Abstract
RICK (receptor-interacting protein-like interacting caspase-like apoptosis regulatory protein kinase), a serine-threonine kinase, functions downstream of the pattern recognition receptors Nod1 and Nod2 to mediate NF-κB and mitogen-activated protein kinase (MAPK) activation in response to specific microbial stimuli. However, the function of RICK in the recognition and host defense of gram-negative bacteria remains poorly understood. We report here that infection of wild-type and RICK-deficient macrophages with Pseudomonas aeruginosa and Escherichia coli elicited comparable activation of NF-κB and MAPKs as well as secretion of proinflammatory cytokines. However, production of interleukin 6 (IL-6) and IL-1β induced by these gram-negative bacteria was impaired in RICK-deficient macrophages when the cells had previously been stimulated with lipopolysaccharide (LPS) or E. coli. The diminished proinflammatory response of RICK-deficient macrophages to bacteria was associated with reduced activation of NF-κB and MAPKs. Importantly, mutant mice deficient in RICK were less susceptible than wild-type mice to P. aeruginosa infection when the animals had previously been stimulated with LPS. The reduced lethality of RICK-deficient mice infected with P. aeruginosa was independent of pathogen clearance but was associated with diminished production of proinflammatory molecules in vivo. These results demonstrate that RICK contributes to the induction of proinflammatory responses and susceptibility to gram-negative bacteria after exposure to LPS, a condition that is associated with reduced Toll-like receptor signaling.
Infection of the host by bacteria leads to the activation of multiple defense signaling pathways that are critical for the removal of the invading pathogen. These signaling events induce the production of proinflammatory cytokines and antimicrobial molecules that are important for bacterial clearance. At the cellular level, detection of bacteria is mediated through pattern recognition receptors that sense conserved and unique microbial structures (2, 23). Toll-like receptors (TLRs) sense bacterial molecules, such as lipopolysaccharide (LPS) or nucleic acids, at the cell surface or within endosomes (2), whereas Nod-like receptors recognize microbial components in the host cytosol (23, 36). Two Nod-like receptor family members, Nod1 and Nod2, are activated by bacterial molecules produced during the synthesis and/or degradation of peptidoglycan (5, 17, 18, 25). Nod1 recognizes peptidoglycan-related molecules containing the amino acid meso-diaminopimelic acid that are produced by most gram-negative bacteria and certain gram-positive bacteria (5, 17). In contrast, Nod2 is activated by muramyl dipeptide, which is a conserved structure present in virtually all types of peptidoglycan (18, 25). TLRs, Nod1, and Nod2 induce the production of proinflammatory molecules through the NF-κB transcription factor and the mitogen-activated protein kinase (MAPK) signaling pathways (19, 22, 24, 28).
RICK (receptor-interacting protein-like interacting caspase-like apoptosis regulatory protein kinase) (also called RIP2 [receptor-interacting protein 2] and CARDIAK [caspase activation and recruitment domain-containing interleukin 1β-converting enzyme-associated kinase]) is a caspase recruitment domain (CARD)-containing kinase that acts downstream of Nod1 and Nod2 to mediate signaling and induction of proinflammatory cytokines. Stimulation of Nod1 or Nod2 by their specific bacterial activators causes the recruitment and ubiquitinylation of RICK, leading to the interaction with TAK1 (tumor growth factor β-activated kinase 1) and activation of the IKK (IκB kinase) complex (1, 20). Mice deficient in RICK exhibit increased susceptibility to systemic infection with the intracellular pathogen Listeria monocytogenes (6, 28), indicating that RICK plays a role in host defense against certain intracellular bacteria. The function of RICK as well as Nod1 and Nod2 in host defense against L. monocytogenes is particularly important under conditions in which TLR signaling is downregulated by previous exposure to TLR ligands (26). However, the roles of Nod1 and Nod2 signaling in the recognition of gram-negative bacteria and host defense remain largely unexplored.
In sepsis, interactions between microorganisms and host cells trigger inflammatory responses that include the production of proinflammatory molecules. Although proinflammatory cytokines are critical for bacterial killing and activation of adaptive immunity, the excessive amounts of these cytokines produced during infection are harmful to the host and can lead to septic shock and multiorgan failure (7, 8). During infection with gram-negative organisms, LPS and other bacterial molecules are released into the blood circulation system, and systemic exposure to these stimuli is known to be harmful to the host (8, 9, 21). Stimulation of host cells with LPS is known to induce the production of proinflammatory cytokines through the activation of Toll-like receptor 4 (TLR4) and to affect host survival and immune responses to invading gram-negative bacteria (30, 38). However, the interplay between TLR4 and other innate signaling pathways in the regulation of proinflammatory responses and host survival after gram-negative infection remains poorly understood. In the current work, we studied the function of RICK in the recognition of Pseudomonas aeruginosa and Escherichia coli, two extracellular gram-negative bacteria that are commonly involved in human disease (3, 14). RICK was found to be important for production of proinflammatory cytokines and regulation of host survival in response to gram-negative bacteria only when macrophages or mice had been prestimulated with LPS. These results highlight a differential function for RICK in the host defense against L. monocytogenes and gram-negative bacteria and suggest a role for RICK in promoting lethality in mice.
MATERIALS AND METHODS
Mice.
Mice deficient in RICK backcrossed eight times onto the C57BL/6 background have been described previously (33). C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). The animal studies were conducted under approved protocols by the University of Michigan Committee on Use and Care of Animals.
Reagents and bacterial infection.
Ultrapure LPS from Escherichia coli 0111:B4 was purchased from Invivogen. Wild-type Pseudomonas aeruginosa strain PAK and its isogenic mutant lacking PscC, an essential component of the type III secretion system apparatus, were a gift of Stephen Lory, Harvard University. E. coli strain K-12 (a gift of M. O'Riordan, University of Michigan) were used in this study. Heat-killed E. coli was prepared by boiling for 10 min. For bacterial preparation, single colonies were inoculated into 6 ml of brain heart infusion medium and grown overnight at 37°C with shaking. A 1:4 dilution of the culture grown overnight was allowed to grow in fresh medium at 37°C with shaking for an additional hour. Bacteria were diluted to the desired concentration and used in subsequent experiments.
Preparation and stimulation of murine macrophages.
Bone marrow-derived macrophages were prepared as previously described (4) and finally cultured in 48-well plates at a concentration of 2 × 105/well or in 6-well plates at a concentration of 2 × 106 cells/well. The day after plating, cells were left untreated with or treated with LPS (100 ng/ml) or heat-killed E. coli for 24 h and then infected with P. aeruginosa strains or E. coli at different infection ratios.
Measurement of cytokines.
Mouse cytokines were measured in culture supernatants using enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems.
Immunoblotting.
Cells were lysed in buffer containing 1% NP-40 supplemented with complete protease inhibitor cocktail (Roche, Mannheim, Germany) and 2 mM dithiothreitol. Lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes by electroblotting. The antibodies for mouse IκΒ-α, phospho-IκΒ-α, p38, phospho-p38, Jun N-terminal protein kinase (JNK), and phospho-JNK were from Cell Signaling (Beverly, MA). The band densities were measured by image analysis software (Kodak Digital Science 1D).
In vivo experiments.
Wild-type and RICK-deficient mice were injected intraperitoneally (i.p.) with phosphate-buffered saline (PBS) or LPS (10 μg/animal) once a day for 2 days. Peritoneal neutrophil recruitment was assessed by flow cytometry using anti-GR1 (BD Bioscience) and anti-7/4 (Serotec) antibodies 24 h after the last LPS injection. Similarly, 24 h after the last injection, the mice were infected i.p. with P. aeruginosa strain PAK (2 × 107 CFU/animal), and serum cytokine and chemokine levels were determined 3 h postinfection. In addition, bacterial load in blood and lung samples after challenge with P. aeruginosa 8 h after infection was enumerated.
Statistical analysis.
Statistical significance between two groups was determined by two-tailed t test with unequal variance (Aspin-Welch's t test; Excel [Microsoft]). The mortality of infected mice was analyzed by Kaplan-Meier survival curves and log rank test (GraphPad Prism). Differences were considered significant when the P values were <0.05.
RESULTS
RICK deficiency is associated with decreased production of IL-1β and IL-6 in response to Pseudomonas infection in macrophages prestimulated with LPS.
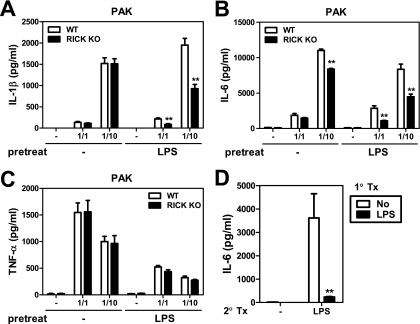
To assess the contribution of RICK to the host response against P. aeruginosa, we initially infected bone marrow-derived macrophages with bacteria at different macrophage/bacterium ratios and measured the amounts of cytokines secreted in the culture supernatants. In unstimulated macrophages, RICK deficiency was associated with unimpaired production of interleukin 1β (IL-1β) and tumor necrosis factor alpha (TNF-α) and a slight reduction of IL-6, which was observed only at the highest macrophage/bacterium ratio of infection (Fig. 1A and B, no pretreatment with LPS). In contrast, RICK-deficient macrophages produced lower amounts of IL-1β and IL-6 than wild-type macrophages in response to P. aeruginosa when the macrophages were prestimulated with LPS for 24 h (Fig. 1A and B). Similar results were obtained when macrophages were infected with an P. aeruginosa mutant that lacks PscC, an essential component of the type III secretion system (data not shown). The production of TNF-α was reduced in macrophages prestimulated with LPS, but the levels of this cytokine were comparable in RICK-deficient and wild-type macrophages (Fig. 1C). Under the same conditions, the IL-6 response to LPS was reduced in macrophages pretreated with LPS (Fig. 1D), indicating that the cells are tolerized to TLR4 signaling.
FIG. 1.
RICK deficiency leads to reduced IL-1β and IL-6 production in response to P. aeruginosa infection in macrophages prestimulated with LPS. Macrophages from wild-type (WT) and RICK-deficient (RICK knockout [KO]) mice were left untreated (−) or stimulated with LPS (100 ng/ml) for 24 h and then infected with live P. aeruginosa strain PAK at various macrophage/bacterium ratios for 24 h (A to C) or treated with LPS (10 ng/ml) for 24 h (D). Cell-free supernatants were analyzed for the production of IL-1β (A), TNF-α (C), and IL-6 (B and D) by ELISAs. Values that were significantly different from the control value (no pretreatment) are indicated as follows: *, P < 0.05; **, P < 0.01. Results are shown as the means plus standard deviations (error bars) of triplicate samples. The results of this experiment are representative of those of five independent experiments. 1° Tx, first treatment; 2° tx, second treatment.
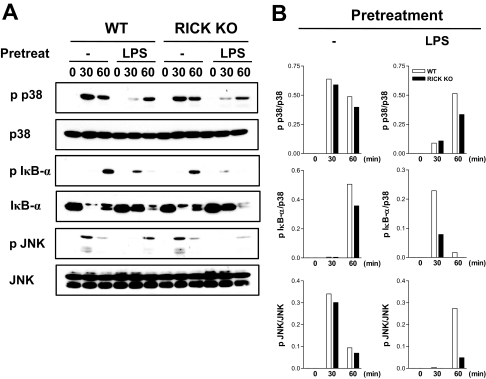
RICK deficiency is associated with reduced Pseudomonas-induced NF-κB and MAPK activation in macrophages prestimulated with LPS.
Bacteria induce the secretion of proinflammatory cytokines via NF-κB and MAPK activation (2, 23). To determine whether RICK deficiency is associated with alteration in these signaling pathways, protein extracts were prepared from unstimulated and LPS-stimulated macrophages infected with P. aeruginosa and immunoblotted with antibodies that recognize activated forms of NF-κB, JNK, and p38. P. aeruginosa infection resulted in phosphorylation and degradation of IκB-α as well as phosphorylation of p38, and JNK in untreated macrophages that was unimpaired or slightly decreased in RICK-deficient macrophages (Fig. 2A). The degradation of IκB-α and activation of MAPKs was inhibited or delayed in wild-type macrophages prestimulated with LPS (Fig. 2A). In line with the cytokine results shown in Fig. 1, there was reduced phosphorylation of p38, IκB-α, and JNK in RICK-deficient macrophages compared to wild-type macrophages in LPS-treated cells (Fig. 2A and B). These results indicate that RICK enhances signaling, which is necessary for a robust proinflammatory response triggered by P. aeruginosa infection in macrophages prestimulated with LPS.
FIG. 2.
NF-κB and MAPK activation in wild-type and RICK-deficient macrophages infected with P. aeruginosa. Macrophages from wild-type (WT) and RICK-deficient (RICK knockout [KO]) mice were left untreated (−) or stimulated with LPS (100 ng/ml) for 24 h and then infected with P. aeruginosa at a macrophage/bacterium ratio of 1/10 for the indicated times (in minutes). (A) Whole-cell extracts were immunoblotted with antibodies that detect unphosphorylated and phosphorylated (activated) forms of the indicated proteins (p p38, phosphorylated p38). (B) The band density was determined by dividing the density of phosphorylated forms by unphosphorylated forms. The results of this experiment are representative of those of three independent experiments.
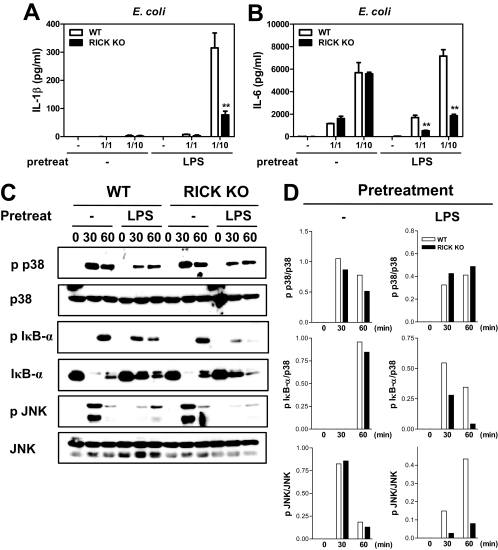
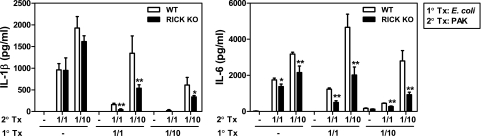
RICK deficiency is associated with decreased proinflammatory responses to E. coli in macrophages prestimulated with LPS.
To determine whether the role of RICK in the proinflammatory response to P. aeruginosa also applies to other gram-negative bacteria, macrophages were infected with E. coli at different macrophage/bacterium ratios. RICK deficiency was associated with unimpaired production of IL-6 in unstimulated macrophages (Fig. 3A). In contrast, the amounts of IL-6 produced in response to E. coli infection were reduced in RICK-deficient macrophages when the cells were prestimulated with LPS (Fig. 3A). Infection of unstimulated wild-type or RICK-deficient macrophages with E. coli did not induce IL-1β secretion in wild-type macrophages (Fig. 3B), which is consistent with previous results (12). In contrast, infection with E. coli induced the secretion of IL-1β in macrophages prestimulated with LPS (Fig. 3B). Importantly, the production of IL-1β in LPS-stimulated macrophages was reduced in RICK-deficient macrophages (Fig. 3B). The latter finding could be explained, at least in part, by decreased degradation and phosphorylation of IκB-α and JNK activation in LPS-stimulated RICK-deficient macrophages (Fig. 3C and D).
FIG. 3.
RICK is required for optimal production of IL-1β and IL-6 and activation of NF-κB and MAPK in response to E. coli infection in macrophages prestimulated with LPS. Macrophages from wild-type (WT) and RICK-deficient (RICK knockout [KO]) mice were left untreated (−) or stimulated with LPS (100 ng/ml) for 24 h. For cytokine analysis, the cells were infected with E. coli at various macrophage/bacterium ratios for 24 h. Cell-free supernatants were analyzed by ELISAs for the production of IL-1β (A) and IL-6 (B). Values that were significantly different (P < 0.01) from the control value (no pretreatment) are indicated by two asterisks. (C) For Western blot analysis, the cells were infected with E. coli at the macrophage/bacterium ratio of 1/10 for the indicated times (in minutes). Whole-cell extracts were immunoblotted with antibodies that detect unphosphorylated and phosphorylated (activated) forms of the indicated proteins (p p38, phosphorylated p38). (D) The band density was determined by dividing the density of phosphorylated forms by unphosphorylated forms. The results of this experiment are representative of those of three independent experiments.
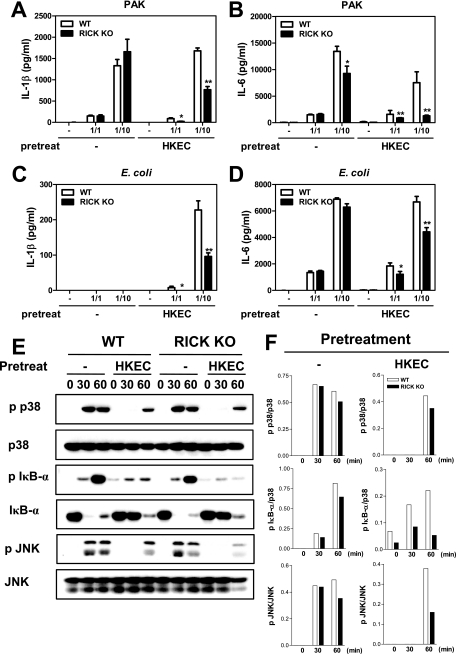
RICK deficiency is associated with decreased proinflammatory responses to gram-negative bacteria in macrophages prestimulated with heat-killed or live E. coli.
The experiments presented thus far revealed that the absence of RICK is associated with reduced proinflammatory responses after infection with gram-negative bacteria in macrophages prestimulated with LPS. We determined next whether the regulation of proinflammatory responses by RICK is also observed when the macrophages are prestimulated with whole bacteria. Consistent with results presented above, IL-1β and IL-6 production induced by P. aeruginosa and E. coli infection was largely unimpaired in wild-type and RICK-deficient cells (Fig. 4A to D). The only exception was a slight reduction in the amount of IL-6 induced by P. aeruginosa infection in RICK-deficient macrophages, which is in line with the results shown in Fig. 1. In contrast, there was a significant reduction in the amounts of IL-6 and IL-1β induced by P. aeruginosa and E. coli infection in RICK-deficient macrophages prestimulated with heat-killed E. coli (Fig. 4A to D). In agreement with these results, phosphorylation of IκB-α and JNK in response to P. aeruginosa was reduced in RICK-deficient macrophages prestimulated with heat-killed E. coli, whereas phosphorylation of IκB-α and JNK induced in unstimulated macrophages was comparable in wild-type and RICK-deficient macrophages (Fig. 4E and F). Similarly, prestimulation of macrophages with live E. coli elicited reduced production of IL-6 and IL-1β in RICK-deficient macrophages in response to P. aeruginosa, compared to wild-type cells (Fig. 5A and B). These results indicate that RICK regulates the production of proinflammatory responses in response to P. aeruginosa and E. coli infection in macrophages stimulated with LPS or E. coli.
FIG. 4.
Cytokine production and NF-κB and MAPK activation in response to P. aeruginosa or E. coli in wild-type (WT) and RICK-deficient (RICK knockout [KO]) macrophages prestimulated with heat-killed E. coli. (A to D) Macrophages were left untreated (−) or stimulated with heat-killed E. coli (HKEC) for 24 h and then infected with P. aeruginosa strain PAK (A and B) or E. coli (C and D) at various macrophage/bacterium ratios for 24 h. Cell-free supernatants were analyzed for the production of IL-1β and IL-6 by ELISAs. Values that were significantly different from the control value (no pretreatment) are indicated as follows: *, P < 0.05; **, P < 0.01. The results of this experiment are representative of those of three independent experiments. (E) Whole-cell extracts were immunoblotted with the indicated antibodies (p p38, phosphorylated p38), and (F) the band density was determined by dividing the density of phosphorylated forms by unphosphorylated forms.
FIG. 5.
Cytokine production in response to P. aeruginosa or E. coli in wild-type (WT) and RICK-deficient (RICK knockout [KO]) macrophages prestimulated with live E. coli. Macrophages were left untreated (−) or infected with live E. coli at the indicated macrophage/bacterium ratio for 24 h and then infected with P. aeruginosa (PAK) at the indicated macrophage/bacterium ratio for 24 h. Cell-free supernatants were analyzed for the production of IL-1β and IL-6 by ELISAs. Values that were significantly different from the control value (no pretreatment) are indicated as follows: *, P < 0.05; **, P < 0.01. The results of this experiment are representative of those of two independent experiments. 1° Tx, first treatment; 2° Tx, second treatment.
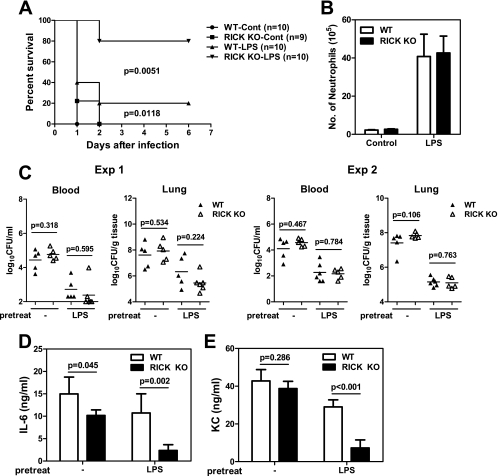
RICK deficiency is associated with reduced production of proinflammatory molecules and protection against P. aeruginosa-induced lethality in LPS-treated mice.
Next we determined the role of RICK in host defense against P. aeruginosa in vivo. In these experiments, wild-type and RICK-deficient mice were given LPS i.p. or PBS as a control 24 h prior to infection with P. aeruginosa, and mouse survival was monitored over time. In untreated mice, both wild-type and RICK-deficient mice die within 2 days when the mice were infected with 2 × 107 CFU of P. aeruginosa i.p. (Fig. 6A). Prestimulation of wild-type mice with LPS was associated with modest improvement in survival compared to untreated mice (20% versus 0%) after P. aeruginosa infection (Fig. 6A). Importantly, 80% of the RICK-deficient mice survived infection with P. aeruginosa compared to only 20% of wild-type mice when the mice were prestimulated with LPS (P < 0.005). The increased survival of RICK-deficient mice was not due to alteration in LPS-induced recruitment of neutrophils. Thus, i.p. injection of LPS elicited the recruitment of neutrophils in the peritoneal cavity, but the number of neutrophils was comparable in RICK-deficient and wild-type mice in the absence of P. aeruginosa infection (Fig. 6B). Furthermore, prestimulation with LPS reduced the bacterial loads in the blood and lungs of both wild-type and RICK-deficient mice (Fig. 6C). However, the improved survival of RICK-deficient mice was independent of bacterial clearance in that comparable loads of P. aeruginosa were found in the blood and lungs of wild-type and RICK-deficient mice (Fig. 6C). Notably, increased survival of P. aeruginosa-infected RICK-deficient mice prestimulated with LPS was associated with reduced amounts of IL-6 and KC in the serum compared to those found in wild-type mice (Fig. 6D and E). These results indicate that RICK promotes bacterium-induced lethality independently of bacterial clearance in mice stimulated with LPS and suggest that this harmful effect is mediated, at least in part, by increased production of proinflammatory cytokines.
FIG. 6.
RICK contributes to lethality after intraperitoneal P. aeruginosa infection in mice stimulated with LPS. (A) Wild-type (WT) and RICK-deficient (RICK knockout [KO]) mice were injected i.p. with PBS (control [Cont]) or LPS (10 μg/animal) once a day for two days and infected with P. aeruginosa 2 × 107 CFU/animal 24 h after the second LPS injection. Lethality was monitored for more than 6 days after infection. The results from two experiments were pooled together. (B) Neutrophil (GR1+/7/4+) recruitment was assessed at 48 h in peritoneal fluid samples from WT and RICK-deficient mice injected with PBS or LPS twice as in panel A, in the absence of P. aeruginosa infection. (C to E) Mice were treated with PBS or LPS as in panel A and infected with P. aeruginosa (2 × 107 CFU/animal). (C) Bacterial loads in blood and lung samples were determined at 8 h postinfection in two separate experiments. The short black horizontal lines show the mean values for the groups. Each symbol shows the value for one animal. (D and E) At 3 h postinfection, the levels of IL-6 (D) and KC (E) in serum were assessed by ELISAs.
DISCUSSION
Recognition of gram-negative bacteria induces the production of proinflammatory cytokines that are critical for cell-mediated bacterial killing and activation of adaptive immunity. Production of these antimicrobial molecules in response to bacterial infection is protective to the host, but excessive amounts of proinflammatory cytokines induce harmful effects that include multiorgan damage and death (7, 8). We show here that RICK, an adaptor that is required for Nod1 and Nod2 signaling, contributes to inflammatory responses against P. aeruginosa and E. coli and that this RICK-dependent response promotes lethality after infection with P. aeruginosa. The role of RICK in the host response to gram-negative bacteria was revealed when macrophages or mice were prestimulated with LPS or E. coli, stimuli that induce tolerization to TLR signaling. During infection with gram-negative bacteria, microbial molecules, such as LPS, or fragments from dead bacteria are released into the blood circulation system, and systemic exposure to these stimuli is likely to affect the host immune response (21). Furthermore, bacterial killing by immune mechanisms and antibiotic therapy is expected to increase the levels of LPS and other microbial molecules in the blood of infected hosts. Under such conditions, our results suggest that RICK signaling is important for bacterial recognition, but it also contributes to harmful proinflammatory responses triggered by gram-negative bacterial infection.
Several studies have shown that TLRs play a critical role in recognition of many gram-negative bacteria, including P. aeruginosa and E. coli (10, 31, 35). In the case of P. aeruginosa, several TLRs, including TLR2, TLR4, and TLR5, contribute to the innate inflammatory response against the pathogen (35). These results are consistent with our observation that RICK signaling alone plays little or no role in the inflammatory response induced by P. aeruginosa and E. coli in unstimulated macrophages. Yet, single deficiency of TLR2, TLR4, or TLR5 does not enhance resistance or susceptibility to P. aeruginosa infection in vivo (11, 15, 34, 35). These results suggest that redundancy among TLRs and between TLR-dependent and TLR-independent signaling pathways contributes to innate immune responses against P. aeruginosa and other gram-negative bacteria. It has been reported that costimulation of human and mouse macrophages with LPS and Nod1 or Nod2 agonists enhances the production of proinflammatory cytokines (5, 13, 33, 39). Thus, during gram-negative bacterial infection, the interplay between TLR and RICK signaling may play a role not only in the induction of protective immune responses but also in inflammation-induced pathology. Because we did not observe defective clearance of P. aeruginosa in RICK-deficient mice, the results indicate that RICK signaling alone does not play a significant role in the host defense to P. aeruginosa in vivo. Yet, RICK deficiency protected mice from Pseudomonas-induced lethality in mice prestimulated with LPS, which correlated with decreased production of proinflammatory cytokines in vivo. Because overproduction of cytokines, such as IL-1β and IL-6, is known to mediate organ damage and lethality, the results suggest that RICK signaling contributes to the harmful effects that are associated with cytokine production after P. aeruginosa infection. Notably, we found that RICK regulates IL-1β and IL-6 production, but not TNF-α production, in LPS-stimulated macrophages. Although the molecular mechanism by which RICK selectively regulates cytokine production is unclear, there is evidence for differential regulation of the IL-6 and TNF-α genes through the use of specific transcriptional activators and repressors in response to bacterial stimuli (16, 27, 29). Furthermore, the expression of TNF-α and IL-6 is differentially regulated in response to P. aeruginosa infection in vivo (34). We also found that pretreatment with LPS reduced the loads of P. aeruginosa in tissues in both wild-type and RICK-deficient mice, which is consistent with a previous study (38). In addition, this effect of LPS is similar to that observed when mice are pretreated with lipopeptide which resulted in increased bacterial clearance after infection with Salmonella (32). The effect of lipopeptide was attributed to an increased number of neutrophils and enhanced intracellular bacterial killing by neutrophils and macrophages (32). Consistent with these findings, we observed increased numbers of neutrophils after LPS treatment of both wild-type and RICK-deficient mice.
The effect of RICK in regulating Pseudomonas-induced lethality was revealed in mice prestimulated with LPS, a condition that is associated with TLR4 tolerization. Collectively, these results suggest that RICK, a molecule required for signaling via the intracellular sensors Nod1 and Nod2, plays a critical role in gram-negative recognition when TLR4 signaling is reduced. Consistently, Nod1 was found to contribute to the early chemokine response induced by P. aeruginosa in mouse embryo fibroblasts, which are largely devoid of TLR signaling (37). Similarly, RICK is critical for recognition of Listeria monocytogenes in macrophages refractory to TLR ligands (26). However, these results are in contrast to those observed during L. monocytogenes infection in which Nod1/Nod2 signaling was critical in controlling pathogen clearance after LPS or E. coli stimulation. Furthermore, deficiency in Nod1 and Nod2 promotes lethality after L. monocytogenes infection in mice prestimulated with LPS (26), which is the opposite of what was found in P. aeruginosa infection. Unlike P. aeruginosa, L. monocytogenes is an intracellular gram-positive bacterium that invades the host cytosol where it triggers a robust innate immune response that relies in part on Nod1 and Nod2 (26, 33). Thus, the different role of RICK signaling in the regulation of host survival after infection with two bacterial pathogens may reflect differences in bacterial lifestyle and bacterium-host interactions between L. monocytogenes and P. aeruginosa.
Acknowledgments
We are grateful to Richard Flavell for providing mutant mice, Stephen Lory for providing bacterial strains, and Joel Whitfield from the Cellular Immunology Core Facility of the University of Michigan Cancer Center for performing ELISAs. We also thank Michael Shaw and Grace Chen for critical review of the manuscript.
This work was supported by NIH grant R01 DK61707. Y.-G. Kim was supported by training funds from the University of Michigan Comprehensive Cancer Center.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 2 February 2009.
REFERENCES
- 1.Abbott, D. W., A. Wilkins, J. M. Asara, and L. C. Cantley. 2004. The Crohn's disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr. Biol. 142217-2227. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124783-801. [DOI] [PubMed] [Google Scholar]
- 3.Bone, R. C. 1993. Gram-negative sepsis: a dilemma of modern medicine. Clin. Microbiol. Rev. 657-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celada, A., P. W. Gray, E. Rinderknecht, and R. D. Schreiber. 1984. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J. Exp. Med. 16055-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4702-707. [DOI] [PubMed] [Google Scholar]
- 6.Chin, A. I., P. W. Dempsey, K. Bruhn, J. F. Miller, Y. Xu, and G. Cheng. 2002. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature 416190-194. [DOI] [PubMed] [Google Scholar]
- 7.Cook, D. N., D. S. Pisetsky, and D. A. Schwartz. 2004. Toll-like receptors in the pathogenesis of human disease. Nat. Immunol. 5975-979. [DOI] [PubMed] [Google Scholar]
- 8.Danner, R. L., R. J. Elin, J. M. Hosseini, R. A. Wesley, J. M. Reilly, and J. E. Parillo. 1991. Endotoxemia in human septic shock. Chest 99169-175. [DOI] [PubMed] [Google Scholar]
- 9.Dobrovolskaia, M. A., and S. N. Vogel. 2002. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 4903-914. [DOI] [PubMed] [Google Scholar]
- 10.Elson, G., I. Dunn-Siegrist, B. Daubeuf, and J. Pugin. 2007. Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood 1091574-1583. [DOI] [PubMed] [Google Scholar]
- 11.Feuillet, V., S. Medjane, I. Mondor, O. Demaria, P. P. Pagni, J. E. Galan, R. A. Flavell, and L. Alexopoulou. 2006. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc. Natl. Acad. Sci. USA 10312487-12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franchi, L., T. D. Kanneganti, G. R. Dubyak, and G. Nunez. 2007. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J. Biol. Chem. 28218810-18818. [DOI] [PubMed] [Google Scholar]
- 13.Fritz, J. H., S. E. Girardin, C. Fitting, C. Werts, D. Mengin-Lecreulx, M. Caroff, J. M. Cavaillon, D. J. Philpott, and M. Adib-Conquy. 2005. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur. J. Immunol. 352459-2470. [DOI] [PubMed] [Google Scholar]
- 14.Garau, J., and L. Gomez. 2003. Pseudomonas aeruginosa pneumonia. Curr. Opin. Infect. Dis. 16135-143. [DOI] [PubMed] [Google Scholar]
- 15.George, S. E., M. J. Kohan, M. I. Gilmour, M. S. Taylor, H. G. Brooks, J. P. Creason, and L. D. Claxton. 1993. Pulmonary clearance and inflammatory response in C3H/HeJ mice after intranasal exposure to Pseudomonas spp. Appl. Environ. Microbiol. 593585-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilchrist, M., V. Thorsson, B. Li, A. G. Rust, M. Korb, J. C. Roach, K. Kennedy, T. Hai, H. Bolouri, and A. Aderem. 2006. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature 441173-178. [DOI] [PubMed] [Google Scholar]
- 17.Girardin, S. E., I. G. Boneca, L. A. Carneiro, A. Antignac, M. Jehanno, J. Viala, K. Tedin, M. K. Taha, A. Labigne, U. Zahringer, A. J. Coyle, P. S. DiStefano, J. Bertin, P. J. Sansonetti, and D. J. Philpott. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 3001584-1587. [DOI] [PubMed] [Google Scholar]
- 18.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2788869-8872. [DOI] [PubMed] [Google Scholar]
- 19.Girardin, S. E., R. Tournebize, M. Mavris, A. L. Page, X. Li, G. R. Stark, J. Bertin, P. S. DiStefano, M. Yaniv, P. J. Sansonetti, and D. J. Philpott. 2001. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa, M., Y. Fujimoto, P. C. Lucas, H. Nakano, K. Fukase, G. Nunez, and N. Inohara. 2008. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. EMBO J. 27373-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellman, J., P. M. Loiselle, M. M. Tehan, J. E. Allaire, L. A. Boyle, J. T. Kurnick, D. M. Andrews, K. Sik Kim, and H. S. Warren. 2000. Outer membrane protein A, peptidoglycan-associated lipoprotein, and murein lipoprotein are released by Escherichia coli bacteria into serum. Infect. Immun. 682566-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu, Y. M., Y. Zhang, Y. You, D. Wang, H. Li, O. Duramad, X. F. Qin, C. Dong, and X. Lin. 2007. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat. Immunol. 8198-205. [DOI] [PubMed] [Google Scholar]
- 23.Inohara, N., M. Chamaillard, C. McDonald, and G. Nunez. 2005. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 74355-383. [DOI] [PubMed] [Google Scholar]
- 24.Inohara, N., T. Koseki, J. Lin, L. del Peso, P. C. Lucas, F. F. Chen, Y. Ogura, and G. Nunez. 2000. An induced proximity model for NF-kappa B activation in the Nod1/RICK and RIP signaling pathways. J. Biol. Chem. 27527823-27831. [DOI] [PubMed] [Google Scholar]
- 25.Inohara, N., Y. Ogura, A. Fontalba, O. Gutierrez, F. Pons, J. Crespo, K. Fukase, S. Inamura, S. Kusumoto, M. Hashimoto, S. J. Foster, A. P. Moran, J. L. Fernandez-Luna, and G. Nunez. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 2785509-5512. [DOI] [PubMed] [Google Scholar]
- 26.Kim, Y. G., J. H. Park, M. H. Shaw, L. Franchi, N. Inohara, and G. Nunez. 2008. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity 28246-257. [DOI] [PubMed] [Google Scholar]
- 27.Kimura, A., T. Naka, T. Muta, O. Takeuchi, S. Akira, I. Kawase, and T. Kishimoto. 2005. Suppressor of cytokine signaling-1 selectively inhibits LPS-induced IL-6 production by regulating JAK-STAT. Proc. Natl. Acad. Sci. USA 10217089-17094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi, K., N. Inohara, L. D. Hernandez, J. E. Galan, G. Nunez, C. A. Janeway, R. Medzhitov, and R. A. Flavell. 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416194-199. [DOI] [PubMed] [Google Scholar]
- 29.Kuwata, H., M. Matsumoto, K. Atarashi, H. Morishita, T. Hirotani, R. Koga, and K. Takeda. 2006. IkappaBNS inhibits induction of a subset of Toll-like receptor-dependent genes and limits inflammation. Immunity 2441-51. [DOI] [PubMed] [Google Scholar]
- 30.Lehner, M. D., J. Ittner, D. S. Bundschuh, N. van Rooijen, A. Wendel, and T. Hartung. 2001. Improved innate immunity of endotoxin-tolerant mice increases resistance to Salmonella enterica serovar Typhimurium infection despite attenuated cytokine response. Infect. Immun. 69463-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer, A. K., M. Muehmer, J. Mages, K. Gueinzius, C. Hess, K. Heeg, R. Bals, R. Lang, and A. H. Dalpke. 2007. Differential recognition of TLR-dependent microbial ligands in human bronchial epithelial cells. J. Immunol. 1783134-3142. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien, G. C., J. H. Wang, and H. P. Redmond. 2005. Bacterial lipoprotein induces resistance to Gram-negative sepsis in TLR4-deficient mice via enhanced bacterial clearance. J. Immunol. 1741020-1026. [DOI] [PubMed] [Google Scholar]
- 33.Park, J. H., Y. G. Kim, C. McDonald, T. D. Kanneganti, M. Hasegawa, M. Body-Malapel, N. Inohara, and G. Nunez. 2007. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J. Immunol. 1782380-2386. [DOI] [PubMed] [Google Scholar]
- 34.Ramphal, R., V. Balloy, M. Huerre, M. Si-Tahar, and M. Chignard. 2005. TLRs 2 and 4 are not involved in hypersusceptibility to acute Pseudomonas aeruginosa lung infections. J. Immunol. 1753927-3934. [DOI] [PubMed] [Google Scholar]
- 35.Skerrett, S. J., C. B. Wilson, H. D. Liggitt, and A. M. Hajjar. 2007. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 292L312-L322. [DOI] [PubMed] [Google Scholar]
- 36.Ting, J. P., and B. K. Davis. 2005. CATERPILLER: a novel gene family important in immunity, cell death, and diseases. Annu. Rev. Immunol. 23387-414. [DOI] [PubMed] [Google Scholar]
- 37.Travassos, L. H., L. A. Carneiro, S. E. Girardin, I. G. Boneca, R. Lemos, M. T. Bozza, R. C. Domingues, A. J. Coyle, J. Bertin, D. J. Philpott, and M. C. Plotkowski. 2005. Nod1 participates in the innate immune response to Pseudomonas aeruginosa. J. Biol. Chem. 28036714-36718. [DOI] [PubMed] [Google Scholar]
- 38.Varma, T. K., M. Durham, E. D. Murphey, W. Cui, Z. Huang, C. Y. Lin, T. Toliver-Kinsky, and E. R. Sherwood. 2005. Endotoxin priming improves clearance of Pseudomonas aeruginosa in wild-type and interleukin-10 knockout mice. Infect. Immun. 737340-7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang, S., R. Tamai, S. Akashi, O. Takeuchi, S. Akira, S. Sugawara, and H. Takada. 2001. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect. Immun. 692045-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]