Abstract
Enterotoxigenic Bacteroides fragilis (ETBF) causes diarrhea and is implicated in inflammatory bowel diseases and colorectal cancer. The only known ETBF virulence factor is the Bacteroides fragilis toxin (BFT), which induces E-cadherin cleavage, interleukin-8 secretion, and epithelial cell proliferation. A murine model for ETBF has not been characterized. Specific pathogen-free (SPF) C57BL/6J or germfree 129S6/SvEv mice were orally inoculated with wild-type ETBF (WT-ETBF) strains, a nontoxigenic WT strain of B. fragilis (WT-NTBF), WT-NTBF overexpressing bft (rETBF), or WT-NTBF overexpressing a biologically inactive mutated bft (rNTBF). In SPF and germfree mice, ETBF caused colitis but was lethal only in germfree mice. Colonic histopathology demonstrated mucosal thickening with inflammatory cell infiltration, crypt abscesses, and epithelial cell exfoliation, erosion, and ulceration. SPF mice colonized with rETBF mimicked WT-ETBF, whereas rNTBF caused no histopathology. Intestinal epithelial E-cadherin was rapidly cleaved in vivo in WT-ETBF-colonized mice and in vitro in intestinal tissues cultured with purified BFT. ETBF mice colonized for 16 months exhibited persistent colitis. BFT did not directly induce lymphocyte proliferation, dendritic cell stimulation, or Toll-like receptor activation. In conclusion, WT-ETBF induced acute then persistent colitis in SPF mice and rapidly lethal colitis in WT germfree mice. Our data support the hypothesis that chronic colonization with the human commensal ETBF can induce persistent, subclinical colitis in humans.
In 1984, a molecular subgroup of Bacteroides fragilis, enterotoxigenic B. fragilis (ETBF), was identified to cause diarrheal illnesses in livestock (44) and, in 1992, in humans (40). Subsequently, ETBF has been associated with diarrheal disease globally and, in limited data, active inflammatory bowel diseases (IBD) (2, 36) and colorectal cancer (50). However, all human studies of ETBF infection identify a subset of individuals (∼4 to 30%) asymptomatically colonized with ETBF. Most recently, ETBF was shown to stimulate inflammatory diarrhea in humans, similar to Shigella spp. (45). The only known virulence factor for ETBF is a 20-kDa zinc-dependent metalloprotease called B. fragilis toxin (BFT) or fragilysin (10, 27, 52) that has three distinct molecular isoforms (bft-1, bft-2, and bft-3) (4, 10, 19). All BFT isoforms exhibit similar biologic activity. Consistent with the clinical observations of ETBF disease, BFT injected into lamb, rabbit, or rat ligated intestinal loops induced inflammation and fluid accumulation (33). In human colon cell (38) and colonic epithelial cell (CEC) lines in vitro (3, 32), BFT increases epithelial barrier permeability associated with E-cadherin shedding and changes CEC morphology and actin structure as well as interleukin-8 (IL-8) secretion and CEC proliferation (57). The lack of murine models for studying ETBF infections in vivo has impeded understanding of the molecular mechanisms of ETBF infections (29, 30). Recently, we described that ETBF colonization in C57BL/6J mice results in early colitis (by 1 to 2 weeks) and augments dextran sodium sulfate (DSS)-induced colitis (37). One brief study limited to histopathology reported that ETBF induces mild colitis in outbred germfree mice (31). Pharmacologic doses of BFT are reported to induce fluid accumulation in ligated murine intestinal loops (17). None of these reports characterized the murine models or provided insights into ETBF disease pathogenesis. Herein, we detail both acute and persistent ETBF colitis models in C57BL/6J mice and provide evidence that BFT expression is essential to ETBF disease pathogenesis. Our data suggest the hypothesis that asymptomatic ETBF colonization in humans may result in persistent, potentially deleterious, colitis.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains used in this study are listed in Table 1. Nontoxigenic wild-type B. fragilis (WT-NTBF) overexpressing bft (rETBF; bft-2) and WT-NTBF overexpressing a biologically inactive mutated bft (rNTBF; bft-2 H352Y) differ by a single base pair resulting in a single amino acid change in the BFT-2 catalytic domain; rETBF and rNTBF secrete biologically active and inactive BFT-2, respectively (8). VPI13784 (Δbft1) is an isogenic mutant of VPI13784 containing an in-frame chromosomal deletion of bft-1 (see “Creation of bft-1 isogenic mutant”). Bacteroides thetaiotaomicron expressing bft-2 was constructed as previously described (9). pFD340 is a plasmid vector conferring clindamycin resistance to transformed Bacteroides strains. All Bacteroides strains used in this study are naturally resistant to gentamicin. Bacteroides strains were grown overnight at 37°C under anaerobic conditions (Pack-Anaero; Mitsubishi Gas Chemical Co., Inc., NY) in brain heart infusion broth supplemented with hemin, vitamin K1, and cysteine; clindamycin was added into brain heart infusion broth for transformed strains (46, 54).
TABLE 1.
Bacterial strains used in this study
| Designation | BFT isoform and activity | Straina | Relevant characteristics | Source or reference |
|---|---|---|---|---|
| WT-ETBF (bft-2) | BFT-2 | B. fragilis 86-5443-2-2 | Piglet isolate | 29 |
| WT-NTBF | None | B. fragilis NCTC 9343 | Transformed with pFD340; human isolate | 9 |
| rETBF (bft-2) | BFT-2 | B. fragilis NCTC 9343 | Transformed with bft2::pFD340 | 9 |
| rNTBF (bft-2 H352Y) | Noneb | B. fragilis NCTC 9343 | Transformed with inactive bft2 H352Y::pFD340 | 8 |
| WT-ETBF (bft-1) | BFT-1 | B. fragilis VPI 13784c | Transformed with pFD340; lamb isolate | This paper |
| WT-ETBF (Δbft1) | Noneb | B. fragilis VPI 13784c | Transformed with pFD340; in-frame chromosomal deletion of bft-1 | This paper |
| WT-ETBF (bft-3) | BFT-3 | B. fragilis Korea 570 | Human blood isolate | 4 |
| BT4001 | None | B. thetaiotaomicron 4001d | Transformed with pFD340 | This paper |
| BT4001 (bft-2) | BFT-2 | B. thetaiotaomicron 4001d | Transformed with bft-2::pFD340 | This paper |
All strains used in this study are resistant to clindamycin either naturally or by introduction of pFD340.
Biologically inactive BFT or bft deletion mutant.
B. fragilis VPI 13784 was originally from Tracy Wilkins (51).
Creation of bft-1 isogenic mutant.
A bft-1 isogenic mutant was created using the method of Coyne et al. (5). Briefly, a primer internal to and oriented upstream of bft (primer 2; XhoI, 5′-GGAAGCTGTAACTCGAGTATCAATAGA) was used in a PCR analysis with primer 1 (BamHI, 5′-TTTACATTGGATCCCATGAGATTGGC) located approximately 3 kb upstream of primer 2 (restriction sites are underlined). A second PCR analysis used a primer within bft oriented downstream (primer 3; XhoI, 5′-CATGCGGATGCTCGAGAAGATTTGAT) with a primer located approximately 3 kb downstream of primer 3 (primer 4; BamHI, 5′-CTAAAAGTTGGATCCGTCCCACTGGA) (restriction sites are underlined). PCRs with primers 1 and 2 and primers 3 and 4 were performed with high-fidelity DNA polymerase (Life Technologies, Gaithersburg, MD). The PCR products were digested with BamHI and XhoI cloned by three-way ligation into the Bacteroides suicide vector pNJR6 at the BamHI site (47). Ligation of the XhoI sites created an in-frame deletion, removing 90% of the 1,191 bp of bft-1. A plasmid containing the correct orientation of the PCR products was selected by PCR and introduced into VPI13784 by mobilization from Escherichia coli using the conjugal helper plasmid pRK231 (51). Single homologous recombination mutants were selected with clindamycin and, subsequently, double homologous recombination mutants as being clindamycin sensitive. VPI13784 bft-1 isogenic mutants were confirmed by PCR analysis, sequence analyses, and BFT biologic activity on HT29/C1 cells (28).
Mouse infection.
Specific pathogen-free (SPF) 3-week-old male C57BL/6J and 129S6/SvEv mice were purchased from Jackson Laboratories and housed under SPF conditions. Experimental protocols were approved by the Johns Hopkins University Animal Care and Use Committee in accordance with the regulations of the Association for the Assessment and Accreditation of Laboratory Animal Care International. Based on other mouse enteric colonization models (15), mice were given water with clindamycin (100 mg/liter) and gentamicin (300 mg/liter) to promote B. fragilis colonization. Antibiotic water was initiated 7 days prior to bacterial inoculations and continued for the duration of the experiments. Bacteria were washed with filter-sterilized 0.1 N sodium bicarbonate and adjusted to 1 × 109 CFU/200 μl for mouse oral inoculations. Germfree 129S6/SvEv mice and germfree IL-10 knockout (KO) 129S6/SvEv mice were maintained in the Gnotobiotic Core of the Center for Gastrointestinal Biology and Disease at North Carolina State University (NCSU), College of Veterinary Medicine, and the National Gnotobiotic Rodent Resource Center, University of North Carolina (UNC) at Chapel Hill. Gnotobiotic animal use protocols were approved by the Institutional Animal Care and Use Committees, NCSU and UNC. Germfree mice were monoassociated with B. fragilis strains between ∼4 and 10 months of age.
Fecal analysis.
Total fecal bacteria were estimated using a bacterial counting kit (Molecular Probes). Briefly, a diluted stool sample was stained with a nucleic acid-specific fluorescent dye, mixed with a known number of microspheres, and analyzed by flow cytometry. The bacterial density in the sample is calculated from the ratio of bacterial signals to microsphere signals. B. fragilis colonization (CFU/g stool) was monitored microbiologically as previously described (37). B. fragilis was not present in baseline cultures of any mouse (>100 analyzed), indicating that B. fragilis bacteria are not normal colonic flora in C57BL/6J mice. ETBF and NTBF strains were verified by testing for BFT biologic activity on HT29/C1 cells or by analysis for the bft gene by PCR as previously described (37).
Histology.
Formalin-fixed (10%), paraffin-embedded intestinal tissues were sectioned (5 μm) and stained with hematoxylin and eosin (H&E). Inflammation during acute infection (up to 1 week) was graded blindly by a board-certified pathologist as follows: 0, normal; 1, mild increase in inflammatory cells and no mucosal epithelial changes (no proliferation or loss of crypt structure); 2, moderate increase in inflammatory cells and mild scattered mucosal epithelial proliferation with or without focal loss of crypt architecture; 3, moderate increase in inflammatory cells, diffuse or nearly diffuse (more than two sites) mucosal epithelial proliferation, and edema with or without focal loss of crypt architecture; 4, severe increase in inflammatory cells and marked consistent proliferation with extensive loss (more than two sites) of crypt architecture; and 5, complete destruction of mucosa. Inflammation during chronic infection (≥4 weeks) was graded as follows: 0, normal; 1, mild (few infiltrating inflammatory cells and edema); 2, moderate (focal area affected and mild to moderate infiltration of inflammatory cells); and 3, marked (focally extensive or diffuse area affected, with extensive infiltration of inflammatory cells accompanied by either erosion and/or ulceration). Hyperplasia was graded as follows: 0, normal; 1, slight increase in crypt length and normal CEC; 2, moderate (less than twofold) crypt length with hyperchromatic CEC and goblet cell loss; and 3, severe (more than twofold) increase in crypt length with arborized crypts and high mitotic index. Images were taken using a Nikon E800 camera and rendered using Adobe Photoshop.
Anti-B. fragilis Western blot analysis.
B. fragilis lysates or BFT (purified as described previously [55]) were electrophoresed under reducing conditions on precast 4 to 12% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (NuPage; Invitrogen). Proteins were transferred to a 0.22-μm nitrocellulose membrane (Protran BA83; Whatman), probed overnight with diluted murine sera (see Results), and developed with goat anti-mouse immunoglobulin horseradish peroxidase (ImmunoResearch Laboratories, Bar Harbor, ME) and SuperSignal West Pico chemiluminescent substrate (Pierce). B. fragilis lysates were prepared by mechanically disrupting the bacterial cells with glass beads (Mini-Beadbeater 3110BX; BioSpec Products, Bartlesville, OK) for 3 min at 5,000 rpm, clarified by centrifugation, and protein quantified using the bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL).
In vitro and ex vivo E-cadherin cleavage.
Mouse intestines were excised, opened longitudinally, washed extensively with cold phosphate-buffered saline to remove all fecal material, and laid flat onto a petri dish to obtain full-thickness punch biopsies (3 mm in diameter). Biopsies were incubated with or without purified BFT (5 nM) in serum-free Dulbecco's modified Eagle's medium for 15 min and then lysed on ice for 15 min in radioimmunoprecipitation assay lysis buffer (Sigma). Clarified intestinal lysates and culture supernatants were stored at −20°C until analyzed by Western blot analysis using monoclonal antibodies against E-cadherin (N-terminal specific; clone ECCD-2; Invitrogen), transferrin receptor (clone H68.4; Invitrogen), pan-cytokeratin (clone C-11; Invitrogen), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH; clone 6C5; Calbiochem). Ex vivo experiments to detect E-cadherin cleavage were also performed with ceca from mice infected with B. fragilis strains as described above (“Mouse infection”) for 12, 18, and 24 h. Ceca were evaluated by histopathology and processed for Western blot analysis in parallel as described above.
TLR activation.
HEK 293 stable transfectants expressing human Toll-like receptor 2 (TLR-2), -3, -4, -7, or -9 were stimulated with either purified BFT (5 nM), TLR ligands (positive controls), or unstimulated (negative controls) for 24 h and conditioned medium frozen at −80°C until assayed for IL-8 secretion by enzyme-linked immunosorbent assay (R&D Systems) (14). HEK 293 cell TLR transfectants secrete IL-8 upon stimulation with TLR ligands. HEK 293 cells naturally express TLR-5 (23).
Splenocyte proliferation assay.
Splenocytes from C57BL/6J mice were prepared by gentle sieving on a nylon mesh (100 μm; BD Falcon) followed by lysis of red blood cells (ACK lysing buffer; Biosource). The cells were washed extensively, resuspended in 2% fetal bovine serum RPMI 1640 medium at a final concentration of 1 × 106 cells/ml and divided into aliquots (100 μl per well) into flat-bottomed 96-well plates in triplicate. Purified BFT (5 nM [100 ng/ml final]), anti-mouse CD3ɛ (clone 145-2C11, 100 ng/ml), lipopolysaccharide (100 ng/ml; Alexis, San Diego, CA), or culture medium alone was added to the cells, followed by 48-h culture. Cells were pulsed with 1 μCi of [3H]thymidine for the final 12 h of the culture period. After lysis, the [3H]thymidine incorporated into the splenocytes was counted with a liquid scintillation counter.
BMDCs.
Bone marrow-derived dendritic cells (BMDCs) from C57BL/6J mice were generated as reported by Lu et al. (24). Briefly, bone marrow was collected from tibias and femurs by flushing with RPMI 1640 culture medium. After lysing red blood cells, cells were cultured with recombinant granulocyte-macrophage colony-stimulating factor (1,000 U/ml; R&D Systems) to induce dendritic cell differentiation. After 6 days, BMDCs were incubated with purified BFT (5 nM [100 ng/ml final]), lipopolysaccharide (100 ng/ml), or culture medium alone (2% fetal bovine serum RPMI 1640 medium). After 20 h, cells were harvested for flow cytometric analysis (anti-CD11c, anti-MHC-II, anti-CD86, and anti-CD40; all from BD Biosciences), and conditioned medium was collected for cytokine secretion analysis (BD cytometric bead array, catalogue no. 552364; detects murine IL-6, IL-10, monocyte chemoattractant protein 1, gamma interferon, tumor necrosis factor alpha, and IL-12 p70).
Data analysis.
For statistical analysis of inflammation and hyperplasia scores, the Mann-Whitney U test was used to compare between-group distributions for unpaired data. All other data are presented as means ± standard errors of the means. Comparison of means was done by unpaired Student's t test. A P value of ≤0.05 was considered statistically significant.
RESULTS
Acute ETBF infection (1 to 2 weeks). (i) Clinical characteristics.
After 1 week of antibiotic pretreatment, stool bacterial counts analyzed by fluorescence-activated cell sorting dropped by ∼10- to 100-fold to ∼1010 to 1011 bacterial cells/g stool. Acute infection experiments were initially performed using WT-ETBF (bft-2), WT-NTBF, and rETBF (bft-2) (Table 1). At 24 h postinoculation, all three B. fragilis strains were recovered in high numbers from the stool (∼109 to 1010 CFU/g stool), and this level of bacterial colonization density persisted for the duration of the experiment (data not shown). Therefore, a high level of colonization of mice with B. fragilis strains is rapid and stable in our mouse model.
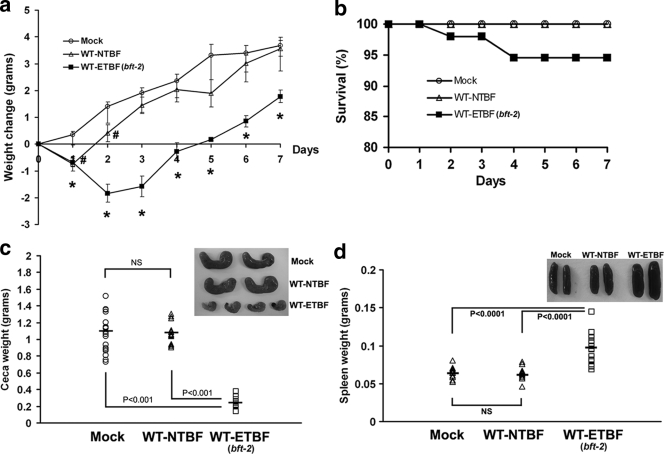
WT-ETBF (bft-2)-colonized mice were usually lethargic at ∼2 to 3 days postinoculation, with ruffled fur and a hunched posture with transient weight loss (Fig. 1a) and occasional rectal bleeding. A small percentage (5.4%; 3 of 55 mice) of WT-ETBF (bft-2)-colonized mice died at 2 to 3 days postcolonization (Fig. 1b). Mortality did not appear to be due to bacteremia as aerobic and anaerobic blood cultures of WT-ETBF (bft-2)-infected mice (n = 5) at 2 days postinfection were negative for B. fragilis or other bacterial species. The stool of WT-ETBF (bft-2)-colonized mice ranged from moist, but formed, to transient diarrhea, usually at ∼1 to 3 days postcolonization. Thus, the deaths may be related to fluid or electrolyte imbalances secondary to enteric disease. All surviving WT-ETBF (bft-2)-colonized mice were healthy in appearance and activity by approximately day 4 to 5 in association with the onset of weight gain (Fig. 1a). WT-NTBF-colonized and mock-infected mice appeared healthy at all times and, except for the first day in NTBF-colonized mice, continuously gained weight (Fig. 1a).
FIG. 1.
Clinicopathologic analysis of C57BL/6 mice inoculated with B. fragilis (∼1 × 109 CFU) for 7 days. (a) Weight change. The daily body weight of individual mice was normalized to the starting body weight. Shown are pooled data from three independent experiments, with ∼4 to 15 mice per experiment. The baseline weight (in grams) of individual mice was similar between the three mouse groups as follows: mock-infected mice (Mock), 14.42 ± 0.35 (standard error of the mean); WT-NTBF-infected mice, 14.62 ± 0.43; and WT-ETBF (bft-2)-infected mice, 14.47 ± 0.42. *, a P value of ≤0.001 for WT-ETBF (bft-2)-infected mice compared with either WT-NTBF- or mock-infected mice, except for day 1 which is significant (P < 0.005) only compared with mock-infected mice; #, a P value of <0.01 for WT-NTBF-infected mice compared with mock-infected mice. (b) Mortality of WT-ETBF (bft-2)-infected (n = 55), WT-NTBF-infected (n = 44), or mock-infected (n = 35) mice. Shown are pooled data from seven independent experiments. (c) Cecal weight and gross morphology (a total of ∼5 to 7 mice) (inset). (d) Spleen weight and gross morphology (a total of ∼5 to 7 mice) (inset). Bars, median weight. NS, statistically not significant.
(ii) Gross morphology and histology.
By 7 days postcolonization, the ceca of WT-ETBF (bft-2)-colonized mice appeared smaller and, in some mice, lacked cecal contents or contained blood clots compared to those of WT-NTBF-colonized mice or mock-infected mice (Fig. 1c and inset). Consistent with the observed decrease in the size of ceca in WT-ETBF (bft-2)-colonized mice, cecal weights were significantly less in WT-ETBF (bft-2)-colonized mice compared to those in mock-infected and WT-NTBF-colonized mice. The decreased sizes of ceca in WT-ETBF (bft-2)-colonized mice occurred within 2 days of oral colonization (data not shown). Decreases in the sizes of ceca with marked inflammation have also been noted in Citrobacter rodentium (25) and Salmonella enterica serovar Typhimurium (1) murine models and likely represent a response to cecal injury. Colon length decreased substantially in DSS-treated mice, and this correlates with the extent of inflammation (34). The average colon length of WT-ETBF (bft-2)-colonized mice was similar to that of WT-NTBF-colonized mice or mock-infected mice (data not shown). The only gross difference between the colons of WT-ETBF (bft-2)-colonized mice and those of WT-NTBF-colonized mice was that the proximal colons of WT-ETBF (bft-2)-colonized mice contained semisolid stool, whereas the WT-NTBF-colonized mice had well-formed stool. Although there is some variability between individual ETBF-colonized mice, splenomegaly (P < 0.0001) as well as enlarged mesenteric lymph nodes (data not shown) were noted in most WT-ETBF (bft-2)-colonized mice (Fig. 1d and inset).
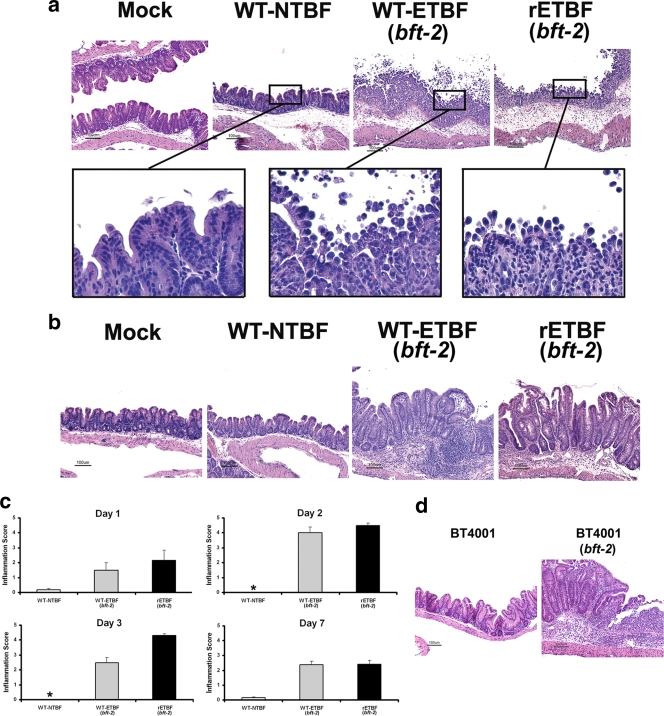
Histopathology of the colons of WT-ETBF (bft-2)-colonized mice revealed edema and inflammatory cell infiltration with rounding and detachment of enterocytes by day 2 postinfection (Fig. 2a and c). By day 7 postinfection, the WT-ETBF (bft-2)-colonized mice had mucosal thickening with mucosal and submucosal edema, crypt hyperplasia, and elongation and extensive neutrophilic infiltration into both the mucosa and submucosa with epithelial cell destruction resulting in erosions and ulceration (Fig. 2b and c). Scattered crypt abscesses were observed in the colons. Inflammation and hyperplasia were usually more severe in the proximal than distal colons in WT-ETBF (bft-2)-colonized mice. The jejunums and ileums were normal. WT-NTBF-colonized mice and mock-infected mice exhibited no colonic histopathology abnormalities.
FIG. 2.
ETBF induces acute colitis. H&E-stained tissue sections of mock-, WT-NTBF-, WT-ETBF (bft-2)-, and rETBF (bft-2)-infected mice for 2 days (a) and 7 days (b). Boxed areas were digitally magnified (×100). Bars, 100 μm. (c) Histologic inflammation scores of large bowels of infected mice at day 1, day 2, day 3, and day 7. Results of mock-infected mice were identical to those of WT-NTBF-infected mice (data not shown). Asterisks denote a lack of inflammation (an inflammation score of <0.2). (d) H&E-stained cecal tissue sections from BT4001- and BT4001 (bft-2)-infected mice.
Antibody responses to B. fragilis and BFT.
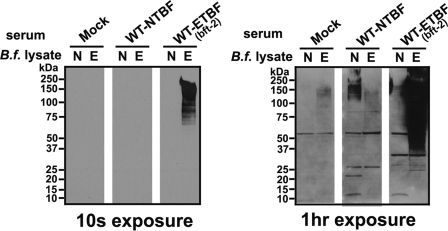
Splenomegaly and enlarged mesenteric lymph nodes in WT-ETBF (bft-2)-colonized mice suggested activation of a systemic immune response. Thus, we examined sera for anti-Bacteroides antibodies at day 7 postcolonization. Pooled sera from three WT-ETBF (bft-2)-colonized mice reacted specifically to diffuse high-molecular-weight bands in WT-ETBF (bft-2), but not WT-NTBF, lysates; pooled sera from WT-NTBF-colonized and mock-infected mice did not react to either WT-ETBF (bft-2) or WT-NTBF lysates (Fig. 3). However, Western blots exposed for a longer amount of time (1 h) revealed that sera from WT-NTBF-colonized mice reacted specifically to WT-NTBF lysates (Fig. 3). These results suggest that WT-ETBF (bft-2)- and WT-NTBF-colonized mice both develop B. fragilis strain-specific antibodies. No BFT-specific antibodies were detected in mice colonized with WT-ETBF (bft-2) for up to 8 months (data not shown).
FIG. 3.
Western blot analysis of mouse serum against B. fragilis (B.f.) lysates. Shown are pooled sera (1:500 dilution) from mock-infected (Mock), WT-NTBF-infected, or WT-ETBF (bft-2)-infected mice evaluated on B. fragilis lysates. Films were exposed for 10 s and 1 h.
BFT and ETBF induce E-cadherin cleavage in mouse intestine.
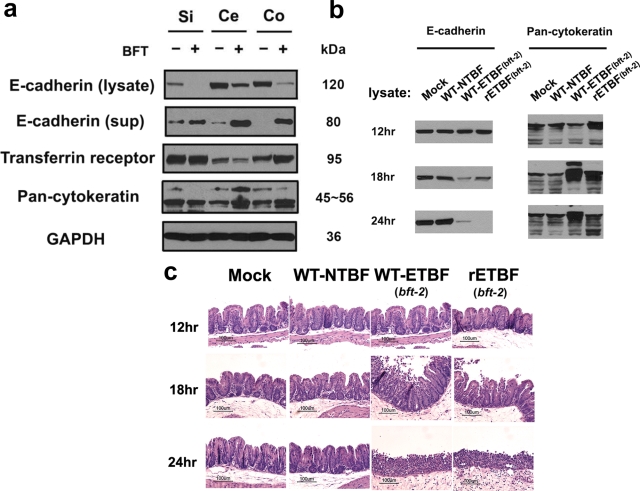
BFT induces E-cadherin cleavage with shedding of the 80-kDa extracellular E-cadherin domain from the surface of human CEC lines in vitro (56, 59). It is unknown whether BFT induces cleavage of E-cadherin of other species or if ETBF induces E-cadherin cleavage in vivo. Thus, we analyzed punch biopsies of small intestines, ceca, and colons from naïve mice incubated with purified BFT (5 nM) in vitro by Western blot analysis for E-cadherin cleavage. Both a decrease in the intensity of full-length membrane-bound E-cadherin (∼120 kDa) and accumulation of the “shed” extracellular fragment of E-cadherin (∼80 kDa) into the culture supernatants were observed in all tissues examined (Fig. 4a). BFT-induced cleavage of mouse E-cadherin is likely specific since cleavage of another transmembrane protein, the transferrin receptor, was not observed.
FIG. 4.
ETBF induces E-cadherin cleavage in vitro and ex vivo. (a) Uninfected mouse small intestine (Si), cecum (Ce), and mid-colon (Co) tissues cultured with (+) or without (−) purified BFT (5 nM) for 15 min and probed for E-cadherin (N terminus specific) in the lysate and the supernatant. Results are representative of three independent experiments. Pan-cytokeratin was used as a CEC loading control, and GAPDH was used as a total protein loading control. (b) Mice were infected for 12, 18, and 24 h, and the ceca were cut lengthwise into two pieces. (c) One piece was used for Western blot analysis and the second half was used for H&E sections. Results are representative of two independent experiments (a total of three mice per experiment).
To determine if E-cadherin cleavage occurs in WT-ETBF (bft-2)-colonized mice, cecal tissues were examined in mice at 12, 18, and 24 h postinfection. At 12 h postinfection, cecal lysates from WT-ETBF (bft-2)-colonized mice showed no difference in full-length membrane-bound E-cadherin compared to WT-NTBF-colonized and mock-infected mice (Fig. 4b). In contrast, progressive loss of the E-cadherin band occurred at 18 h and 24 h in WT-ETBF (bft-2)-colonized mice compared to that in WT-NTBF-colonized mice. Loss of full-length E-cadherin correlated with the onset of colitis (Fig. 4c). Pan-cytokeratin (a CEC marker) and transferrin receptor levels (data not shown) were unaffected, suggesting that CEC loading was uniform and that the observed E-cadherin cleavage is not due to nonspecific, inflammation-associated degradation of transmembrane proteins.
Catalytically active BFT expressed by Bacteroides species is necessary and sufficient to induce colitis.
To date, BFT is the only known virulence factor specific to ETBF strains. To determine the contribution of biologically active BFT to ETBF colitis, mice were colonized with rETBF (bft-2) and rNTBF (bft-2 H352Y) that differ by one amino acid, resulting in expression of biologically active or inactive BFT-2, respectively (Table 1; see Materials and Methods). rETBF (bft-2)-colonized mice were similar to WT-ETBF (bft-2)-colonized mice, developing lethargy, ruffled fur, and, in several mice, rectal bleeding. Mortality was usually similar to that of mice infected with WT-ETBF (bft-2), but it did reach 90% by 7 days in two of five experiments (5 to 7 mice per group; data not shown). The time course and extent of colon inflammation as well as E-cadherin cleavage were similar in WT-ETBF (bft-2)- and rETBF (bft-2)-colonized mice (Fig. 2 and 4). rNTBF (bft-2 H352Y)-colonized mice exhibited no gross or histopathologic colon abnormalities. BFT in stool from both rETBF (bft-2)- and rNTBF (bft-2 H352Y)-infected mice was detected by Western blot analysis, demonstrating in vivo expression of both WT-BFT and mutated BFT, respectively (data not shown). To determine if bft-2 expressed by a non-Bacteroides fragilis strain can induce inflammation, we infected mice with a Bacteroides thetaiotaomicron strain overexpressing bft-2 (Table 1) (Fig. 2d) and found that colitis ensued (Table 2). In addition, mice colonized with a bft-1-expressing strain of WT-ETBF (Table 1) exhibited colitis, whereas no colitis occurred in mice colonized with an isogenic mutant strain, WT-ETBF (Δbft-1) (Table 1), in which an in-frame chromosomal deletion of bft-1 was constructed. Finally, a bft-3-expressing WT-ETBF strain (Table 1) also induced colitis (Table 2). These results show that WT-ETBF strains expressing each of the three bft isoforms induce colon inflammation in WT C57BL/6 mice and, furthermore, that biologically active BFT is necessary and sufficient to induce colitis.
TABLE 2.
Inflammation scores of mice acutely infected with different Bacteroides strainsa
| Designation | Wk evaluated | Median (range) histologic inflammation score | No. of mice |
|---|---|---|---|
| rETBF (bft-2) | 2 | 3.0 (1.5-3.5)* | 6 |
| rNTBF (bft-2 H352Y) | 2 | 0.0 (0) | 3 |
| WT-ETBF (bft-1) | 2 | 2.5 (2-3)** | 6 |
| WT-ETBF (Δbft-1) | 2 | 0.0 (0-0.5) | 6 |
| WT-ETBF (bft-3) | 1-2 | 1.5 (1-2.5) | 6 |
| BT4001 | 1 | 0.0 (0-1) | 4 |
| BT4001 (bft-2) | 1 | 3.5 (2.5-3.5)*** | 6 |
The Mann-Whitney U test was used to compare between-group distributions for unpaired data. *, a P value of <0.05 compared with rNTBF-infected mice; **, a P value of <0.05 compared with WT-ETBF (Δbft-1)-infected mice; ***, a P value of <0.05 compared with BT4001 (bft-1)-infected mice.
Persistent ETBF infection (1 month to 16 months).
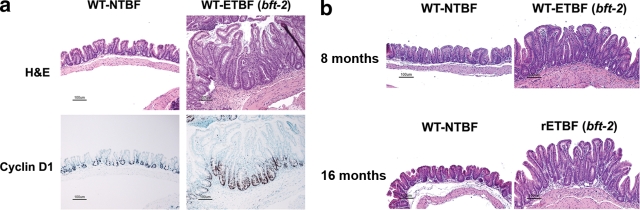
WT C57BL/6 mice colonized for a period spanning ∼1 to 16 months demonstrated consistently high colonization (range, ∼109 to 1010 CFU/g stool) with the B. fragilis strains [WT-ETBF (bft-2), WT-NTBF, rETBF (bft-2), and rNTBF (bft-2 H352Y)]. Mice persistently colonized with WT-ETBF (bft-2) and rETBF (bft-2) appeared healthy, similar to WT-NTBF-, rNTBF (bft-2 H352Y)-, or mock-infected mice. At 3 months, WT-ETBF (bft-2)-colonized mice were similar in weight to WT-NTBF-colonized mice and mock-infected mice [mock infected, 30 ± 2 g; WT-NTBF infected, 33 ± 2 g; WT-ETBF (bft-2) infected, 30 ± 2 g; the P value was not statistically significant; ∼5 to 7 mice per group]. However, some of the WT-ETBF (bft-2)- and rETBF (bft-2)-infected mice still exhibited visible blood in the cecal lumens as well as colon inflammation and hyperplasia at 1, 3, 8, and 16 months (Tables 3 and 4). To verify hyperplasia, the nuclear protein Ki67 was used to detect proliferating cells. Colons from WT-ETBF (bft-2)-colonized mice exhibited an increased percentage of Ki67-positive crypt cells (46.9% ± 2.1%) compared to those from WT-NTBF-colonized mice (36.1% ± 2.5%) or mock-infected controls (31.5% ± 2.1%) [a P value of <0.004 for WT-ETBF (bft-2)- versus WT-NTBF-infected mice or controls at 1 month]. BFT stimulates β-catenin nuclear signaling in vitro with induction of c-Myc expression (57), a protein critical to cell growth. To examine if ETBF hyperplasia may involve c-Myc-regulated mechanisms, expression of cyclin D1, a cell growth gene regulated by c-Myc, was examined in mice colonized with WT-ETBF (bft-2) for 1 month. Enhanced cyclin D1 expression was detected in WT-ETBF (bft-2)-colonized mice compared to that in control mice (Fig. 5). Mice persistently infected with ETBF for 8 months and 16 months also showed epithelial hyperplasia. These results suggest that ETBF and NTBF strains can persistently colonize WT C57BL/6 mice but only ETBF strains induce chronic colon inflammation and hyperplasia.
TABLE 3.
Cecum and colon inflammation scores of mice persistently infected with different B. fragilis strainsa
| Strain or type of infection | Median (range) inflammation score, no. of mice at:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 mo
|
3 mo
|
8 mo
|
16 mo
|
|||||
| Cecum | Colon | Cecum | Colon | Cecum | Colon | Cecum | Colon | |
| Mock | 0.0 (0-1), 6 | 0.0 (0-0.5), 3 | ND | ND | 0.0 (0), 3 | 0.0 (0), 3 | 0.0 (0-1), 4 | 0.0 (0), 4 |
| WT-ETBF (bft-2) | 2.25 (2-3), 8* | 1.0 (0.5-1.5), 5* | 2.0 (2-3), 5* | 2.0 (1-2), 5* | 2.0 (1-2), 3* | 1.0 (0-1), 3 | 2.5 (2-3), 6* | 2.5 (2-3), 6* |
| WT-NTBF | 0.0 (0-1), 7 | 0.0 (0), 4 | 0.5 (0-1), 4 | 0.0 (0), 4 | 0.0 (0), 3 | 0.0 (0), 3 | 0.0 (0-1), 8 | 0.0 (0-1), 8 |
| rETBF (bft-2) | 3.0 (3), 3** | ND | ND | ND | ND | ND | 1.0 (1-3), 9** | 1.0 (0-3), 9** |
| rNTBF (bft-2 H352Y) | 0.0 (0-1), 3 | ND | ND | ND | ND | ND | ND | ND |
The Mann-Whitney U test was used to compare between-group distributions for unpaired data. *, a P value of <0.05 compared with mock-infected and WT-NTBF-infected mice; **, a P value of <0.05 compared with rNTBF- and WT-NTBF-infected mice. ND, not determined.
TABLE 4.
Cecum and colon hyperplasia scores of mice persistently infected with different B. fragilis strainsa
| Strain or type of infection | Median (range) hyperplasia score, no. of mice at:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 mo
|
3 mo
|
8 mo
|
16 mo
|
|||||
| Cecum | Colon | Cecum | Colon | Cecum | Colon | Cecum | Colon | |
| Mock | 0.0 (0), 6 | 0.5 (0-0.5), 3 | ND | ND | 0.0 (0), 3 | 0.0 (0), 3 | 0.0 (0-1), 4 | 0.0 (0-1), 4 |
| WT-ETBF (bft-2) | 2.0 (1.5-3), 8* | 1.5 (1-2), 5* | 3.0 (2-3), 5* | 2.0 (2-3), 5* | 3.0 (1-3), 3* | 1.0 (0-2), 3 | 3.0 (2-3), 6* | 2.0 (2-3), 6* |
| WT-NTBF | 0.0 (0-1), 7 | 0.25 (0-0.5), 4 | 0.5 (0-1), 4 | 0.0 (0-1), 4 | 0.0 (0), 3 | 0.0 (0), 3 | 0.0 (0-2), 8 | 0.0 (0-1), 8 |
| rETBF (bft-2) | 2.0 (2), 3** | ND | ND | ND | ND | ND | 2.0 (1-3), 9** | 2.0 (1-3), 9** |
| rNTBF (bft-2 H352Y) | 0.0 (0-1), 3 | ND | ND | ND | ND | ND | ND | ND |
The Mann-Whitney U test was used to compare between-group distributions for unpaired data. *, a P value of <0.05 compared with mock-infected and WT-NTBF-infected mice; **, a P value of <0.05 compared with rNTBF- and WT-NTBF-infected mice. ND, not determined.
FIG. 5.
ETBF chronic infection enhances epithelial hyperplasia. (a) H&E staining and anti-cyclin D1 immunohistochemistry of ceca from mice persistently infected (for 1 month) with WT-NTBF and WT-ETBF (bft-2). Results for mock-infected mice are identical to those for WT-NTBF-infected mice (data not shown). (b) H&E staining of ceca from mice persistently infected for 8 months [WT-NTBF and WT-ETBF (bft-2)] and 16 months [WT-NTBF and rETBF(bft-2)].
ETBF infection of germfree mice.
Thus far, our results demonstrate that infection with various ETBF strains induced colitis in conventional WT mice. However, it is possible that ETBF acts cooperatively with other microbial flora to induce colitis and that ETBF alone does not induce inflammation. Thus, to determine if ETBF alone induces colitis, 129S6/SvEv germfree mice were infected with either WT-ETBF (bft-2) or WT-NTBF. Unexpectedly, 100% of germfree mice inoculated with WT-ETBF (bft-2) (n = 6) died abruptly by day 3 postinoculation. Histopathology showed severe colitis in all WT-ETBF (bft-2)-colonized mice (Fig. 6). Histopathology of the lungs, livers, and spleens was unremarkable (data not shown). Germfree mice inoculated with WT-NTBF (n = 10) did not develop intestinal inflammation even when persistently colonized for up to 13 weeks. In a parallel experiment, germfree IL-10 KO 129S6/SvEv mice infected with WT-ETBF (bft-2) also developed fatal, rapid onset colitis, whereas germfree IL-10 KO 129S6/SvEv mice infected with WT-NTBF remained healthy with normal colonic histology after 13 weeks (data not shown).
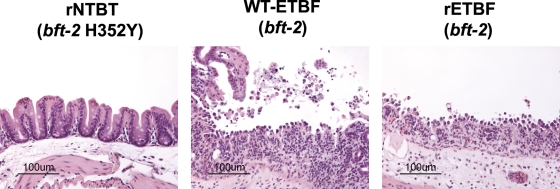
FIG. 6.
ETBF induces colitis in germfree mice. Germfree 129S6/SvEv mice (∼4 to 10 months old) were inoculated with WT-ETBF (bft-2), rETBF (bft-2), or rNTBF (bft-2 H352Y), and ceca were prepared for histology 3 days later. Bars, 100 μm.
We next determined whether colonization of germfree mice with rETBF (bft-2) was sufficient to induce colitis as we observed in conventional mice. In contrast to the rapid onset of lethal colitis noted with WT-ETBF (bft-2)-colonized germfree 129S6/SvEv mice, all mice monoassociated with rETBF (bft-2) (n = 9) or rNTBF (bft-2 H352Y) (n = 10) for 3 days appeared healthy and exhibited a high level of colonization (∼1010 CFU/g stool). Histopathology at 3 days postinoculation revealed marked cecal inflammation with ulcerations in mice infected with rETBF (bft-2) but not rNTBF (bft-2 H352Y) (Fig. 6). These results indicate that ETBF alone induces colitis and confirm that BFT expression alone is sufficient to induce colitis. However, rETBF (bft-2) did not recapitulate the complete phenotype of WT-ETBF (bft-2) in germfree mice, suggesting that other virulence factors of WT-ETBF (bft-2) contribute to rapidly progressive colitis in these mice. The sudden death observed in germfree mice after WT-ETBF (bft-2) inoculation is not due to the 129S6/SvEv mouse strain background, as SPF 129S6/SvEv mice infected with WT-ETBF (bft-2) exhibited colonic inflammation with little lethality (<5%) similar to C57BL/6 mice (data not shown).
Purified BFT does not stimulate lymphocytes, TLRs, or dendritic cells.
The rapid, lethal colitis observed in WT-ETBF (bft-2)-infected germfree 129S6/SvEv mice prompted us to test if BFT was acting as a bacterial superantigen. Bacterial superantigens such as staphylococcal enterotoxin B are low-molecular-weight proteins that directly ligate major histocompatibility complex class II (MHC-II) molecules and the variable chain of the T-cell receptor, triggering vigorous T-cell proliferation, cytokine production, and rapidly progressive, potentially fatal illnesses (e.g., staphylococcal toxic shock syndrome). Compared to anti-CD3-treated mouse splenocytes (positive control), BFT (5 nM) did not induce splenocyte proliferation ([3H]thymidine uptake, 496 ± 19 cpm BFT versus 33,333 ± 3,528 cpm T-cell receptor antibodies; a total of three experiments), indicating that BFT is not a superantigen. Due to the rapid onset of colonic disease with a marked infiltration of neutrophils, we determined if BFT activates TLRs, mediators of innate immune responses. HEK 293 cells do not bind to BFT and exhibit no biologic responses to BFT (data not shown). HEK 293 cells overexpressing TLR-2, -3, -4, -7, or -9 were tested for reconstitution of BFT biologic activity and secretion of IL-8. Compared to the positive control TLR ligands for each receptor, BFT did not stimulate IL-8 secretion from any TLR-expressing HEK 293 cell lines, suggesting that BFT does not directly activate the TLRs tested (data not shown). Lastly, we determined whether purified BFT can activate BMDCs in vitro. BMDCs from C57BL/6J mice cultured with purified BFT (5 nM) were not activated based on fluorescence-activated cell sorting analysis of BMDC activation markers (i.e., MHC-II, CD80, and CD86) and based on secretion of inflammatory cytokines (data not shown). These results suggest that BFT does not directly stimulate lymphocytes and dendritic cells nor does it activate TLR pathways in vitro.
DISCUSSION
Using a murine model, we demonstrate for the first time that ETBF, often a human commensal in clinical studies conducted to date, induces not only acute, symptomatic colitis but persistent subclinical colonic inflammation and hyperplasia. These observations reinforce and extend results from our prior report indicating that ETBF induces murine colitis (37). This new model of colonic inflammation differs substantially from the majority of other reported inbred murine IBD models (>30) in which intestinal inflammation develops in mice with host immunoregulatory defects (e.g., IL-2- or IL-10-deficient mice) (22, 41). In contrast, C57BL/6 mice are viewed as colitis resistant (7). A unique aspect of the ETBF C57BL/6 murine colitis model is the ability of both ETBF and NTBF strains to persistently colonize the murine colon, akin to what is observed for B. fragilis in humans and distinct, for example, from Citrobacter rodentium murine colitis in which the mice clear C. rodentium in ∼4 to 6 weeks (25).
BFT is the only known virulence factor described specifically for ETBF strains. Our experiments demonstrate that all three isoforms of BFT stimulate colonic inflammation and that biologically active BFT expression is necessary and sufficient to induce murine colitis. This conclusion is supported by our studies of a WT-NTBF strain engineered to express either biologically active or inactive BFT (i.e., rETBF or rNTBF, respectively); demonstration of colitis induced by a WT-ETBF (bft-1) strain but not its isogenic deletion mutant and by the induction of colitis by the nonpathogenic Bacteroides thetaiotaomicron transformed with bft-2. Reported data suggest that B. thetaiotaomicron is a lumen-residing commensal species, whereas B. fragilis associates with the colonic mucosa, possibly due to its ability to change capsular antigenicity (6, 21). Our observations that the BFT-expressing B. thetaiotaomicron induces colitis suggest that close delivery of BFT to the mucosa by B. fragilis may not be required for the induction of inflammation. Alternatively, expression of BFT by B. thetaiotaomicron and/or B. thetaiotaomicron colonization in a murine model treated with antibiotics enhances B. thetaiotaomicron mucosal adherence. Although our data strongly suggest a central role for BFT secretion in ETBF disease pathogenesis, our germfree mouse studies using rETBF (bft-2) and rNTBF (bft-2 H352Y) suggest that other virulence factors may drive the early mortality associated with colitis observed in this murine model (35, 39).
Our in vivo murine data confirm the relevance of prior in vitro studies of the BFT mechanism of action to understanding the pathogenesis of ETBF disease. In vitro studies indicated that BFT binds to a putative intestinal epithelial cell receptor stimulating signal transduction pathways, resulting in the following: (i) E-cadherin cleavage with reduced barrier function in CEC monolayers (59), (ii) β-catenin signaling with induction of c-Myc expression and CEC proliferation (57), and (iii) activation of NF-κB with expression and secretion of IL-8 and other cytokines (18, 42, 57, 58). The studies herein confirm for the first time that E-cadherin cleavage occurs in vivo early in ETBF-induced murine colitis. E-cadherin is important for intestinal barrier function (61), and chimeric mice expressing a dominant negative N-cadherin in CECs lose intercellular E-cadherin associations and develop colitis (13). Our preliminary data (unpublished data) indicate that early ETBF colitis is also associated with expression of the chemokine KC, the murine IL-8 analog. Our data support the concept that diminished barrier function is an early step in the development of colonic inflammation as proposed for human IBD (16, 48). Herein, we also demonstrate ETBF-induced CEC hyperplasia (Fig. 5a) with increased Ki67 and cyclin D1 expression, the latter a downstream target of β-catenin signaling and c-Myc induction known to be triggered by BFT (12, 49). While crypt hyperplasia is common with experimental colitis, the colon histopathology from mice colonized with ETBF for 16 months (Fig. 5b) reveals hyperplasia without gross CEC barrier disruption and inflammation (compare Fig. 2a and 5b). More detailed studies are needed to discern the mechanisms of ETBF-induced inflammation and hyperplasia and to determine if these are molecularly linked mucosal events. Lastly, our results did not show direct activation of TLRs or immune cells by BFT consistent with in vitro data, indicating that BFT specifically binds only to epithelial cell lines that express E-cadherin and polarize in vitro (60). Additional studies are necessary to define the mechanisms by which ETBF and BFT activate the mucosal and systemic immune system and to identify the specific BFT cell receptor. Our data suggest that the BFT receptor is present throughout the intestine since BFT stimulated E-cadherin cleavage in small intestinal and colonic tissues (Fig. 4a). Thus, the colonic localization of WT-ETBF-induced disease likely relates to the colonic environment and as-yet-uncharacterized B. fragilis adherence factors.
The potent antibody response specific to WT-ETBF (bft-2) was unexpected considering the short-term infection but likely was facilitated by BFT-induced colitis with translocation of luminal antigens and systemic immune activation as evidenced by splenomegaly in ETBF-colonized mice. Although blood and peripheral tissue cultures of WT-ETBF (bft-2)- and rETBF (bft-2)-infected mice were negative (n = 5), it is possible that B. fragilis transiently invaded the periphery. The size and diffuse appearance of the WT-NTBF and WT-ETBF (bft-2) antigens detected by Western blot analysis (Fig. 3) suggest antibody reactivity to the unique and antigenically potent B. fragilis polysaccharide capsules of the inoculated B. fragilis strains. In contrast, murine BFT-specific antibodies were not detected despite the known immunogenicity of BFT in rabbits and humans (45). It is possible that Western blot analysis is too insensitive or that only murine mucosal antibodies are produced.
Although WT-NTBF-colonized mice did not exhibit any colonic inflammation, we detected weak antibody responses to NTBF, indicating a systemic adaptive immune response to NTBF, a finding consistent with a recent report of peripheral CD4+ T lymphocyte expansion in NTBF-colonized germfree mice (26). Our results extend these prior observations, showing initiation of adaptive immune responses to NTBF within 1 week of colonization, but differ from results of Konrad et al. (20) who reported that the murine immune responses to enteric bacteria were tightly compartmentalized to the mucosa in conventional mice. These differences suggest that the interaction of B. fragilis with the host is complex and differs from other common fecal flora.
Recently, Nakano et al. (31) reported that outbred germfree NIH (Swiss Webster) mice housed under short-term germfree conditions and infected with a human strain of WT-ETBF developed only mild, nonlethal colitis. Potential reasons for the experimental discrepancies include differences in susceptibility to colitis by different mouse strains and different amounts of BFT secretion among clinical strains of ETBF (4, 43, 53). Nonetheless, our experiments indicate that monoinfection with at least some WT-ETBF strains is sufficient to induce profound colonic inflammation.
In small studies, ETBF colonization in humans has been associated with active IBD (2, 36) and colorectal cancer (50). Reports estimate that ∼4 to 30% of healthy individuals harbor ETBF without obvious symptoms. We hypothesize that humans harboring ETBF or potentially similar as-yet-unidentified commensals may be at risk for the deleterious consequences of persistent, subclinical colonic inflammation such as IBD, oncogenic sequelae, and/or irritable bowel syndrome. Consistent with our hypothesis, we previously reported that mice given subclinical doses of DSS followed by ETBF colonization show exacerbated colitis (37) and that in Bangladeshi patients with ETBF, persistent intestinal inflammation was present 3 weeks postinfection even if ETBF was cleared with antibiotic therapy (45). To date, we have not observed colonic neoplasms in mice persistently colonized with WT-ETBF (bft-2) or rETBF (bft-2) at up to 16 months of age. Well-designed clinical studies are necessary to determine the long-term consequences of ETBF colonization.
In summary, we describe ETBF-induced colitis in colitis-resistant antibiotic-treated SPF C57BL/6 mice that mirrors acute and persistent infection observed in ETBF-infected individuals. Our data indicate that BFT is necessary and sufficient to induce colitis in both conventional and germfree mice. Studies to define the immunopathogenesis of ETBF colitis are in progress. The future translational challenge will be to identify if select members of the intestinal flora such as ETBF are key to the pathogenesis of colonic inflammatory conditions and their sequelae.
Acknowledgments
We appreciate the technical assistance of Melanie Adams and the gifts of Bacteroides strains by Lyle Myers, Tracy Wilkins, and Nadia Shoemaker.
This work was supported by the Crohn's and Colitis Foundation of America (CCFA) Research Fellowship Award to K.-J.R. and through a CCFA Senior Investigator Award to C.L.S. as well as NIH grants RO1 DK45496, RO1 DK080817 (to C.L.S.), NIH KO1 A1066994 (to J.E.G.), CA62924 (to Scott Kern), R24 DK64388 (to Mark Donowitz), and the NCI Division of Cancer Prevention contracts HHSN261200433002C (to Bert Vogelstein), RR21362 (to B.K.), P40 RR020764 (to R.B.S.), RO1 DK53347 (to R.B.S.), P30 DK34987 (to Robert S. Sandler), and RR00171 (to D.L.H.).
The authors have no financial conflict of interest.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 2 February 2009.
REFERENCES
- 1.Barthel, M., S. Hapfelmeier, L. Quintanilla-Martinez, M. Kremer, M. Rohde, M. Hogardt, K. Pfeffer, H. Russmann, and W. D. Hardt. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 712839-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basset, C., J. Holton, A. Bazeos, D. Vaira, and S. Bloom. 2004. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig. Dis. Sci. 491425-1432. [DOI] [PubMed] [Google Scholar]
- 3.Chambers, F. G., S. S. Koshy, R. F. Saidi, D. P. Clark, R. D. Moore, and C. L. Sears. 1997. Bacteroides fragilis toxin exhibits polar activity on monolayers of human intestinal epithelial cells (T84 cells) in vitro. Infect. Immun. 653561-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung, G. T., A. A. Franco, S. Wu, G. E. Rhie, R. Cheng, H. B. Oh, and C. L. Sears. 1999. Identification of a third metalloprotease toxin gene in extraintestinal isolates of Bacteroides fragilis. Infect. Immun. 674945-4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coyne, M. J., W. Kalka-Moll, A. O. Tzianabos, D. L. Kasper, and L. E. Comstock. 2000. Bacteroides fragilis NCTC9343 produces at least three distinct capsular polysaccharides: cloning, characterization, and reassignment of polysaccharide B and C biosynthesis loci. Infect. Immun. 686176-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coyne, M. J., K. G. Weinacht, C. M. Krinos, and L. E. Comstock. 2003. Mpi recombinase globally modulates the surface architecture of a human commensal bacterium. Proc. Natl. Acad. Sci. USA 10010446-10451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elson, C. O., Y. Cong, V. J. McCracken, R. A. Dimmitt, R. G. Lorenz, and C. T. Weaver. 2005. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol. Rev. 206260-276. [DOI] [PubMed] [Google Scholar]
- 8.Franco, A. A., S. L. Buckwold, J. W. Shin, M. Ascon, and C. L. Sears. 2005. Mutation of the zinc-binding metalloprotease motif affects Bacteroides fragilis toxin activity but does not affect propeptide processing. Infect. Immun. 735273-5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco, A. A., R. K. Cheng, A. Goodman, and C. L. Sears. 2002. Modulation of bft expression by the Bacteroides fragilis pathogenicity island and its flanking region. Mol. Microbiol. 451067-1077. [DOI] [PubMed] [Google Scholar]
- 10.Franco, A. A., L. M. Mundy, M. Trucksis, S. Wu, J. B. Kaper, and C. L. Sears. 1997. Cloning and characterization of the Bacteroides fragilis metalloprotease toxin gene. Infect. Immun. 651007-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta, A., H. Vlamakis, N. Shoemaker, and A. A. Salyers. 2003. A new Bacteroides conjugative transposon that carries an ermB gene. Appl. Environ. Microbiol. 696455-6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He, T. C., A. B. Sparks, C. Rago, H. Hermeking, L. Zawel, L. T. da Costa, P. J. Morin, B. Vogelstein, and K. W. Kinzler. 1998. Identification of c-MYC as a target of the APC pathway. Science 2811509-1512. [DOI] [PubMed] [Google Scholar]
- 13.Hermiston, M. L., M. H. Wong, and J. I. Gordon. 1996. Forced expression of E-cadherin in the mouse intestinal epithelium slows cell migration and provides evidence for nonautonomous regulation of cell fate in a self-renewing system. Genes Dev. 10985-996. [DOI] [PubMed] [Google Scholar]
- 14.Hise, A. G., K. Daehnel, I. Gillette-Ferguson, E. Cho, H. F. McGarry, M. J. Taylor, D. T. Golenbock, K. A. Fitzgerald, J. W. Kazura, and E. Pearlman. 2007. Innate immune responses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and Mal, but not TLR4, TRIF, or TRAM. J. Immunol. 1781068-1076. [DOI] [PubMed] [Google Scholar]
- 15.Högenauer, C., C. Langner, E. Beubler, I. T. Lippe, R. Schicho, G. Gorkiewicz, R. Krause, N. Gerstgrasser, G. J. Krejs, and T. A. Hinterleitner. 2006. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. N. Engl. J. Med. 3552418-2426. [DOI] [PubMed] [Google Scholar]
- 16.Hollander, D., C. M. Vadheim, E. Brettholz, G. M. Petersen, T. Delahunty, and J. I. Rotter. 1986. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann. Intern. Med. 105883-885. [DOI] [PubMed] [Google Scholar]
- 17.Kim, J. M., J. Y. Lee, Y. M. Yoon, Y. K. Oh, J. S. Kang, Y. J. Kim, and K. H. Kim. 2006. Bacteroides fragilis enterotoxin induces cyclooxygenase-2 and fluid secretion in intestinal epithelial cells through NF-κB activation. Eur. J. Immunol. 362446-2456. [DOI] [PubMed] [Google Scholar]
- 18.Kim, J. M., Y. K. Oh, Y. J. Kim, H. B. Oh, and Y. J. Cho. 2001. Polarized secretion of CXC chemokines by human intestinal epithelial cells in response to Bacteroides fragilis enterotoxin: NF-κB plays a major role in the regulation of IL-8 expression. Clin. Exp. Immunol. 123421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kling, J. J., R. L. Wright, J. S. Moncrief, and T. D. Wilkins. 1997. Cloning and characterization of the gene for the metalloprotease enterotoxin of Bacteroides fragilis. FEMS Microbiol. Lett. 146279-284. [DOI] [PubMed] [Google Scholar]
- 20.Konrad, A., Y. Cong, W. Duck, R. Borlaza, and C. O. Elson. 2006. Tight mucosal compartmentation of the murine immune response to antigens of the enteric microbiota. Gastroenterology 1302050-2059. [DOI] [PubMed] [Google Scholar]
- 21.Krinos, C. M., M. J. Coyne, K. G. Weinacht, A. O. Tzianabos, D. L. Kasper, and L. E. Comstock. 2001. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature 414555-558. [DOI] [PubMed] [Google Scholar]
- 22.Kühn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75263-274. [DOI] [PubMed] [Google Scholar]
- 23.Lu, W., A. Hisatsune, T. Koga, K. Kato, I. Kuwahara, E. P. Lillehoj, W. Chen, A. S. Cross, S. J. Gendler, A. T. Gewirtz, and K. C. Kim. 2006. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J. Immunol. 1763890-3894. [DOI] [PubMed] [Google Scholar]
- 24.Lu, Z., L. Yuan, X. Zhou, E. Sotomayor, H. I. Levitsky, and D. M. Pardoll. 2000. CD40-independent pathways of T cell help for priming of CD8+ cytotoxic T lymphocytes. J. Exp. Med. 191541-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luperchio, S. A., and D. B. Schauer. 2001. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3333-340. [DOI] [PubMed] [Google Scholar]
- 26.Mazmanian, S. K., C. H. Liu, A. O. Tzianabos, and D. L. Kasper. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122107-118. [DOI] [PubMed] [Google Scholar]
- 27.Moncrief, J. S., R. Obiso, Jr., L. A. Barroso, J. J. Kling, R. L. Wright, R. L. Van Tassell, D. M. Lyerly, and T. D. Wilkins. 1995. The enterotoxin of Bacteroides fragilis is a metalloprotease. Infect. Immun. 63175-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mundy, L. M., and C. L. Sears. 1996. Detection of toxin production by Bacteroides fragilis: assay development and screening of extraintestinal clinical isolates. Clin. Infect. Dis. 23269-276. [DOI] [PubMed] [Google Scholar]
- 29.Myers, L. L., and D. S. Shoop. 1987. Association of enterotoxigenic Bacteroides fragilis with diarrheal disease in young pigs. Am. J. Vet. Res. 48774-775. [PubMed] [Google Scholar]
- 30.Myers, L. L., D. S. Shoop, J. E. Collins, and W. C. Bradbury. 1989. Diarrheal disease caused by enterotoxigenic Bacteroides fragilis in infant rabbits. J. Clin. Microbiol. 272025-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano, V., D. A. Gomes, R. M. Arantes, J. R. Nicoli, and M. J. Avila-Campos. 2006. Evaluation of the pathogenicity of the Bacteroides fragilis toxin gene subtypes in gnotobiotic mice. Curr. Microbiol. 53113-117. [DOI] [PubMed] [Google Scholar]
- 32.Obiso, R. J., Jr., A. O. Azghani, and T. D. Wilkins. 1997. The Bacteroides fragilis toxin fragilysin disrupts the paracellular barrier of epithelial cells. Infect. Immun. 651431-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obiso, R. J., Jr., D. M. Lyerly, R. L. Van Tassell, and T. D. Wilkins. 1995. Proteolytic activity of the Bacteroides fragilis enterotoxin causes fluid secretion and intestinal damage in vivo. Infect. Immun. 633820-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okayasu, I., S. Hatakeyama, M. Yamada, T. Ohkusa, Y. Inagaki, and R. Nakaya. 1990. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98694-702. [DOI] [PubMed] [Google Scholar]
- 35.Pantosti, A., A. O. Tzianabos, B. G. Reinap, A. B. Onderdonk, and D. L. Kasper. 1993. Bacteroides fragilis strains express multiple capsular polysaccharides. J. Clin. Microbiol. 311850-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prindiville, T. P., R. A. Sheikh, S. H. Cohen, Y. J. Tang, M. C. Cantrell, and J. Silva, Jr. 2000. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg. Infect. Dis. 6171-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabizadeh, S., K.-J. Rhee, S. Wu, D. Huso, C. M. Gan, J. E. Golub, X. Wu, M. Zhang, and C. L. Sears. 2007. Enterotoxigenic Bacteroides fragilis: a potential instigator of colitis. Inflamm. Bowel Dis. 131475-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riegler, M., M. Lotz, C. Sears, C. Pothoulakis, I. Castagliuolo, C. C. Wang, R. Sedivy, T. Sogukoglu, E. Cosentini, G. Bischof, W. Feil, B. Teleky, G. Hamilton, J. T. LaMont, and E. Wenzl. 1999. Bacteroides fragilis toxin 2 damages human colonic mucosa in vitro. Gut 44504-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson, K. P., C. J. Smith, A. M. Gough, and E. R. Rocha. 2006. Characterization of Bacteroides fragilis hemolysins and regulation and synergistic interactions of HlyA and HlyB. Infect. Immun. 742304-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sack, R. B., L. L. Myers, J. Almeido-Hill, D. S. Shoop, W. C. Bradbury, R. Reid, and M. Santosham. 1992. Enterotoxigenic Bacteroides fragilis: epidemiologic studies of its role as a human diarrhoeal pathogen. J. Diarrhoeal Dis. Res. 104-9. [PubMed] [Google Scholar]
- 41.Sadlack, B., H. Merz, H. Schorle, A. Schimpl, A. C. Feller, and I. Horak. 1993. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75253-261. [DOI] [PubMed] [Google Scholar]
- 42.Sanfilippo, L., C. K. Li, R. Seth, T. J. Balwin, M. G. Menozzi, and Y. R. Mahida. 2000. Bacteroides fragilis enterotoxin induces the expression of IL-8 and transforming growth factor-beta (TGF-β) by human colonic epithelial cells. Clin. Exp. Immunol. 119456-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scotto d'Abusco, A. S., L. Sanfilippo, M. G. Menozzi, and A. Pantosti. 2000. Activity and role of BFT, an enterotoxin produced by Bacteroides fragilis J. Nat. Toxins 9267-280. [PubMed] [Google Scholar]
- 44.Sears, C. L. 2001. The toxins of Bacteroides fragilis. Toxicon 391737-1746. [DOI] [PubMed] [Google Scholar]
- 45.Sears, C. L., S. Islam, A. Saha, M. Arjumand, N. H. Alam, A. S. Faruque, M. A. Salam, J. Shin, D. Hecht, A. Weintraub, R. B. Sack, and F. Qadri. 2008. Association of enterotoxigenic Bacteroides fragilis infection with inflammatory diarrhea. Clin. Infect. Dis. 47797-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shanson, D. C., and J. Singh. 1981. Effect of adding cysteine to brain-heart infusion broth on the isolation of Bacteroides fragilis from experimental blood cultures. J. Clin. Pathol. 34221-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens, A. M., N. B. Shoemaker, and A. A. Salyers. 1990. The region of a Bacteroides conjugal chromosomal tetracycline resistance element which is responsible for production of plasmidlike forms from unlinked chromosomal DNA might also be involved in transfer of the element. J. Bacteriol. 1724271-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teahon, K., P. Smethurst, A. J. Levi, I. S. Menzies, and I. Bjarnason. 1992. Intestinal permeability in patients with Crohn's disease and their first degree relatives. Gut 33320-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tetsu, O., and F. McCormick. 1999. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398422-426. [DOI] [PubMed] [Google Scholar]
- 50.Toprak, N. U., A. Yagci, B. M. Gulluoglu, M. L. Akin, P. Demirkalem, T. Celenk, and G. Soyletir. 2006. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin. Microbiol. Infect. 12782-786. [DOI] [PubMed] [Google Scholar]
- 51.Van Tassell, R. L., D. M. Lyerly, and T. D. Wilkins. 1992. Purification and characterization of an enterotoxin from Bacteroides fragilis. Infect. Immun. 601343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vines, R. R., S. S. Perdue, J. S. Moncrief, D. R. Sentz, L. A. Barroso, R. L. Wright, and T. D. Wilkins. 2000. Fragilysin, the enterotoxin from Bacteroides fragilis, enhances the serum antibody response to antigen co-administered by the intranasal route. Vaccine 19655-660. [DOI] [PubMed] [Google Scholar]
- 53.Weikel, C. S., F. D. Grieco, J. Reuben, L. L. Myers, and R. B. Sack. 1992. Human colonic epithelial cells, HT29/C1, treated with crude Bacteroides fragilis enterotoxin dramatically alter their morphology. Infect. Immun. 60321-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkins, T. D., and S. Chalgren. 1976. Medium for use in antibiotic susceptibility testing of anaerobic bacteria. Antimicrob. Agents Chemother. 10926-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, S., L. A. Dreyfus, A. O. Tzianabos, C. Hayashi, and C. L. Sears. 2002. Diversity of the metalloprotease toxin produced by enterotoxigenic Bacteroides fragilis. Infect. Immun. 702463-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu, S., K. C. Lim, J. Huang, R. F. Saidi, and C. L. Sears. 1998. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc. Natl. Acad. Sci. USA 9514979-14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu, S., P. J. Morin, D. Maouyo, and C. L. Sears. 2003. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology 124392-400. [DOI] [PubMed] [Google Scholar]
- 58.Wu, S., J. Powell, N. Mathioudakis, S. Kane, E. Fernandez, and C. L. Sears. 2004. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-κB pathway. Infect. Immun. 725832-5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu, S., K.-J. Rhee, M. Zhang, A. Franco, and C. L. Sears. 2007. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and γ-secretase-dependent E-cadherin cleavage. J. Cell Sci. 1201944-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu, S., J. Shin, G. Zhang, M. Cohen, A. Franco, and C. L. Sears. 2006. The Bacteroides fragilis toxin binds to a specific intestinal epithelial cell receptor. Infect. Immun. 745382-5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zbar, A. P., C. Simopoulos, and A. J. Karayiannakis. 2004. Cadherins: an integral role in inflammatory bowel disease and mucosal restitution. J. Gastroenterol. 39413-421. [DOI] [PubMed] [Google Scholar]