/kg of xylazine (both from União Química Farmacêutica, Brazil). After approximately 10 min, the necks were extended and the tracheas exposed at the level of the thyroid. For the i.t. inoculation, a 26-gauge needle was used and the incisions were sutured, right afterwards, with a 5 to 0 silk thread.
Fungal burden in organs of infected mice.
Mice were sacrificed 60 days after i.t. infection, and the fungal burden was measured by CFU. Sections of the lungs, livers, and spleens were removed, weighed, and homogenized using a tissue grinder in 10 ml of sterile PBS. The corresponding pellets were resuspended and homogenized each in 1 ml of PBS. A 100-μl sample of this suspension was plated on solid brain heart infusion medium supplemented with 4% fetal calf serum (Gibco, NY), 5% spent P. brasiliensis (strain 192) culture supernatant, streptomycin/penicillin (10 IU/ml) (Cultilab, Brazil), and cycloheximide (500 mg/ml) (Sigma, St. Louis, MO). Petri dishes were incubated at 36°C for at least 20 days, and colonies were counted (1 colony = 1 CFU) (35).
Lung histopathology.
Following immunization with different vaccine formulations, BALB/c mice were i.t. infected and sacrificed after 2 months. The lungs were excised, fixed in 10% buffered formalin, and embedded in paraffin for sectioning. The sections were stained with hematoxylin-eosin and examined microscopically (Optiphot-2; Nikon, Tokyo, Japan).
Cytokine determination by enzyme-linked immunosorbent assay.
Cytokine analysis was performed 7 days after the immunizations and 2 months after infection of the animals. Lung sections (right and left alternating) of mice were homogenized in 2 ml of PBS in the presence of protease inhibitors (Boehringer Mannheim, Indianapolis, IN). The homogenates were centrifuged, and the supernatants were frozen at −80°C until tested. The supernatants were assayed for IL-4, IL-10, IL-12, and gamma interferon (IFN-γ) using enzyme-linked immunosorbent assay kits (BD PharMingen, San Diego, CA). The detection limits of such assays were as follows: 7.8 pg/ml for IL-4, 31.25 pg/ml for IL-10 and IFN-γ, and 62.5 pg/ml for IL-12p40, as indicated by the manufacturer.
Statistical analysis.
Data were analyzed by one-way analysis of variance and Student's t test followed by Tukey's honestly significant difference test and Dunnett's multiple comparison tests to compare the differences between the mean values of the immunization groups studied.
RESULTS
Mice immunized with purified gp43 admixed with Salmonella FliC flagellin develop specific antibody responses.
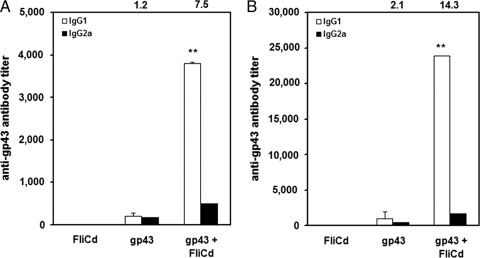
Male BALB/c mice received three i.n. doses of purified gp43 alone or admixed with purified FliC, and the serum gp43-specific immunoglobulin G (IgG) responses were measured 7 days after the last immunization and 60 days after the i.t. challenge with P. brasiliensis strain Pb18. Enhanced gp43-specific IgG1 antibodies were detected in noninfected mice immunized with the FliC-containing vaccine formulation (IgG1 titer, 3,804 ± 28.83) but not in animals immunized with purified gp43 (IgG1 titer, 207 ± 75.12) (Fig. 1A). A further 6.2-fold increase in the anti-gp43 serum IgG1 was detected in vaccinated mice following challenge with Pb18 (average IgG1 titer, 23,924 ± 21.00) in mice immunized with the FliC-containing vaccine formulation (Fig. 1B). Determination of the specific IgG subclasses elicited in mice immunized with gp43/FliC vaccine formulation revealed a biased type 2 response with IgG1/IgGa ratios of 7.5 and 14.3 before and after the fungus challenge, respectively (Fig. 1). These results indicate that Salmonella FliC flagellin acted as a strong antibody-inducing adjuvant when admixed with purified P. brasiliensis gp43 following mucosal administration in BALB/c mice.
FIG. 1.
Characterization of the specific serum IgG subclass response elicited in mice i.n. immunized with gp43 or gp43 admixed with FliC. (A) Anti-gp43-specific IgG subclass detected 1 week after the last immunization dose. (B) Anti-gp43-specific IgG subclass detected in vaccinated mice 2 months after i.t. challenge with the Pb18 strain. gp43, mice immunized with 3 doses of purified gp43; gp43 + FliCd, mice immunized with purified gp43 admixed with FliC. For the other groups (FliCd, FliCd-P10, FliCd-P10L, and P10 + FliCd), a gp43-specific antibody response was not detected. Values are means of endpoint titers plus standard deviations for serum pools (n = 8) prepared from each mouse group. Data are representative of two independent experiments with similar results. The IgG1/IgG2a ratio of each immunization group is indicated at the top of the figure. Asterisks indicate a statistically significant difference observed between mice immunized with gp43 and mice immunized with gp43 admixed with FliC (P ≤ 0.01).
Generation of vaccine formulations based on P10 genetically fused or admixed with Salmonella FliC.
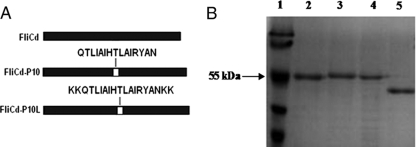
Additional anti-P. brasiliensis vaccine formulations were tested and employed the P10 peptide in combination with FliC. The first approach relied on the generation of hybrid flagellins in which the P10 minigene was wedged in frame at the central hypervariable domain of FliC, a permissive insertion site that did not affect both the inflammatory and adjuvant properties of Salmonella flagellin (Fig. 2A). The resulting recombinant S. Dublin SLP10 (encoding FliCd-P10) and SLP10L (encoding FliCd-P10L) strains were nonmotile in semisolid agar plates but expressed abundant extracellular flagella composed by hybrid flagellins with slightly altered molecular weights, as demonstrated by SDS-PAGE of purified flagella (Fig. 2B). After purification, the flagellin preparations were >95% pure and endotoxin levels were below 3.0 endotoxin units/μg of protein. Another P10-based vaccine formulation involved admixing of purified native FliC (5 μg) with the synthetic P10 peptide (20 μg).
FIG. 2.
Generation of recombinant hybrid flagellins genetically fused to the CD4+ T-cell-specific gp43-derived P10 epitope. (A) Schematic representation of the recombinant flagellins after P10 in-frame insertion at FliC central hypervariable domain. The two recombinant flagellins carried the P10 peptide (FliCd-P10) or the P10 peptide with lysine residues on each side of the fusion site (FliCd-P10L). (B) Coomassie blue-stained 12% polyacrylamide gel loaded with flagellins extracted from different Salmonella strains. Lane 1, molecular mass markers (Fermentas); lane 2, FliCd flagellin extracted from S. Dublin strain SL5930 (with no insert); lane 3, FliCd-P10 flagellin extracted from S. Dublin strain SLP10; lane 4, FliCd-P10L flagellin extracted from S. Dublin strain SLP10L; lane 5, purified gp43. Each well was loaded with approximately 2 μg of purified flagellins or gp43.
Cytokine expression patterns in mice immunized with different vaccine formulations containing Salmonella FliC flagellin.
The cytokine (IL-4, IL-10, IL-12, and IFN-γ) expression pattern in the lung tissue of mice immunized with the different vaccine formulations was determined 7 days after the last immunization dose and 60 days following the i.t. challenge with P. brasiliensis Pb18 yeast forms. As shown in Table 2, the concentrations of IL-4 and IL-10 were low in lung extracts from all immunized groups except in animals immunized with purified gp43 admixed with FliC, in which IL-4 and mainly IL-10 appeared in higher concentrations than in nonimmunized mice or mice vaccinated with FliC only. This picture changed considerably when the same cytokines were measured in vaccinated mice 2 months after the challenge with the Pb18 strain. The IL-4 and IL-10 concentrations in mice immunized with gp43 were approximately 30% lower than the values detected in nonimmunized animals, but the addition of FliC enhanced production of cytokines to levels similar to those found in the nonimmunized control group. Mice immunized with the recombinant hybrid P10/flagellins had IL-4 and IL-10 levels similar to animals immunized only with gp43 or FliC. On the other hand, mice immunized with FliC admixed with the synthetic P10 peptide had IL-4 and IL-10 values equal to those in nonchallenged vaccinated animals, which were statistically different from the values in animals immunized only with FliC.
TABLE 2.
Cytokine levels detected in lungs of mice immunized with different vaccine formulationsa
| Vaccine group | Level (ng/ml) (mean ± SD) of cytokine:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IL-4
|
IL-10
|
IL-12
|
IFN-γ
|
|||||
| BC | AC | BC | AC | BC | AC | BC | AC | |
| PBS | 0.08 ± 0.03 | 6.22 ± 0.41 | 0.07 ± 0.07 | 14.42 ± 1.95 | 0.25 ± 0.44 | 25.90 ± 3.86 | 0.05 ± 0.03 | 4.37 ± 0.53 |
| FliCd | 1.50 ± 0.24 | 2.93 ± 0.64 | 2.76 ± 0.29 | 7.52 ± 1.97 | 5.33 ± 1.34 | 22.00 ± 1.56 | 1.23 ± 0.07 | 9.96 ± 1.08 |
| gp43 | 0.20 ± 0.01b | 4.77 ± 0.55b | 0.18 ± 0.10b | 10.25 ± 2.09 | 3.07 ± 0.46b | 30.07 ± 1.44b | 0.66 ± 0.09b | 12.62 ± 0.44b |
| gp43 + FliCd | 3.01 ± 0.82b | 6.05 ± 1.16b | 4.10 ± 0.39c | 14.40 ± 4.90b | 7.46 ± 0.46b | 36.24 ± 2.05c | 2.25 ± 0.49b | 20.61 ± 4.13c |
| FliCd-P10 | 0.82 ± 0.56 | 3.21 ± 0.83 | 2.39 ± 0.15 | 9.48 ± 2.30 | 4.38 ± 0.97 | 30.67 ± 3.10b | 1.26 ± 0.25 | 4.89 ± 1.05 |
| FliCd-P10L | 0.60 ± 0.30b | 3.16 ± 0.22 | 1.00 ± 0.70b | 8.95 ± 0.93 | 3.65 ± 0.83 | 22.76 ± 2.26 | 0.44 ± 0.06b | 7.16 ± 1.40b |
| P10 + FliCd | 1.50 ± 0.49 | 2.80 ± 0.06 | 1.47 ± 0.88b | 1.46 ± 0.66b | 9.40 ± 0.86c | 36.63 ± 2.37c | 2.35 ± 0.19b | 18.10 ± 0.25b |
Immunization groups (5 to 8 animals/group) are as described in the legend to Fig. 2. BC, before challenge; AC, after challenge.
P < 0.05; statistically significant difference relative to that of mice immunized only with the FliCd flagellin.
P < 0.01; statistically significant difference relative to that of mice immunized only with the FliCd flagellin.
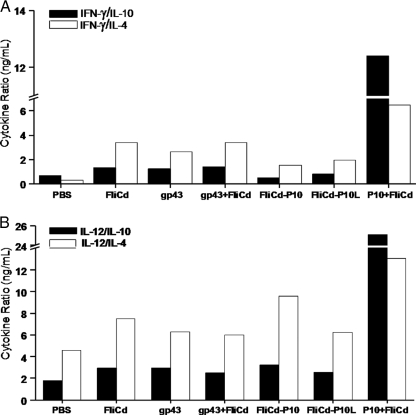
Measurements of IL-12 and IFN-γ in vaccinated mice showed that all tested vaccines led to enhanced cytokine expression in lung cells, but statistically significant differences with regard to nonimmunized animals were observed only in animals immunized with gp43 and P10 admixed with FliC (Table 2). Interestingly, there was no significant enhancement in IL-12 and IFN-γ levels detected in mice immunized with the recombinant flagellins compared to mice immunized only with FliC, both before and after i.t. challenge with Pb18. However, mice immunized with the P10 and FliC mixture, as well as those immunized with gp43 plus FliC, produced enhanced IL-12 and IFN-γ levels similar to those detected in mice immunized with gp43 admixed with FliC. On the other hand, the IL-10 levels of P10 plus FliC-vaccinated mice remained lower than those detected in mice immunized with FliC, particularly after challenge with Pb18. Collectively, these data suggest that the vaccine formulation based on P10 admixed with FliC induced a predominant Th1 immune response compared to mice immunized with the other tested vaccine formulations. Determination of the IFN-γ/IL-4 (or IFN-γ/IL-10) and IL-12/IL-4 (or IL-12/IL-10) ratios clearly demonstrated that mice immunized with P10 admixed with FliC developed a more pronounced Th1-biased immune response compared to animals immunized with the other vaccine formulations (Fig. 3).
FIG. 3.
Cytokine relative ratios measured in lungs of mice after challenge with the Pb18 strain. All cytokines were measured in whole extracts of lung cells. Immunization groups and specific cytokine values used in the cytokine ratio determination were as depicted in Table 2.
Growth of P. brasiliensis in lung tissues of mice vaccinated with the different vaccine formulations.
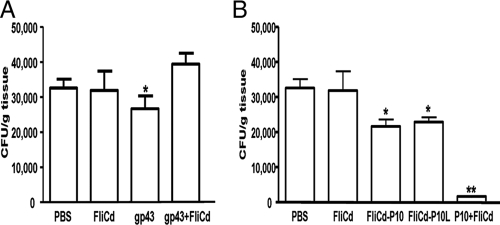
The protective effects of the vaccine formulations were determined 2 months after the i.t. challenge with the virulent Pb18 strain. CFU counts were determined in homogenized lung tissue from vaccinated animals as well as from nonimmunized mice and those inoculated only with purified FliC (Fig. 4). In mice i.n. immunized with gp43 only, there was a significant reduction in the number of fungal colonies compared to the nonimmunized (PBS) or the FliC-immunized groups, in agreement with previously described results based on parenteral immunizations (37). Mice immunized with gp43 admixed with FliC, however, showed enhanced fungal proliferation in the lung tissues compared to the nonimmunized (PBS) or the FliC-immunized groups. Mice immunized with the hybrid flagellins (FliCd-P10 or FliCd-P10L) showed a reduction in the number of viable yeast cells similar to that observed in mice immunized with gp43 without adjuvant. Furthermore, mice immunized with the vaccine formulation prepared with the synthetic P10 peptide admixed with FliC had a drastically reduced number of viable fungal cells recovered from their lung tissues (<100 CFU/g lung tissue) 2 months after challenge with the Pb18 strain. In contrast to mice immunized with gp43 or gp43 plus FliC, no viable yeast cells were recovered from spleen and liver homogenates from mice vaccinated with P10 admixed with FliC or the hybrid flagellins (data not shown).
FIG. 4.
Detection of viable fungal cells in lung tissues of vaccinated mice after i.t. challenge with P. brasiliensis Pb18. (A) Detection of viable fungi in mice i.n. immunized with purified gp43 or gp43 admixed with FliC. Immunization groups are as described in the legend to Fig. 2. (B) Detection of viable fungal cells in mice i.n. immunized with P10 epitope genetically fused with flagellin (FliCd-P10 or FliCd-P10L) or synthetic P10 peptide admixed with FliC flagellin. PBS, mice immunized only with PBS; FliCd, mice immunized only with FliC; FliCd-P10 and FliCd-P10L, mice immunized with purified recombinant FliCd-P10 and FliCd-P10L, respectively; P10+FliCd, mice immunized with P10 admixed with FliCd. All mice groups were i.t. challenged with the Pb18 strain and sacrificed 2 months later for determination of CFU in homogenized lung tissue. The same experiments were repeated three times. Each bar represents the medium number plus standard deviation in organs collected from 8 to 10 animals in each group. Asterisks indicate statistically significant differences between results detected in mice immunized with gp43 and P10 and those in mice immunized only with FliC (*, P ≤ 0.05; **, P ≤ 0.01).
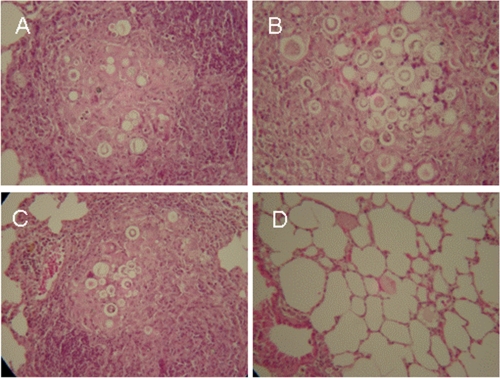
Histological analysis of lung tissue damage in immunized mice challenged with P. brasiliensis.
Histopathological analyses of lung tissue samples collected from mice submitted to different vaccine formulations showed that mice immunized with gp43 admixed with FliC had extensive tissue destruction, with abundant cellular infiltration (Fig. 5B). Exudative epithelioid granulomas containing giant cells with multiplying fungal cells were also observed in this vaccination group to a higher extent than in nonimmunized animals (Fig. 5A). Mice immunized with the hybrid recombinant FliCd-P10 and FliCd-P10L flagellins showed lower numbers of exudative lesions than those detected in animals immunized with FliC only or nonimmunized animals (Fig. 5C). The number of visible, detectable, and viable fungi was also lower than in the control groups. In contrast, lung tissues of mice immunized with P10 admixed with flagellin were virtually devoid of granulomas and viable fungal cells. The lung tissues of mice immunized with P10 plus FliC showed preserved alveolar organization with no indication of phagocytic cell infiltration (Fig. 5D).
FIG. 5.
Representative histopathology of lung lesions caused by P. brasiliensis strain Pb18 in mice immunized with different vaccine formulations. Tissue samples were collected 2 months after i.t. challenge with strain Pb18. (A) Lung section from PBS-immunized mouse with granuloma containing multiple viable fungal cells. (B) Lung section from a mouse immunized with gp43 admixed with FliC. Observe the extensive granulomatous lesions with intense cellular infiltration and large number of multiplying fungal cells. (C) Lung section from mouse immunized with the hybrid FliCd-P10L. (D) Lung section from mouse immunized with P10 admixed with FliC showing preserved alveolar structure and absence of granulomatous lesions and fungal cells. All sections were amplified 40-fold and stained with hematoxylin-eosin.
DISCUSSION
In the present study, mucus-delivered vaccine formulations based on the Salmonella FliC flagellin revealed contrasting, but rather interesting, results regarding induction of specific immune response and prophylactic protection against a virulent P. brasiliensis strain in the murine model. While i.n. administration of purified gp43 admixed with FliC induced a Th-2-biased immune response, leading to exacerbated fungal multiplication and tissue damage, vaccine formulations based on hybrid flagellins genetically fused with the gp43-derived P10 sequence, and mainly purified FliC admixed with synthetic P10 peptide, resulted in a Th1 predominant immune response and enhanced protection to the fungal challenge in vaccinated mice. The present evidence represents the first attempt to develop experimental anti-PCM mucosal vaccines and extends the knowledge about Salmonella flagellin adjuvant effects associated with different antigens.
The mucus-delivered vaccines have several advantages over conventional parenteral vaccines. For example, mucosal vaccines are easier to administer, lack iatrogenic infection risks, and more importantly, induce broader immunity, including activation of both systemic and local immune responses, a feature that may be particularly relevant for airborne infections. Therefore, the incorporation of Salmonella flagellin, a potent adjuvant known to act both parenterally and at mucosal sites, to P10-based vaccine formulations represented an alternative to the Freund adjuvant previously used in other anti-PCM vaccines. Indeed, it opens a renewed perspective for a future clinical use.
The epitope-based vaccine concept was designed as a strategy to preserve antigen immunogenicity but avoid potential undesirable effects, such as activation of suppressive immune responses or induction of self-reacting antibodies (21). The low immunogenicity of synthetic peptides, which represents a major drawback in the development of effective peptide-based vaccines, stimulated parallel experimental procedures, such as the use of potent adjuvants, synthesis of tandem repeats or MAPs, and genetic fusion with carrier proteins (25). In the present study, we have shown that the combination of the synthetic P10 peptide and the Salmonella FliC flagellin elicited strong activation of CD4+ T-cell-dependent immune responses leading to the efficient control of fungal infection in vaccinated mice. The best results were achieved with the peptide admixed with the adjuvant, thus avoiding complex and expensive chemical synthetic procedures or generation and purification of hybrid peptides by genetic engineering methods. The rather promiscuous binding of P10 to several major histocompatibility complex class II molecules, both from mice and humans (17, 37), in combination with the strong mucosal adjuvant effects of Salmonella flagellins resulted in enhanced vaccine efficacy, leading to more efficient control of fungus multiplication.
The genetic fusion of ovalbumin or the influenza virus M2 protein to Salmonella flagellins was required to generate antigen-specific B- and T-cell-dependent responses (15, 16). In our hands, coadministration of flagellin and the P10 peptide resulted in a higher Th1-biased immune response than that in mice immunized with hybrid flagellin genetically fused with P10. Linking antigens to flagellin would supply in a single molecule the signals required for activation and maturation of APC, but the present results based on a mucus-delivered formulation as well as other parenterally delivered vaccines (4, 23) clearly show that genetic fusion of flagellin to the target antigen does not represent a requirement for proper stimulation of the immune system by flagellin-containing vaccine formulations.
Quantification of IFN-γ and IL-12 indicated that mice immunized with P10 admixed with FliC developed a more pronounced Th1 immune response than mice immunized with the recombinant hybrid flagellins (FliCd-P10 and FliCd-P10L). Additionally, determination of the IFN-γ/IL-4 and IFN-γ/IL-10 ratios (as well as the IL-12/IL-4 and IL-12/IL-10 ratios) showed that mice immunized with FliC and P10 elicited a predominant Th1 immune response to other immunization groups, including mice immunized with gp43 and FliC and those immunized with the recombinant hybrid P10-containing flagellins. Indeed, induction of a Th1-biased immune response positively correlated with asymptomatic and mild forms of PCM in humans as well as resistance to P. brasiliensis infection in mice (9, 22, 26, 32). Additionally, the lack of anti-gp43 antibodies in animals immunized with the P10/FliC formulation further showed that immunization with P10 peptide avoids other gp43 sequences involved in nonprotective anti-P. brasiliensis immune responses. The possibility to add another gp43-derived peptide, which reacts with a protective monoclonal antibody (7), may further enhance the efficacy of the vaccine formulation by means of a proper Th1 response in combination with a protective antibody response.
Innate immunity has a pivotal role on the control of P. brasiliensis replication, as well as other microbial pathogens, in different mammalian hosts (8). TLR ligands directly interact with macrophages and dendritic cells, leading to inflammatory responses required for the direct elimination of the pathogen and generation of protective adaptive responses. In this study, we demonstrated that incorporation of Salmonella FliC, a TLR-5 ligand, may trigger anti-PCM immune responses ranging from complete prophylactic protection to exacerbated parasite multiplication according to the nature of the antigen tested, thus offering new tools for the understanding of the immunological mechanisms leading to resistance or sensitivity to P. brasiliensis. In addition, the evidence that the immunogenicity of epitope-based vaccines, when associated with Salmonella FliC, may elicit a protective immune response in vaccinated mice raises new perspectives for the development of improved vaccine formulations and warrants further studies aimed at the prophylactic and therapeutic control of PCM.
Acknowledgments
This work was supported by a grant from Fapesp (São Paulo State Foundation for Research Support) and a Brazilian federal government grant for the Millennium Institute for Vaccine Development and Technology (CNPq).
We acknowledge the valuable technical assistance of L. C. Silva.
Editor: A. Casadevall
Footnotes
Published ahead of print on 9 February 2009.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signaling. Nat. Rev. Immunol. 4499-511. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124783-801. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72248-254. [DOI] [PubMed] [Google Scholar]
- 4.Braga, C. J. M., L. M. Massis, B. C. G. Alencar, M. M. Rodrigues, M. E. Sbrogio-Almeida, and L. C. S. Ferreira. 2008. Cytotoxic T cell adjuvant effects of three Salmonella enterica flagellins. Braz. J. Microbiol. 3944-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borges-Walmsley, M. I., D. Chen, X. Shu, and A. R. Walmsley. 2002. The pathobiology of Paracoccidioides brasiliensis. Trends Microbiol. 1080-87. [DOI] [PubMed] [Google Scholar]
- 6.Brummer, E., E. Castaneda, and A. Restrepo. 1993. Paracoccidioidomycosis: an update. Clin. Microbiol. Rev. 689-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buissa-Filho, R., R. Puccia, A. F. Marques, F. A. Pinto, J. E. Muñoz, J. D. Nosanchuk, L. R. Travassos, and C. P. Taborda. 2008. The monoclonal antibody against the major diagnostic antigen of Paracoccidioides brasiliensis mediates immune protection in infected BALB/c mice challenged intratracheally with the fungus. Infect. Immun. 763321-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calich, V. L. G., T. A. da Costa, M. Felonato, C. Arruda, S. Bernardino, F. V. Loures, L. R. R. Ribeiro, R. C. Valente-Ferreira, and A. Pina. 2008. Innate immunity to Paracoccidioides brasiliensis infection. Mycopathologia 165223-236. [DOI] [PubMed] [Google Scholar]
- 9.Cano, L. E., S. Y. S. Kashino, C. Arruda, D. Andre, C. F. Xidieh, L. M. Singer-Vermes, C. A. C. Vaz, E. Burger, and V. L. G. Calich. 1998. Protective role of gamma interferon in experimental pulmonary paracoccidioidomycosis. Infect. Immun. 66800-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuadros, C., F. J. Lopez-Hernandez, A. L. Dominguez, M. McClelland, and J. Lustgarten. 2004. Flagellin fusion proteins as adjuvants or vaccines induce specific immune responses. Infect. Immun. 722810-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.do Nascimento Martins, E. M., B. S. Reis, V. C. Fernandes, M. M. S. Costa, A. M. Góes, and A. S. R. de Andrade. 2007. Immunization with radioattenuated yeast cells of Paracoccidioides brasiliensis induces a long lasting protection in BALB/c mice. Vaccine 257893-7899. [DOI] [PubMed] [Google Scholar]
- 12.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowisk, and J. L. Madara. 2001. Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflamatory gene expression. J. Immunol. 1671882-1885. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 4101099-1103. [DOI] [PubMed] [Google Scholar]
- 14.Honko, A., N. Sriranganathan, C. J. Lees, and S. B. Mizel. 2006. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect. Immun. 741113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huleatt, J. W., A. R. Jacobs, J. Tang, P. Desai, E. B. Kopp, Y. Huang, L. Song, V. Nakaar, and T. J. Powell. 2007. Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine 25763-775. [DOI] [PubMed] [Google Scholar]
- 16.Huleatt, J. W., V. Nakaar, P. Desai, Y. Huang, D. Hewitt, A. Jacobs, J. Tang, W. McDonald, L. Song, R. K. Evans, S. Umlauf, L. Tussey, and T. J. Powell. 2008. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine 26201-214. [DOI] [PubMed] [Google Scholar]
- 17.Iwai, L. K., M. Yoshida, J. Sidney, M. A. Shikanai-Yasuda, A. C. Goldberg, M. A. Juliano, J. Hammer, L. Juliano, A. Sette, J. Kalil, L. R. Travassos, and E. Cunha-Neto. 2003. In silico prediction of peptides binding to multiple HLA-DR molecules accurately identifies immunodominant epitopes from gp43 of Paracoccidioides brasiliensis frequently recognized in primary peripheral blood mononuclear cell responses from sensitized individuals. Mol. Med. 9209-219. [PMC free article] [PubMed] [Google Scholar]
- 18.Iwai, L. K., M. Yoshida, A. Sadahiro, R. W. da Silva, M. L. Marin, A. C. Goldberg, M. A. Juliano, L. Juliano, M. A. Shikanai-Yasuda, J. Kalil, E. Cunha-Neto, and L. R. Travassos. 2007. T cell recognition of Paracoccidioides brasiliensis gp43-derived peptides in patients with paracoccidioidomycosis and healthy individuals. Clin. Vaccine Immunol. 14474-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5987-995. [DOI] [PubMed] [Google Scholar]
- 20.Lee, S. E., S. Y. Kim, B. C. Jeong, Y. R. Kim, S. J. Bae, O. S. Ahn, J. J. Lee, H. C. Song, J. M. Kim, H. E. Choy, S. S. Chung, M. N. Kweon, and J. H. Rhee. 2006. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect. Immun. 74694-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, Z., Y. Xian, and Y. Chen. 2003. Epitope-vaccine strategy against HIV-1: today and tomorrow. Immunobiology 208423-428. [DOI] [PubMed] [Google Scholar]
- 22.Livonesi, M. C., J. T. Souto, A. P. Campanelli, C. M. L. Maffei, R. Martinez, M. A. Rossi, and J. S. Da Silva. 2008. Deficiency of IL-12p40 subunit determines severe paracoccidioidomycosis in mice. Med. Mycol. 46637-646. [DOI] [PubMed] [Google Scholar]
- 23.McSorley, S. J., B. D. Ehst, Y. Yu, and A. T. Gewirtz. 2002. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J. Immunol. 1693914-3919. [DOI] [PubMed] [Google Scholar]
- 24.Newton, S. M., C. O. Jacob, and B. A. Stocker. 1989. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science 24470-72. [DOI] [PubMed] [Google Scholar]
- 25.Olive, C. T. T., and D. Jackson. 2001. Technological advances in antigen delivery and synthetic peptide vaccine development strategies. Mini-Rev. Med. Chem. 1429-438. [DOI] [PubMed] [Google Scholar]
- 26.Pina, A., R. C. Valente-Ferreira, E. E. W. Molinari-Madlum, C. A. C. Vaz, A. C. Keller, and V. L. G. Calich. 2004. Absence of interleukin-4 determines less severe pulmonary paracoccidioidomycosis associated with impaired Th2 response. Infect. Immun. 722369-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pino, O., M. Martin, and S. M. Michalek. 2005. Cellular mechanisms of the adjuvant activity of the flagellin component FljB of Salmonella enterica serovar Typhimurium to potentiate mucosal and systemic responses. Infect. Immun. 736763-6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto, A. R., R. Puccia, S. N. Diniz, M. F. Franco, and L. R. Travassos. 2000. DNA based vaccination against murine paracoccidioidomycosis using the gp43 gene from Paracoccidioides brasiliensis. Vaccine 183050-3058. [DOI] [PubMed] [Google Scholar]
- 29.Puccia, R., L. R. Travassos, E. G. Rodrigues, A. K. Carmona, M. C. Oliveira, and L. Juliano. 1994. Purification of the specific exocellular antigen gp43 from Paracoccidioides brasiliensis: immunological and proteolytic activities, p. 507-515. In B. Maresca and G. S. Kobayashi (ed.), Molecular biology of pathogenic fungi: a laboratory manual. Telos Press, New York, NY.
- 30.Ramos, H. C., M. Rumbo, and J. C. Sirard. 2004. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 12509-517. [DOI] [PubMed] [Google Scholar]
- 31.Restrepo, A., and M. D. Arango. 1980. In vitro susceptibility testing of Paracoccidioides brasiliensis to sulfonamides. Antimicrob. Agents Chemother. 18190-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romano, C. C., M. J. S. Mendes-Giannini, A. J. S. Duarte, and G. Benard. 2002. IL-12 and neutralization of endogenous IL-10 revert the in vitro antigen-specific cellular immunosuppression of paracoccidioidomycosis patients. Cytokine 18149-157. [DOI] [PubMed] [Google Scholar]
- 33.San-Blas, G., A. Restrepo, K. Clemons, D. A. Stevens, F. San-Blas, R. Puccia, et al. 1992. Paracoccidioidomycosis. J. Med. Vet. Mycol. 3059-71. [DOI] [PubMed] [Google Scholar]
- 34.Sbrogio-Almeida, M. E., T. Mosca, L. M. Massis, I. A. Abrahamsohn, and L. C. Ferreira. 2004. Host and bacterial factors affecting induction of immune responses to flagellin expressed by attenuated Salmonella vaccine strains. Infect. Immun. 722546-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer-Vermes, L. M., M. C. Ciavaglia, S. S. Kashino, E. Burger, and V. L. Calich. 1992. The source of the growth-promoting factor(s) affects the plating efficiency of Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 30261-264. [DOI] [PubMed] [Google Scholar]
- 36.Souza, E. B., J. D. Lopes, and S. R. Almeida. 2004. B and T cell responses elicited by monoclonal anti-idiotypic antibody (Ab2b) mimicking gp43 from Paracoccidioides brasiliensis. Clin. Exp. Immunol. 137123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taborda, C. P., M. A. Juliano, R. Puccia, M. Franco, and L. R. Travassos. 1998. Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice. Infect. Immun. 66786-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taborda, C. P., C. R. Nakaie, E. M. Cilli, E. G. Rodrigues, L. S. Silva, M. F. Franco, and L. R. Travassos. 2004. Synthesis and immunological activity of a branched peptide carrying the T-cell epitope of gp43, the major exocellular antigen of Paracoccidioides brasiliensis. Scand. J. Immunol. 5958-65. [DOI] [PubMed] [Google Scholar]
- 39.Travassos, L. R., G. Goldman, C. P. Taborda, and R. Puccia. 2007. Insights in Paracoccidioides brasiliensis pathogenicity, p. 241-265. In K. Kavanagh (ed.), New insights in medical mycology. Springer, Dordrecht, The Netherlands.
- 40.Travassos, L. R., C. P. Taborda, and A. L. Colombo. 2008. Treatment options for paracoccidioidomycosis and new strategies investigated. Expert Rev. Anti. Infect. Ther. 6251-262. [DOI] [PubMed] [Google Scholar]
- 41.Travassos, L. R., E. G. Rodrigues, L. K. Iwai, and C. P. Taborda. 2008. Attempts at a peptide vaccine against paracoccidioidomycosis, adjuvant to chemotherapy. Mycopathologia 165341-352. [DOI] [PubMed] [Google Scholar]
- 42.Verma, N. K., H. K. Ziegler, B. A. D. Stocker, and G. K. Schoolnik. 1995. Induction of a cellular immune response to a defined T-cell epitope as an insert in the flagellin of a live vaccine strain of Salmonella. Vaccine 13235-244. [DOI] [PubMed] [Google Scholar]