Abstract
Trypanosoma cruzi, the agent of Chagas' disease, promotes neuron survival through receptor tyrosine kinase TrkA and glycosylphosphatidylinositol-anchored glial cell-derived family ligand receptors (GFRα). However, these receptors are expressed by only a subset of neurons and at low levels or not at all in glial cells. Thus, T. cruzi might exploit an additional neurotrophic receptor(s) to maximize host-parasite equilibrium in the nervous system. We show here that T. cruzi binds TrkC, a neurotrophic receptor expressed by glial cells and many types of neurons, and that the binding is specifically inhibited by neurotrophin-3, the natural TrkC ligand. Coimmunoprecipitation and competition assays show that the trans-sialidase/parasite-derived neurotrophic factor (PDNF), previously identified as a TrkA ligand, mediates the T. cruzi-TrkC interaction. PDNF promotes TrkC-dependent mitogen-activated protein kinase signaling, neurite outgrowth, and survival of genetically engineered PC12 neuronal cells and glial Schwann cells in a TrkC-dependent manner. Thus, TrkC is a new neurotrophic receptor that T. cruzi engages to promote the survival of neuronal and glial cells. The results raise the possibility that T. cruzi recognition of TrkC underlies regenerative events in nervous tissues of patients with Chagas' disease.
Trypanosoma cruzi causes Chagas' disease, a chronic, incurable, debilitating condition widespread in Latin America and increasingly prevalent in the United States (5, 19). T. cruzi preferentially invades Schwann cells and enteric glial cells in the peripheral nervous system (PNS) (45) and astrocytes in the central nervous system (CNS) (15). T. cruzi also invades neurons in both the PNS and CNS (38, 40). The interaction of T. cruzi with the nervous system may trigger cell survival mechanisms, as judged by nerve tissue-regenerative events in patients in the chronic indeterminate phase of Chagas' disease (16, 17, 25). For example, although the number of neurons in chagasic patients is lower than that of age-matched healthy individuals, the average number of neurons in both cardiac and gastrointestinal ganglia actually increases with the age of patients, contrary to the age-related physiological reduction in nonchagasic individuals (25). Furthermore, T. cruzi infects the CNS, where the parasites are found in the spinal fluid of patients with acute Chagas' disease (22), yet T. cruzi invasion of the CNS produces few, if any, symptoms and pathology unless patients are coinfected with human immunodeficiency virus or undergo treatment with immunosuppressants (13, 37). Animals infected with T. cruzi also present evidence of neuroregeneration such as neurite outgrowth and the absence of neurodegeneration in T. cruzi-infected foci in the brain (30, 32, 33).
Host cell protection against injury could confer obvious benefits to an obligate intracellular parasite like T. cruzi. Although host responses likely underlie tissue repair mechanisms, increasing evidence suggests that T. cruzi may play a direct role in enhancing cell survival (7, 8). Similar to the activation of TrkA receptor by nerve growth factor (NGF), T. cruzi binds TrkA and activates mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase, and Akt kinase signaling pathways (9, 11, 12). T. cruzi recognition of TrkA is via the neuraminidase/trans-sialidase, also called parasite-derived neurotrophic factor (PDNF) (7, 8). The activation of these TrkA-dependent signaling pathways leads to the survival of neurons and other TrkA-expressing cell types subjected to various insults such as infection and starvation (11, 12). Thus, PDNF may be one of the factors that may mitigate host nerve tissue damage.
TrkA is a member of the tyrosine kinase Trk receptor family, which also includes the receptors TrkB and TrkC. Trk signaling helps mediate the differentiation, continued survival, and regeneration of cells throughout the CNS and PNS (24). Despite a 60 to 80% homology, Trk receptors have preferred ligands called neurotrophins, a distinct pattern of expression, and nonredundant functions. For example, the preferred ligands for TrkA and TrkB are NGF and brain-derived NGF (BDNF), respectively, which do not bind TrkC. TrkC is recognized only by neurotrophin-3 (NT-3), yet NT-3 is the most promiscuous of the neurotrophins, as it binds TrkA and TrkB albeit with a 102 to 104 lower affinity (24). The binding of NT-3 to each Trk receptor with various affinities may activate signaling pathways differentially (27). However, it is not clear whether low-affinity binding to nonpreferred receptors is relevant in vivo, as the decreased affinity of binding of NT-3 to TrkB may be magnified in vivo, resulting in selective signaling through TrkC but not TrkB (44). The divergence in Trk family receptors can also be seen by examining cell-specific Trk expression, as any given neuron or glial cell often expresses only one Trk receptor type. Defined segments of the nervous system often depend on a specific Trk receptor for differentiation and continued survival (6, 24).
Some nerve tissues highly parasitized by T. cruzi such as those in the heart and gastrointestinal tract depend on the expression of TrkC but not of TrkA or TrkB (6, 24). It also depends on the expression of receptors for glial cell-derived family ligands (GFRα and Ret) (1, 3). The tissue specificity of Trk receptors, GFRα, and Ret raises the possibility of T. cruzi optimizing its interactions with cells throughout the mammalian nervous system by binding multiple neurotrophic receptors. For example, Schwann cells, which myelinate neurons in the PNS and express TrkB and TrkC but not TrkA and GFRα (50), undergo regeneration in animal models of Chagas' disease (34). Although T. cruzi activates TrkA (11) and GFRα (31) prosurvival signalings, an alternative mechanism(s) must be employed if T. cruzi is to promote the maintenance of parasitized cells that do not express TrkA and GFRα receptors. In ways that mimic, yet that are distinct from the endogenous actions of NT-3, we show here that T. cruzi mediates neuronal and Schwann cell survival through the binding and activation of the neurotrophic receptor TrkC.
MATERIALS AND METHODS
Parasites and cell lines.
T. cruzi trypomastigotes (Silvio X-10/4 strain) were propagated in Vero cell cultures (11). PC12nnr5 cells were gifts from Lloyd Green (College of Physicians and Surgeons, Columbia University, NY). Trk receptor-deficient PC12 cell mutant NNR5 (20) was cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco) supplemented with 10% FBS (Gemini Bio Products), 100 U/ml penicillin-streptomycin (Gibco), 2 mM l-glutamine (Gibco), 1× nonessential amino acids (Gibco), and 1 mM sodium pyruvate (Gibco). Human Schwann cells (permanent cell line) (7) were maintained in DMEM supplemented with 10% FBS (Gemini Bio Products) and 100 U/ml penicillin-streptomycin (Gibco).
Cloning and transfection.
TrkB and TrkC were directionally cloned from human RNA (RNA was a gift from Tugba Bagci, Neuroscience Department, Tufts Medical School, Tufts University) via reverse transcription-PCR into the pIRES-dsRed mammalian expression vector (Clontech). TrkC was amplified using forward primer 5′-CCGCTCGAGATGGATGTCTCTCTT (incorporating the XhoI site, which is underlined) and reverse primer 5′-CTAGCCAAGAATGTCCAGGTAGATTG. The PCR product was ligated into the Topo vector (Invitrogen), excised, and religated into the pIRES vector using XhoI and EcoRI sites. A similar cloning strategy was employed for TrkB using 5′-CCGCTCGAGATGTCGTCCTGGATA and 5′-CTAGCCTAGAATGTCCAGGTAGACCG as forward and reverse primers, respectively. NNR5 cells were transfected with TrkB, TrkC, or empty vector (EV) clones using Fugene HD (Roche) according to the manufacturer's protocol. Transfected cells were selected for in NNR5 medium supplemented with 500 μg/ml Geneticin (selective medium) (Gibco) for 1 week and then sorted by use of a fluorescence-activated cell sorter (FACS) for dsRed expression at 1 week and 1 month posttransfection. Cells were regrown in selective medium and sorted a third time (by FACS) to obtain cell populations with homogenous expression levels of transfected receptors.
PDNF purification.
Full-length PDNF was isolated from T. cruzi cultures by immunoaffinity chromatography as described previously (43).
T. cruzi binding assay.
The extracellular domain of TrkA, TrkB, TrkC, and fibroblast growth factor receptor (FGFR) bound to the immunoglobulin Fc domain were purchased from R&D systems, as was TrkC without the Fc domain. Binding experiments were performed as described previously (18): 5 × 106 trypomastigotes/ml were incubated with each receptor in binding buffer (DMEM, 0.1% bovine serum albumin [BSA]) for 45 min at 4°C and washed four times with binding buffer by centrifugation (6,000 × g for 5 min) to remove unbound receptor. Parasite pellets were resuspended in reducing (2% β-mercaptoethanol) sodium dodecyl sulfate (SDS)-Laemmli sample buffer, run on an SDS-polyacrylamide gel electrophoresis (PAGE) gel (7.5%), transferred onto nitrocellulose, and probed with anti-human immunoglobulin G (IgG) horseradish peroxidase (HRP)-labeled antibody (Promega); blots were quantified in a scanning densitometer (Bio-Rad Laboratories). Blots were stripped and reprobed using human chagasic serum or TCN-2 monoclonal antibody to evaluate loaded parasite and the effect of washing of the parasites. Similar procedures were performed for T. cruzi-TrkC competitive binding experiments except that NT-3, BDNF, NGF (Chemicon), or PDNF was coincubated with T. cruzi and TrkC.
Coimmunoprecipitation assay.
A coimmunoprecipitation assay was performed by a slight modification of a previously reported coimmunoprecipitation procedure (31): PDNF (1 μg) was incubated overnight at 4°C with Fc receptors (1 μg) in binding buffer (DMEM, 0.1% BSA). Fc-receptors were immunoprecipitated on protein G-Sepharose (GE Healthsciences) and washed three times in binding buffer. The pellet was resuspended in SDS-sample buffer, run on reducing (2% β-mercaptoethanol) SDS-PAGE (7.5%) gels, and transferred onto nitrocellulose. PDNF coimmunoprecipitated by the Fc receptors was identified with PDNF-specific monoclonal antibody TCN-2 and anti-mouse-HRP secondary antibodies. Receptors were evaluated using anti-human IgG-HRP antibodies.
Trk signaling evaluation. (i) Neurite extension.
Transfected NNR5 cells were plated in 96-well plates (104 cells/well) and treated with NT-3 or BDNF (100 ng/ml) or PDNF (250 ng/ml) in 10% fetal calf serum (FCS)-DMEM for 48 to 72 h. Cells were fixed in 4% paraformaldehyde, blocked in 5% BSA-phosphate-buffered saline, and probed with anti-neurofilament 200 (Sigma-Aldrich) followed by Alexa 488-conjugated anti-rabbit IgG (Molecular Probes). Cells were imaged (magnification of ×20) by fluorescence microscopy (Olympus IX70). Using SPOT Advanced software, a minimum of 50 cells for each condition were analyzed for neurite length. All cells with at least one neurite greater in length than 100% of its cell body width were counted as being a cell with a neurite.
(ii) Erk phosphorylation.
Subconfluent transfected NNR5 cells were plated in regular medium for 1 day and then cultured in serum-free DMEM overnight. Cultures were then treated with NT-3 or BDNF (100 ng/ml) or PDNF (25 to 200 ng/ml) for 12 min. When indicated, cells were pretreated with the Trk-specific inhibitor K252a (1 μM) (Sigma-Aldrich) for 60 min. Cells were immediately washed with cold phosphate-buffered saline and lysed on ice with 1% NP-40 lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride) for 30 min. Lysates were cleared by centrifugation at 10,000 × g for 10 min, and equal amounts of total protein (20 to 60 μg) in SDS-loading buffer were run under reducing conditions on an SDS-PAGE gel (12.5%), transferred onto nitrocellulose, and probed with antibodies specific for phospho-Erk (P-Erk) (Cell Signaling) and Erk1/2 kinase (Cell Signaling). To determine whether T. cruzi activates Erk1/2, NNR5 transfectants were infected with 107 trypomastigotes/ml for 15 min, and P-Erk was ascertained as described above for PDNF cell activation.
NNR5 survival assay.
Transfected NNR5 cells were plated in 96-well plates (104 cells/well), attached overnight in 10% FCS-DMEM, and grown in 1% FCS-DMEM, serum-free DMEM, or serum-free DMEM supplemented with 250 ng/ml PDNF for 72 h. Cultures were then treated with Hoechst 33342 stain (10 μg/ml) and propidium iodide (PI) (2 μg/ml) in prewarmed (37°C) DMEM for 10 min at 37°C and visualized under UV light (340 to 380 nm). Hoechst 33342 stains live and apoptotic nuclei blue, and PI stains only dead cells (pink).
Schwann cell survival assay.
Schwann cells were plated in 96-well plates (5 × 103 cells/well), allowed to attach overnight to the substratum in 10% FCS-DMEM, and then washed twice in serum-free DMEM. Cells were then maintained in serum-free DMEM for 3 days with daily 1-h treatments of FCS (1%) or serum-free DMEM with or without TrkC-specific antibodies (1 mg/ml) (Upstate Biotech), TrkB-specific antibodies (1 mg/ml) (Upstate Biotech), or PDNF (250 ng/ml) with or without Trk antibodies. Cells were then stained with Hoechst 33342 dye-PI and imaged by fluorescence microscopy as described above.
RESULTS
T. cruzi binds to TrkC.
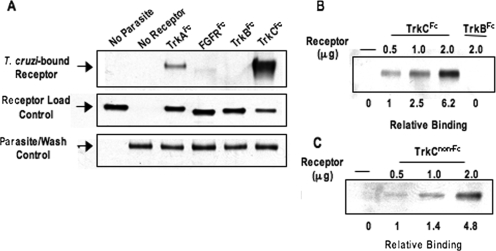
To determine whether T. cruzi binds TrkB and/or TrkC, we followed a procedure similar to the one used previously to study T. cruzi-TrkA interactions (18). First, parasites were incubated with Fc chimera of the extracellular domain (ECD) of TrkB and TrkC receptors, hereafter called TrkB and TrkC unless stated otherwise. Second, parasite-TrkB or -TrkC mixtures were spun down, washed to remove unbound receptors, and lysed with SDS-Laemmli sample buffer. Third, the lysates were analyzed by Western blotting to evaluate T. cruzi-bound receptor using antibody specific for the human IgG Fc domain. An Fc chimera of FGFR1, widely expressed in the nervous system (24), was included as a negative control, and an Fc chimera of TrkA was included as a positive control. The results showed that T. cruzi binds TrkC and not TrkB (Fig. 1A) and that the binding is dose dependent (Fig. 1B). Stripping and redeveloping the blots with a T. cruzi-specific antibody showed that the motile parasites were not lost during washing (Fig. 1A). T. cruzi also binds TrkCECD not linked to Fc (Fig. 1C), suggesting that T. cruzi-TrkC binding is independent of the Fc domain.
FIG. 1.
T. cruzi binds TrkC. (A) Live parasites (5 × 106 parasites) were incubated with equal amounts of the indicated soluble receptors (1 μg), spun down, washed to remove unbound receptors, and lysed to reveal bound receptor by Western blotting using anti-human IgG-HRP. The receptor load control represents an equal volume of supernatant from each sample after the first spin to evaluate unbound receptor by Western blotting. Note that TrkCFc is visibly reduced in the supernatant obtained by incubating parasites with TrkC. The parasite/wash control represents the total number of parasites after washes revealed by Western blotting with T. cruzi-specific antibody TCN-2. Note that parasite load was constant for every lane. (B) Dose response of T. cruzi/TrkCFc binding. (C) Dose response of T. cruzi/TrkCnon-Fc binding. Bound receptor was revealed using anti-TrkC antibody. (B and C) Relative binding was calculated using the parasite/wash control as a standard. Experiments in B and C were done three times, and those in A were done five times, all with similar results.
Binding of T. cruzi to TrkC is specifically inhibited by NT-3.
We used competition assays to further define the specificity of T. cruzi/TrkC molecular interactions. For this, we incubated live parasites with TrkC, with or without the neurotrophins NGF, NT-3, and BDNF, to determine whether TrkC binding to T. cruzi is specifically blocked by NT-3. Indeed, NT-3, but not BDNF and NGF, inhibits TrkC binding to T. cruzi in a dose-dependent manner (Fig. 2A and B).
FIG. 2.
NT-3 specifically inhibits T. cruzi-TrkC binding. (A) A total of 2× molar amounts of recombinant NT-3, BDNF, or NGF (0.6 μg) to TrkCFc were incubated with 5 × 106 T. cruzi parasites and TrkCFc (1 μg). Parasites were then spun down and washed, and bound TrkC was revealed by Western blotting using anti-human IgG antibodies. (B) Dose response of increasing concentrations of NT-3 incubated with live T. cruzi parasites and TrkCFc. Bound TrkCFc was calculated by scanning densitometry, with each sample standardized against its parasite wash control. (Inset) A representative blot from the experiment is shown. Experiments in A and B were done three times, with similar results.
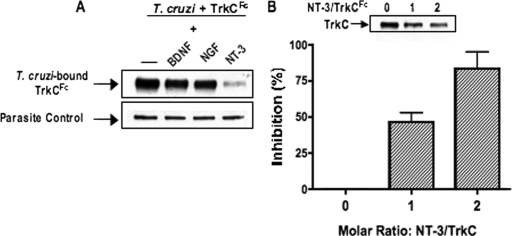
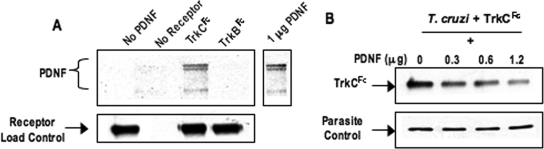
The parasite protein PDNF is responsible for T. cruzi-TrkC binding.
Because PDNF binds TrkA, it may be that PDNF also binds TrkC, which would be in line with the pattern of neurotrophin binding to Trk receptors (23). This possibility was tested by coimmunoprecipitation (31) and T. cruzi-TrkC competition assays (18). PDNF, purified from T. cruzi culture supernatants by affinity chromatography (43), was incubated with TrkC, TrkB, and FGFR. Receptor-PDNF complexes, if any, were pulled down on protein G-Sepharose (via the Fc arm of the chimeric receptors) and evaluated by Western blotting with a monoclonal antibody (TCN-2) specific for PDNF (39). We found that TrkC specifically immunoprecipitates PDNF (Fig. 3A), indicating that PDNF is a TrkC ligand. Additionally, soluble PDNF inhibits TrkC binding to the outer membrane of T. cruzi in a dose-dependent manner (Fig. 3B), further indicating that PDNF is a TrkC ligand. To determine whether additional T. cruzi proteins bind TrkC, we repeated the pull-down experiments using whole T. cruzi lysate and probing with Chagasic serum. The results showed no evidence for TrkC-binding molecules in T. cruzi other than PDNF (data not shown).
FIG. 3.
PDNF binds TrkC. (A) Receptor (1 μg) was incubated with PDNF (1 μg) and mixed with protein G-Sepharose to pull down Fc-receptors. Coimmunoprecipitated PDNF was revealed by Western blotting using TCN-2 monoclonal antibody. Total receptor was revealed using anti-human IgG antibodies. The additional blot shows 1 μg PDNF visualized by a Western blot developed using TCN-2 monoclonal antibody. (B) Parasites were incubated with 1 μg of TrkCFc and increasing concentrations of PDNF. Parasites were washed and evaluated for bound TrkCFc. Bound TrkCFc was calculated by densitometry, with each sample standardized against its parasite wash control, as revealed with chagasic serum (one of several bands shown). Experiments in A and B were done three times, with similar results.
PDNF activates TrkC signaling.
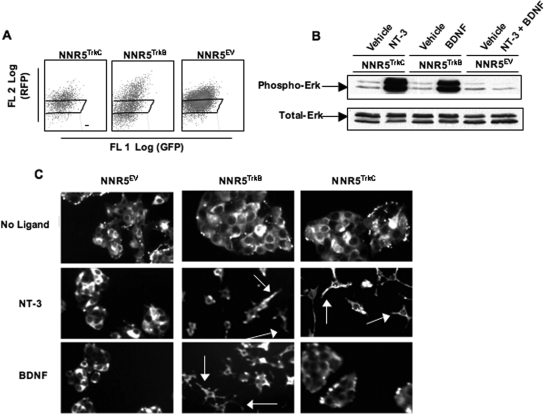
We used a genetic approach to determine whether T. cruzi PDNF activates TrkC signaling. For this, full-length TrkC and TrkB (ECD plus transmembrane domain plus intracellular kinase domain) were cloned from human brain RNA and expressed in PC12NNR5 (NNR5) cells using a bicistronic dsRed mammalian expression vector. We used the neuronal NNR5 cells because they do not express Trk receptors, are readily transfectable, and respond to neurotrophins (20). First, we tested whether transfected cells respond appropriately to physiological ligands. NNR5 cells transfected with TrkB, TrkC, or empty vector were cultured in selective media and sorted by FACS to obtain cell populations with similar expression levels of dsRed and, by extension, of transfected receptors (Fig. 4A). Such sorting was performed to facilitate comparisons between the cell lines stimulated with various agonists. This way, cells transfected with a given receptor responded appropriately and specifically to corresponding ligands (Fig. 4B and C).
FIG. 4.
Trk-deficient PC12-NNR5 (NNR5) cells transfected with TrkC, TrkB, and EV respond appropriately to corresponding ligands. TrkB and TrkC were cloned from human RNA into the bicistronic mammalian expression vector pIRES-dsRed, which was used to transfect PC12NNR5 cells. (A) NNR5TrkC, NNR5TrkB, and NNR5EV (EV) cells were sorted by FACS for dsRed expression to obtain similar expression levels; rectangles represent the cells selected from each population. The x and y axes represent channels that detect green fluorescent protein (GFP) and red fluorescent protein (RFP), respectively. FL, fluorescence. (B) P-Erk was examined by Western blotting with transfected cells cultured in serum-free medium overnight and treated with NT-3 or BDNF (100 ng/ml) for 12 min at 37°C. The upper and lower bands in each blot represent Erk1 (MAPK3) and Erk2 (MAPK1), respectively. (C) Transfected cells were cultured in medium with or without NT-3 (100 ng/ml) or BDNF (100 ng/ml) for 3 days, fixed, probed with anti-neurofilament primary antibody and Alexa 488 secondary antibody, and imaged (magnification, ×20) to visualize neurite extension. Experiments were repeated multiple times at various points to ensure that cells maintained correct responsiveness.
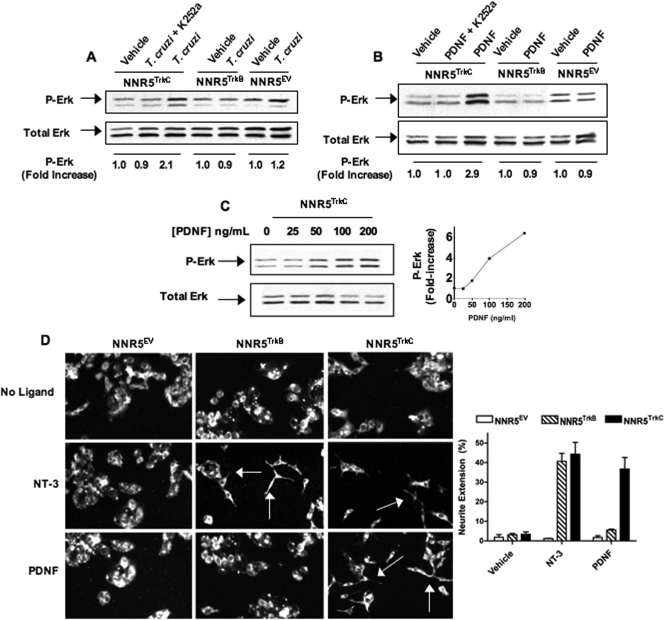
Second, because the activation of MAPK Erk1/2 kinase is key for TrkC-dependent neurite extension, cell differentiation, and cell survival (24), we probed whether PDNF activates TrkC-dependent Erk1/2 signaling by treating NNR5TrkC cells with PDNF (150 ng/ml for 12 min) or T. cruzi (107 trypomastigotes/ml for 15 min) and examining whether such treatment promoted the phosphorylation of Erk1/2 by Western blotting. We found that both PDNF and T. cruzi activate Erk kinase (Fig. 5A and B) in a dose-dependent manner (Fig. 5C). In contrast, purified PDNF and live T. cruzi did not activate Erk in NNR5TrkB and NNR5EV cells (Fig. 5A and B). Furthermore, the observed P-Erk increase in the NNR5TrkC cells was inhibited by K252a, a selective pharmacological inhibitor of Trk signaling (Fig. 5B) (4, 26), further suggesting that T. cruzi and PDNF specifically activate TrkC signaling.
FIG. 5.
T.cruzi/PDNF activates TrkC signaling. (A) Cells were cultured in serum-free medium overnight, treated with the Trk-specific inhibitor K252a (1 μM) for 1 h where indicated, and then treated with 106 parasites or not treated for 12 min at 37°C. Cell lysates were analyzed by Western blotting to evaluate P-Erk and total Erk. (B) Similar to A, but plated cells were treated with PDNF (200 ng/ml) for 12 min. (C) PDNF dose-response treatment of NNR5TrkC as described above (B) with accompanying graph. Phosphorylation (increase) was calculated by scanning densitometry. Each P-Erk band was standardized against its total Erk band and then standardized against vehicle (DMEM), which was arbitrarily set to 1. Experiments in A to C were done twice, with similar results obtained. (D) NNR5TrkC, NNR5TrkB, and NNR5EV cells were plated and cultured for 2 to 3 days with PDNF (250 ng/ml) or NT-3 (100 ng/ml). Cultures were then fixed, probed with anti-neurofilament primary antibody and Alexa 488 secondary antibody, and imaged (magnification, ×20) to visualize neurite extension; arrows point toward neurites. (Inset) Neurite extension was quantified for three individual experiments; error bars represent the standard errors of the means between the three experiments, and 50 or more cells were counted for each condition in each experiment. Neurite extension (percent) is the number of cells with at least one neurite with a length greater than 100% of the diameter of the cell body divided by the total number of cells.
To further examine PDNF-dependent TrkC activation, NNR5 cells were plated and grown in media in the presence or absence of PDNF (250 ng/ml) or NT-3 (100 ng/ml) for 72 h. Cells were then evaluated for neurite extension by fluorescence microscopy. Without the addition of PDNF or NT-3, NNR5TrkB, NNR5TrkC, and NNR5EV cells grew into clusters of small, round cells. However, the treatment of the cells with PDNF resulted in neurite outgrowth in NNR5TrkC cells but not in NNR5TrkB or NNR5EV cells (Fig. 5D). This finding further suggests that PDNF activates TrkC signaling.
PDNF promotes survival of neuronal and glial cells via TrkC.
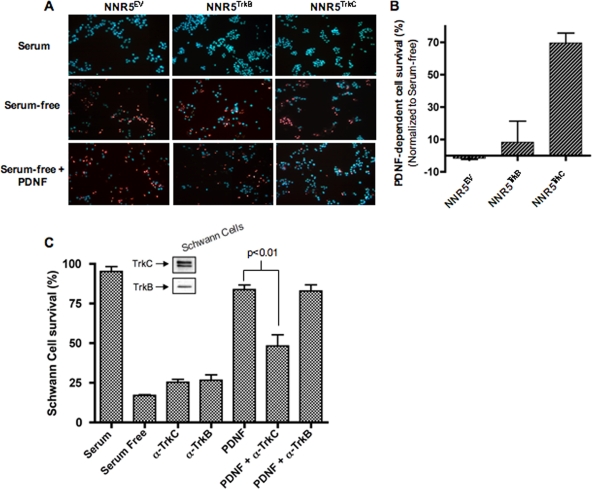
To determine whether PDNF promotes the TrkC-dependent survival of neuronal cells, we induced apoptosis in the NNR5 transfectants by growing the cells in serum-free medium for 3 days in the absence or presence of PDNF (250 ng/ml). Cell death was assessed by fluorescence microscopy using the Hoechst 33343-PI assay. While most NNR5TrkC cells died in serum-free medium, PDNF rescued ∼70% of the serum-starved cells (Fig. 6A and B). In contrast, PDNF did not rescue NNR5TrkB and NNR5EV transfectants (Fig. 6A and B). This result suggests that PDNF treatment protects a neuronal cell line from cell death in a TrkC-dependent manner.
FIG. 6.
PDNF promotes TrkC-dependent neuronal and glial cell survival. (A) NNR5TrkC, NNR5TrkB, and NNR5EV cells were plated in triplicate and cultured for 3 days in 1% FCS, serum-free DMEM, or serum free-medium supplemented with PDNF (250 ng/ml). Cells were stained with propidium iodide and Hoechst nuclear staining reagents and counted by fluorescence microscopy (magnification, ×20). (B) Graph of data for experiment shown in A with PDNF survival normalized against serum-free survival. Error bars represent the standard errors of the means between experiments; an average of three experiments were performed, with each point in triplicate. (C) Schwann cells were plated in triplicate and then cultured under conditions as indicated: 1% FBS or serum free with or without anti-TrkC (1 μg/ml), anti-TrkB (1 μg/ml), PDNF (250 ng/ml), or a combination. Error bars represent the variation between experiments. P values (analysis of variance) were calculated using Prism software. An average of two experiments were performed, each point in triplicate.
To determine whether the survival action of PDNF extends to glial cells, we grew a human Schwann cell line in serum-free medium for 3 days without and with PDNF (250 ng/ml), with or without an antibody specific for TrkC or TrkB. Preliminary experiments showed that the commercial TrkC antibodies reacted with TrkC and not TrkA and TrkB and that the TrkB antibodies were selective for TrkB (not shown). In addition, preliminary experiments confirmed that human Schwann cells express TrkB and TrkC (Fig. 6C, inset) but not TrkA (not shown). We found that PDNF potently promoted the survival of Schwann cells (Fig. 6C, compare the serum bar with the serum-free and PDNF bars). Because PDNF binds TrkC but not TrkB and because Schwann cells do not express TrkA, this result suggests that Schwann cell protection is mediated by PDNF recognition of TrkC, and this was confirmed by inhibition with Trk-specific antibodies. Thus, the PDNF-induced protection of Schwann cells was blocked by a TrkC-specific antibody (P < 0.01) but not by a TrkB-specific antibody (Fig. 6C).
DISCUSSION
Our results demonstrate that T. cruzi PDNF (8), also known as neuraminidase (36) and trans-sialidase (35, 42, 43), engages the neurotrophic receptor TrkC to promote the differentiation of a neuronal cell line and the survival of neuronal and glial Schwann cells. TrkC is widely expressed by neurons in the CNS, particularly in the brain cortex, hippocampus, and cerebellum (29). It is also widely expressed in neurons in the dorsal root ganglia and enteric nervous system (6). In addition to neurons, TrkC is also expressed in Schwann cells and at neuromuscular synapses (21). The expression of TrkC in the CNS is in contrast to that of TrkA, which is restricted to a small subset of cholinergic basal forebrain neurons (28). TrkA is not normally expressed by Schwann cells and other glial cells. Earlier studies showed that T. cruzi uses TrkA to activate antiapoptotic signaling (11). Thus, the discovery that T. cruzi also directly binds and activates TrkC accounts for the protection of the glial cells cocultured with the parasites (7).
PDNF activation of differentiation and survival of cells of the nervous system, through the recognition of TrkC, widens the scope for the possible role that T. cruzi plays in helping repair infected nervous tissues (2, 10, 18). This would be broadened even further by T. cruzi activation of TrkB, as reported, without the use of T. cruzi, with a bacterially expressed truncated form of trans-sialidase (49). Instead of bacterially expressed truncated protein, we used full-length endogenous PDNF isolated from T. cruzi strain Silvio to show that it does not bind TrkB under conditions in which it interacts with and activates TrkC (Fig. 1 to 6). In our experiments, the PDNF/trans-sialidase actions were reproduced by live, invasive parasites (Fig. 1 to 6). Nevertheless, it is possible that T. cruzi (and PDNF) can activate TrkB under conditions distinct from ours, such as concentrations higher than those used here.
T. cruzi PDNF is a functional mimic of neurotrophins inasmuch as it binds and activates TrkA and TrkC. Neurotrophins (NGF, BDNF, and NT-3) interact with two different classes of receptors. The first class is called P75NTR, which belongs to the tumor necrosis factor superfamily and, as such, has a “death” domain; it binds to all neurotrophins with relatively low affinity (∼10−9 M), and it can mediate cell survival or cell death, promote or inhibit axonal growth, and facilitate or attenuate proliferation (14, 23). Although p75NTR is a rabies virus receptor (46), we have been unable to demonstrate a binding of T. cruzi to p75NTR (11; this work).
The second class of neurotrophin receptors is the so-called high affinity (Ko ∼10−11) Trk (tropomyosin-related kinase) receptors. TrkECD is composed of five subdomains, a leucine-rich repeat structure sandwiched between two cysteine-rich cluster domains followed by two Ig-like domains. Neurotrophins bind to the membrane-proximal Ig-like domain, as determined by many criteria including cocrystal structures of the isolated domain or the entire TrkECD region with neurotrophin (47, 48). We hypothesize that T. cruzi interacts with the Ig-2-like domain of TrkC because PDNF (and T. cruzi) binding to TrkCECD is specifically inhibited by the TrkC ligand NT-3 (Fig. 2). Furthermore, each Trk receptor spans the membrane once and ends in the cytoplasm with a tyrosine kinase domain, which becomes activated after ECD dimerization triggered by neurotrophin binding (24, 47). Thus, it may be that T. cruzi, like NT-3, dimerizes TrkC to activate Erk signaling (Fig. 5) and promote cell survival and differentiation (Fig. 6).
Because PDNF is anchored to the trypanosome outer membrane by a glycosylphosphatidylinositol structure (41), the engagement of TrkC by T. cruzi should occur during trypanosome-host cell interactions. Such interactions are required for the parasite to penetrate host cells. T. cruzi-dependent TrkC engagement should extend to uninfected cells, given that PDNF is readily shed from the trypanosome surface into the water-soluble, diffusible factor (41). T. cruzi could emulate NT-3 in vivo by protecting cells against damage in nervous tissues invaded by T. cruzi. Neuroregeneration events occur in the gastrointestinal tract of patients in the indeterminate (asymptomatic) phase of Chagas' disease (25) and in the megacolon of patients with chronic symptomatic disease (16).
Acknowledgments
We thank Tugba Bagci for the human brain RNA and Thereza Imanishi-Kari and Jin Han for help in cloning Trk receptors.
This work was supported by NIH grants NS40574 and NS42960.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 29 January 2009.
REFERENCES
- 1.Airaksinen, M. S., and M. Saarma. 2002. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 3383-394. [DOI] [PubMed] [Google Scholar]
- 2.Akpan, N., K. Caradonna, M. V. Chuenkova, and M. PereiraPerrin. 2008. Chagas' disease parasite-derived neurotrophic factor activates cholinergic gene expression in neuronal PC12 cells. Brain Res. 1217195-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baloh, R. H., H. Enomoto, E. M. Johnson, Jr., and J. Milbrandt. 2000. The GDNF family ligands and receptors—implications for neural development. Curr. Opin. Neurobiol. 10103-110. [DOI] [PubMed] [Google Scholar]
- 4.Berg, M. M., D. W. Sternberg, L. F. Parada, and M. V. Chao. 1992. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J. Biol. Chem. 26713-16. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2007. Blood donor screening for Chagas disease—United States, 2006-2007. MMWR Morb. Mortal. Wkly. Rep. 56141-143. [PubMed] [Google Scholar]
- 6.Chalazonitis, A. 2004. Neurotrophin-3 in the development of the enteric nervous system. Prog. Brain Res. 146243-263. [DOI] [PubMed] [Google Scholar]
- 7.Chuenkova, M. V., F. B. Furnari, W. K. Cavenee, and M. A. Pereira. 2001. Trypanosoma cruzi trans-sialidase: a potent and specific survival factor for human Schwann cells by means of phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl. Acad. Sci. USA 989936-9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuenkova, M. V., and M. A. Pereira. 2003. PDNF, a human parasite-derived mimic of neurotrophic factors, prevents caspase activation, free radical formation, and death of dopaminergic cells exposed to the Parkinsonism-inducing neurotoxin MPP+. Brain Res. Mol. Brain Res. 11950-61. [DOI] [PubMed] [Google Scholar]
- 9.Chuenkova, M. V., and M. A. Pereira. 2001. The T. cruzi trans-sialidase induces PC12 cell differentiation via MAPK/ERK pathway. Neuroreport 123715-3718. [DOI] [PubMed] [Google Scholar]
- 10.Chuenkova, M. V., and M. A. Pereira. 2000. A trypanosomal protein synergizes with the cytokines ciliary neurotrophic factor and leukemia inhibitory factor to prevent apoptosis of neuronal cells. Mol. Biol. Cell 111487-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuenkova, M. V., and M. PereiraPerrin. 2004. Chagas' disease parasite promotes neuron survival and differentiation through TrkA nerve growth factor receptor. J. Neurochem. 91385-394. [DOI] [PubMed] [Google Scholar]
- 12.Chuenkova, M. V., and M. PereiraPerrin. 2005. A synthetic peptide modeled on PDNF, Chagas' disease parasite neurotrophic factor, promotes survival and differentiation of neuronal cells through TrkA receptor. Biochemistry 4415685-15694. [DOI] [PubMed] [Google Scholar]
- 13.Cordova, E., A. Boschi, J. Ambrosioni, C. Cudos, and M. Corti. 2008. Reactivation of Chagas disease with central nervous system involvement in HIV-infected patients in Argentina, 1992-2007. Int. J. Infect. Dis. 12587-592. [DOI] [PubMed] [Google Scholar]
- 14.Cragnolini, A. B., and W. J. Friedman. 2008. The function of p75NTR in glia. Trends Neurosci. 3199-104. [DOI] [PubMed] [Google Scholar]
- 15.Da Mata, J. R., M. R. Camargos, E. Chiari, and C. R. Machado. 2000. Trypanosoma cruzi infection and the rat central nervous system: proliferation of parasites in astrocytes and the brain reaction to parasitism. Brain Res. Bull. 53153-162. [DOI] [PubMed] [Google Scholar]
- 16.da Silveira, A. B., M. A. Freitas, E. C. de Oliveira, S. G. Neto, A. O. Luquetti, J. B. Furness, R. Correa-Oliveira, and D. d'Avila Reis. 2008. Neuronal plasticity of the enteric nervous system is correlated with chagasic megacolon development. Parasitology 1351337-1342. [DOI] [PubMed] [Google Scholar]
- 17.da Silveira, A. B., E. M. Lemos, S. J. Adad, R. Correa-Oliveira, J. B. Furness, and D. D'Avila Reis. 2007. Megacolon in Chagas disease: a study of inflammatory cells, enteric nerves, and glial cells. Hum. Pathol. 381256-1264. [DOI] [PubMed] [Google Scholar]
- 18.de Melo-Jorge, M., and M. PereiraPerrin. 2007. The Chagas' disease parasite Trypanosoma cruzi exploits nerve growth factor receptor TrkA to infect mammalian hosts. Cell Host Microbe 1251-261. [DOI] [PubMed] [Google Scholar]
- 19.Dorn, P. L., L. Perniciaro, M. J. Yabsley, D. M. Roellig, G. Balsamo, J. Diaz, and D. Wesson. 2007. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg. Infect. Dis. 13605-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green, S. H., R. E. Rydel, J. L. Connolly, and L. A. Greene. 1986. PC12 cell mutants that possess low- but not high-affinity nerve growth factor receptors neither respond to nor internalize nerve growth factor. J. Cell Biol. 102830-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hess, D. M., M. O. Scott, S. Potluri, E. V. Pitts, C. Cisterni, and R. J. Balice-Gordon. 2007. Localization of TrkC to Schwann cells and effects of neurotrophin-3 signaling at neuromuscular synapses. J. Comp. Neurol. 501465-482. [DOI] [PubMed] [Google Scholar]
- 22.Hoff, R., R. S. Teixeira, J. S. Carvalho, and K. E. Mott. 1978. Trypanosoma cruzi in the cerebrospinal fluid during the acute stage of Chagas' disease. N. Engl. J. Med. 298604-606. [DOI] [PubMed] [Google Scholar]
- 23.Huang, E. J., and L. F. Reichardt. 2001. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24677-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, E. J., and L. F. Reichardt. 2003. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72609-642. [DOI] [PubMed] [Google Scholar]
- 25.Koberle, F. 1968. Chagas' disease and Chagas' syndromes: the pathology of American trypanosomiasis. Adv. Parasitol. 663-116. [DOI] [PubMed] [Google Scholar]
- 26.Koizumi, S., M. L. Contreras, Y. Matsuda, T. Hama, P. Lazarovici, and G. Guroff. 1988. K-252a: a specific inhibitor of the action of nerve growth factor on PC 12 cells. J. Neurosci. 8715-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuruvilla, R., L. S. Zweifel, N. O. Glebova, B. E. Lonze, G. Valdez, H. Ye, and D. D. Ginty. 2004. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell 118243-255. [DOI] [PubMed] [Google Scholar]
- 28.Lad, S. P., K. E. Neet, and E. J. Mufson. 2003. Nerve growth factor: structure, function and therapeutic implications for Alzheimer's disease. Curr. Drug Targets CNS Neurol. Disord. 2315-334. [DOI] [PubMed] [Google Scholar]
- 29.Lamballe, F., R. Klein, and M. Barbacid. 1991. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell 66967-979. [DOI] [PubMed] [Google Scholar]
- 30.Losavio, A., M. C. Jones, O. P. Sanz, G. Mirkin, S. M. Gonzalez Cappa, S. Muchnik, and R. E. Sica. 1989. A sequential study of the peripheral nervous system involvement in experimental Chagas' disease. Am. J. Trop. Med. Hyg. 41539-547. [DOI] [PubMed] [Google Scholar]
- 31.Lu, B., and M. PereiraPerrin. 2008. A novel immunoprecipitation strategy identifies a unique functional mimic of the glial cell line-derived neurotrophic factor family ligands in the pathogen Trypanosoma cruzi. Infect. Immun. 763530-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machado, C. R., M. V. Caliari, M. de Lana, and W. L. Tafuri. 1998. Heart autonomic innervation during the acute phase of experimental American trypanosomiasis in the dog. Am. J. Trop. Med. Hyg. 59492-496. [DOI] [PubMed] [Google Scholar]
- 33.Machado, C. R., A. B. Machado, and C. A. Chiari. 1978. Recovery from heart norepinephrine depletion in experimental Chagas' disease. Am. J. Trop. Med. Hyg. 2720-24. [DOI] [PubMed] [Google Scholar]
- 34.Molina, H. A., R. L. Cardoni, and M. T. Rimoldi. 1987. The neuromuscular pathology of experimental Chagas' disease. J. Neurol. Sci. 81287-300. [DOI] [PubMed] [Google Scholar]
- 35.Parodi, A. J., G. D. Pollevick, M. Mautner, A. Buschiazzo, D. O. Sanchez, and A. C. Frasch. 1992. Identification of the gene(s) coding for the trans-sialidase of Trypanosoma cruzi. EMBO J. 111705-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira, M. E. 1983. A developmentally regulated neuraminidase activity in Trypanosoma cruzi. Science 2191444-1446. [DOI] [PubMed] [Google Scholar]
- 37.Pittella, J. E. 1993. Central nervous system involvement in Chagas' disease. An updating. Rev. Inst. Med. Trop. Sao Paulo 35111-116. [DOI] [PubMed] [Google Scholar]
- 38.Postan, M., J. A. Dvorak, and J. P. McDaniel. 1983. Studies of Trypanosoma cruzi clones in inbred mice. I. A comparison of the course of infection of C3H/HEN− mice with two clones isolated from a common source. Am. J. Trop. Med. Hyg. 32497-506. [DOI] [PubMed] [Google Scholar]
- 39.Prioli, R. P., J. S. Mejia, and M. E. Pereira. 1990. Monoclonal antibodies against Trypanosoma cruzi neuraminidase reveal enzyme polymorphism, recognize a subset of trypomastigotes, and enhance infection in vitro. J. Immunol. 1444384-4391. [PubMed] [Google Scholar]
- 40.Rosemberg, S., C. J. Chaves, M. L. Higuchi, M. B. Lopes, L. H. Castro, and L. R. Machado. 1992. Fatal meningoencephalitis caused by reactivation of Trypanosoma cruzi infection in a patient with AIDS. Neurology 42640-642. [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg, I., R. P. Prioli, E. Ortega-Barria, and M. E. Pereira. 1991. Stage-specific phospholipase C-mediated release of Trypanosoma cruzi neuraminidase. Mol. Biochem. Parasitol. 46303-305. [DOI] [PubMed] [Google Scholar]
- 42.Schenkman, S., L. Pontes de Carvalho, and V. Nussenzweig. 1992. Trypanosoma cruzi trans-sialidase and neuraminidase activities can be mediated by the same enzymes. J. Exp. Med. 175567-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scudder, P., J. P. Doom, M. Chuenkova, I. D. Manger, and M. E. Pereira. 1993. Enzymatic characterization of beta-D-galactoside alpha 2,3-trans-sialidase from Trypanosoma cruzi. J. Biol. Chem. 2689886-9891. [PubMed] [Google Scholar]
- 44.Stenqvist, A., K. Agerman, F. Marmigere, L. Minichiello, and P. Ernfors. 2005. Genetic evidence for selective neurotrophin 3 signalling through TrkC but not TrkB in vivo. EMBO Rep. 6973-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tafuri, W. L. 1970. Pathogenesis of lesions of the autonomic nervous system of the mouse in experimental acute Chagas' disease. Light and electron microscope studies. Am. J. Trop. Med. Hyg. 19405-417. [DOI] [PubMed] [Google Scholar]
- 46.Tuffereau, C., J. Benejean, D. Blondel, B. Kieffer, and A. Flamand. 1998. Low-affinity nerve-growth factor receptor (P75NTR) can serve as a receptor for rabies virus. EMBO J. 177250-7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wehrman, T., X. He, B. Raab, A. Dukipatti, H. Blau, and K. C. Garcia. 2007. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron 5325-38. [DOI] [PubMed] [Google Scholar]
- 48.Wiesmann, C., M. H. Ultsch, S. H. Bass, and A. M. de Vos. 1999. Crystal structure of nerve growth factor in complex with the ligand-binding domain of the TrkA receptor. Nature 401184-188. [DOI] [PubMed] [Google Scholar]
- 49.Woronowicz, A., S. R. Amith, V. W. Davis, P. Jayanth, K. De Vusser, W. Laroy, R. Contreras, S. O. Meakin, and M. R. Szewczuk. 2007. Trypanosome trans-sialidase mediates neuroprotection against oxidative stress, serum/glucose deprivation, and hypoxia-induced neurite retraction in Trk-expressing PC12 cells. Glycobiology 17725-734. [DOI] [PubMed] [Google Scholar]
- 50.Yamauchi, J., J. R. Chan, and E. M. Shooter. 2003. Neurotrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc. Natl. Acad. Sci. USA 10014421-14426. [DOI] [PMC free article] [PubMed] [Google Scholar]