Abstract
Streptococcus pneumoniae colonization and invasive disease peak around the third and first birthdays, respectively, and decline thereafter. While these declines are attributable in part to immunity acquired via natural exposure, maturation of innate immune responses may also be involved. A mucosally administered candidate whole-cell pneumococcal vaccine (WCV) containing killed pneumococcal antigen (WCA) plus a cholera toxin adjuvant protects against intranasal carriage of pneumococci by a mechanism that is antibody independent and CD4+ TH17 cell dependent. Because infants and children are a key target population for this vaccine, we sought to evaluate the immune responses of neonatal and infant mice to S. pneumoniae and to assess whether the WCV would be effective in these mice. Like human infants, infant mice showed impaired clearance of nasopharyngeal colonization with S. pneumoniae. Macrophages from neonatal and infant mice stimulated with killed pneumococci in vitro showed significantly reduced cytokine production, including that of KC, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, macrophage chemoattractant protein 1, interleukin-6 (IL-6), IL-1α, tumor necrosis factor alpha, and gamma interferon, whereas IL-10 expression was significantly increased compared to that in macrophages from adult mice. IL-17A production from adult immune CD4+ T cells was significantly delayed when neonatal macrophages instead of adult macrophages were used as antigen-presenting cells. Moreover, whole blood from mice immunized as neonates with WCV produced significantly less IL-17A after stimulation with WCA than did blood from mice immunized as adults. Nonetheless, a single immunization of neonatal mice with WCV significantly reduced colonization density. Overall, our data suggest an impairment of both innate and acquired cellular immune responses in neonatal and infant mice. However, WCV confers a significant reduction in colonization following pneumococcal challenge, suggesting that it may still be effective in the setting of immature immune responses.
The incidence of pneumococcal disease in unvaccinated humans peaks around the first birthday and declines rapidly thereafter, while the prevalence and duration of pneumococcal colonization also decline over the course of childhood (5, 14). At least three mechanisms may play a role in this decline. First, acquired immunity to pneumococci has traditionally been attributed to the acquisition of anticapsular antibodies that protect against individual capsular serotypes. There is evidence for the role of anticapsular antibodies in naturally acquired protection against some, though not all, serotypes (11, 13, 42). Second, acquisition of specific immune responses targeting conserved, noncapsular antigens (18, 28, 45, 46) may also play a role. We and others have documented the acquisition of antigen-specific, serotype- and antibody-independent immunity to pneumococcal carriage in mice through a mechanism that depends on the TH17 subset of CD4+ T cells (23, 26, 28, 37, 38, 40). Such a mechanism, possibly in combination with antibody responses to noncapsular antigens (25, 34), could serve as an explanation for the decline in pneumococcal disease and carriage with age. Third, the physiologic maturation of the immune response with age, independent of exposure, may play a role. The importance of the last two mechanisms (rather than anticapsular acquired immunity alone) is suggested by (i) the timing of protection, which appears before anticapsular antibody responses can be detected in most unimmunized children; and (ii) the simultaneity of declines in carriage and disease for many serotypes, which would be unlikely if separate antibody responses were required for protection against each serotype (1, 21). In the current study, we evaluate the third hypothesis, that the immune system matures with age and becomes increasingly capable of mounting both innate and acquired immune responses to pneumococci, thereby leading to reduced susceptibility to carriage.
We have been studying a killed unencapsulated pneumococcal whole-cell antigen (WCA) plus cholera toxin (CT) as an adjuvant (together referred to as a whole-cell vaccine [WCV]) for mucosal immunization against pneumococcal colonization and invasive disease. Because the target population of this vaccine is infants, it is important to determine whether this vaccine can confer protection following a single dose in infancy, especially because invasive pneumococcal disease in developing countries is observed at even younger ages than that in developed countries (32).
Maturation of innate immune responses may play a role in an enhanced ability to mount acquired, antigen-specific responses, because many innate immune components are also involved in the induction of acquired immunity. Previous studies have indicated that an adaptive immune response to whole bacteria is greatly enhanced by the “cargo” effect, the presence of endogenous Toll-like receptor (TLR) agonists that are included within the bacteria, and that the generation of peptide-major histocompatibility complex class II (MHC II) complexes is controlled by these TLRs (4). Furthermore, a recent study showed that the generation of an interleukin-17A (IL-17A) response is also dependent on TLR signaling (10).
Recent studies with infant mouse macrophages and dendritic cells have revealed an impaired cytokine response following stimulation with the TLR agonists lipopolysaccharide (LPS) (TLR4) and heat-killed Listeria monocytogenes (TLR2). Specifically, IL-1 and IL-6 cytokine responses, known to contribute to adaptive immunity, are impaired, while the inhibitory cytokine IL-10 is increased in infant cells, resulting in an inability of neonatal mouse macrophages to stimulate an adequate B-cell response (7, 8).
For these reasons, we wished to evaluate both the innate and acquired immune responses of neonatal mice to pneumococcal antigens. In the following studies, we show that similar to what is observed in humans, the clearance of pneumococcal carriage in infant mice is significantly impaired compared to that in adult mice. Consistent with this finding, the cytokine response of neonatal macrophages is significantly reduced upon stimulation with WCA. Finally, we compare both the cellular and humoral acquired responses of neonatal and adult mice to a single intranasal immunization with WCV and observe the effect of this immunization on subsequent pneumococcal carriage.
(This work was presented in part at the 6th International Symposium on Pneumococci and Pneumococcal Diseases, Reykjavik, Iceland, 8 to 12 June 2008.)
MATERIALS AND METHODS
Mice.
C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME) or from Harlan (Indianapolis, IN). For the purposes of the studies described below, we defined 6- to 8-day-old mice as neonates, 14-day-old mice as infants, and 5- to 6-week-old mice as adults.
Reagents.
Pneumococcal strain RX1AL−, a capsule- and autolysin-negative mutant, was prepared as described previously (25) as a WCA. The WCV contained 108 (killed) CFU equivalents of WCA plus 1 μg of CT (List Biological Laboratories, Campbell, CA) per 10-μl dose. Control mice were immunized with 1 μg of CT in 10 μl of saline. For stimulation of cell cultures, WCA was used at an equivalent concentration of 107 (killed) CFU/ml. The nontoxic pneumolysin derivative and TLR4 agonist PdT (24) was purified under LPS-free conditions from Escherichia coli that overexpress a six-His-tagged fusion protein (36). LPS was purchased from List Biological Laboratories (Campbell, CA). MALP-2 was purchased from Alexis Biochemicals (San Diego, CA), and heat-killed Listeria monocytogenes was obtained from Invivogen (San Diego, CA).
Bacteria for animal challenge.
Streptococcus pneumoniae strain 0603 was originally a clinical isolate of capsular serotype 6B, as described previously (25). This strain was grown to mid-log phase in Todd-Hewitt broth with 0.5% yeast extract, harvested by centrifugation, resuspended in saline at a concentration of 108 CFU/ml, stored at −80°C in Todd-Hewitt broth with 0.5% yeast extract and 10% glycerol, and thawed just prior to challenge. For challenge in mice, frozen suspensions of S. pneumoniae type 6B were thawed and diluted in saline to a concentration of 2 × 106 CFU/10 μl or as otherwise stated; the actual colony count was determined on blood agar.
Cell preparation.
Cellular suspensions of splenocytes were obtained by passing spleens from immunized mice through a 70-μm cell strainer (BD Biosciences, Bedford, MA). Cells were washed, and red blood cells were removed by hemolysis. Macrophages were isolated using adherence to a 100-mm disposable polystyrene petri dish. The purified cells were found to be routinely >90% Mac-3 positive by flow cytometry. For each experiment, the macrophages were pooled from 2 to 4 adult mice and 6 to 12 infant mice.
CD4+ T cells were purified from splenocytes by positive selection of CD4+ cells by use of CD4+ antibody-coupled magnetic beads after negative selection with MHC II antibody-coupled magnetic beads (Miltenyi Biotec, Auburn, CA). To remove any remaining contaminating macrophages, we allowed the cells to sit on plastic (100-mm polystyrene tissue culture dishes; BD Biosciences, Bedford, MA) for 2 h. The absence of antigen-presenting cells (APCs) in this purified CD4+ T-cell population was verified by incubating purified CD4+ cells with antigen and measuring cytokine secretion. Purified CD4+ T cells were considered relatively free of contamination with APCs if incubation with antigen for 72 h did not result in any detectable IL-17A in the supernatant. For each experiment, CD4+ T cells from three or four adult mice were pooled.
MHC II expression on macrophages.
Macrophages were harvested as described previously and stimulated with medium or WCA (10 μg/ml) for 48 h. Cells were washed and stained with anti-MHC II antibody in the presence of Fc block for 30 min at the concentrations recommended by the manufacturer. MHC II expression was analyzed on a Cytomation MoFlo flow cytometer (Beckman Coulter, Fullerton, CA), and results were analyzed with Summit, version 4.3 (Dako, Fort Collins, CO).
Bacterial association.
The capacity of macrophages to bind and phagocytose pneumococci was analyzed by incubating neonatal and adult mouse macrophages with fluorescein isothiocyanate-labeled pneumococcal strain 603 (prepared as described previously [35]). The cells were incubated for 30 min, after which the total of bacterial adherence and uptake (association) was measured and analyzed by flow cytometry.
Cell culture.
Cells were cultured at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium-F-12 with l-glutamine and 15 mM HEPES (BioWhittaker, Walkersville, MD) supplemented with 10% fetal bovine serum (HyClone, Logan, UT) and ciprofloxacin (10 μg/ml). Macrophages (0.25 × 106 cells/well) were cultured in triplicate in 24- or 48-well tissue culture plates (BD Biosciences, Bedford, MA) in 333 μl of medium for 24 h or as otherwise stated. Macrophages (0.25 × 106 cells/well) and CD4+ T cells (0.5 × 106 cells/well) together were cultured in triplicate in a 24- or 48-well tissue culture plate in 555 μl of medium for 72 h or as otherwise stated. In some experiments, recombinant mouse IL-10 (BD Biosciences, Bedford, MA) was added at the indicated concentrations.
Whole-blood stimulation.
Whole blood drawn at 5 weeks postimmunization, diluted 1:10 in Dulbecco's modified Eagle's medium-F-12 with l-glutamine and 15 mM HEPES (BioWhittaker, Walkersville, MD) supplemented with 10% fetal bovine serum (HyClone, Logan, UT) and ciprofloxacin (10 μg/ml), was stimulated with WCA at a concentration corresponding to 107 CFU/ml for 6 days. Supernatants were collected following centrifugation and stored at −80°C until analyzed by enzyme-linked immunosorbent assay (ELISA) or Luminex assay.
Measurement of serum antibodies to WCA by ELISA.
Sera were retrieved 5 weeks after neonatal and adult mice were immunized once with WCV and were assayed for immunoglobulin G (IgG) antibodies to whole bacteria. The serum standard for whole bacterial antibodies was obtained from a pool of sera from adult mice immunized intranasally twice, where the 1:75 dilution was assigned to 1,000 arbitrary units per ml. In short, the ELISA plates were coated with WCA (108 CFU/ml) and incubated for 1 h at room temperature. The plates were washed three times with phosphate-buffered saline (PBS)-Tween and blocked with PBS-1% bovine serum albumin for 1 h. Threefold dilutions of samples and standards in PBS-1% bovine serum albumin were incubated for 2 h. After washing of the samples, horseradish peroxidase-conjugated rat anti-mouse IgG was added to the plate and incubated for another 2 h. The plates were developed using SureBlue TMB microwell peroxidase (KPL, Gaithersburg, MD). The reaction was stopped by the addition of 1 M HCl, and the plate's absorbance was read at 450 to 540 nm.
Measurement of cytokine secretion by ELISA.
Supernatants of cell cultures were collected following centrifugation and stored at −80°C until analyzed by ELISA for KC, IL-6, tumor necrosis factor alpha (TNF-α), and IL-17 concentrations (R&D Systems, Minneapolis, MN). Supernatants were analyzed and read against a standard, following directions provided by the manufacturer.
Measurement of cytokine secretion by Luminex assay.
Supernatants of cell cultures were analyzed on a Luminex 200 machine (Millipore, Billerica, MA) for IL-6, macrophage chemoattractant protein 1 (MCP-1), granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), gamma interferon (IFN-γ), IL-1α, and IL-10, using a LincoPlex multiplex immunoassay kit (Linco Research, Millipore, Billerica, MA). Supernatants were analyzed and read against a standard, following directions provided by the manufacturer. The final analyses were performed using Beadview software (Millipore, Billerica, MA).
Immunization of mice.
Adult C57BL/6 mice were immunized intranasally with the WCV at the age of 5 to 6 weeks as described previously (25). A second dose, when given, was administered 1 week after the first. For in vivo comparison of the effects of vaccination on nasopharyngeal colonization, infant and adult mice were vaccinated just once, at the ages of 7 days and 6 weeks, respectively.
Colonization model.
Nasopharyngeal challenge was performed as described previously (25). Briefly, 2-week-old mice and 5- to 6-week-old mice were challenged intranasally with age-adjusted doses of live strain 0603 (0.6 × 106 CFU and 1.5 × 106 to 2.5 × 106 CFU, respectively). We determined colonization 7 days after challenge. After euthanasia by CO2 inhalation, an upper respiratory tract culture was done by instilling sterile saline retrograde through the transected trachea, collecting the first 6 drops (about 0.1 ml) from the nostrils, and plating diluted samples on blood agar plates containing gentamicin (2.5 μg/ml). For graphical representation and statistical analysis, a sterile sample was assigned one-half of the lower limit of detection, or 5 CFU/ml. To assess the effect of immunization on colonization, we challenged mice vaccinated at 7 days (infant) and 6 weeks (adult) of age at the ages of 7 weeks and 12 weeks, respectively. Thus, in both cases, challenge was performed 6 weeks after the single immunization.
Statistical analysis.
Differences in cytokine concentrations (ELISA and Luminex assay) were compared using the Mann-Whitney U (MWU) test. Proportions of colonized mice at a single time point were compared by Fisher's exact test, and colonization densities were evaluated by the MWU test, using Prism software (version 5.0; GraphPad Software, Inc.). Comparisons of colonization over time in neonatal versus adult mice were powered to detect differences in density at each time point rather than to detect differences in duration. Nonetheless, we assessed whether the duration of detectable colonization was longer in neonates than in adults, using the following nonparametric procedure. For each group i (i = adult or neonate), the proportion of mice cleared at each time point t, pi(t), was calculated. Using the max-min formula for isotonic regression (29), these proportions were smoothed to ensure that they were nondecreasing in t, yielding smoothed proportions qi(t). A test statistic was then calculated to quantify the distance between the smoothed curves for two groups (e.g., WCV versus CT groups), as follows: The significance level of this test statistic was estimated by permuting the group identifiers for the cleared mice at each time point, fixing the total number of mice in each group and the total number cleared at each time point. A total of 100,000 replicates of the permuted data were obtained, and T was calculated for each. The P value was calculated as the fraction of these 100,000 permutations having a test statistic strictly less than that calculated for the data.
RESULTS
Density and duration of pneumococcal colonization in naïve mice.
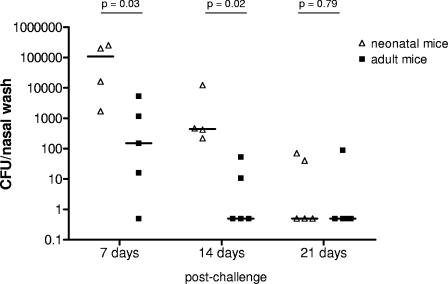
We compared colonization densities and durations in infant and adult mice. The mice were challenged with a weight-adjusted number of CFU: for infant mice, this corresponded to 0.6 × 106 CFU, and for adults, it was 1.5 × 106 to 2.5 × 106 CFU. As shown in Fig. 1, the colonization density was significantly lower in adult mice than in infants 7 and 14 days after challenge. Clearance of colonization (time to disappearance of detectable bacteria by nasal washing) appeared to be faster in adults than in infants, although the difference was not statistically significant (P = 0.081).
FIG. 1.
Effect of age on duration and density of pneumococcal colonization with a serotype 6B strain in mice. Median colonization densities are depicted 7, 14, and 21 days after intranasal inoculation of infant (14-day-old) and adult (5- to 6-week-old) mice. Each symbol represents the density of colonization of an individual animal. Each line represents the median colonization density in each group. The density of colonization was significantly higher in infant mice examined 7 and 14 days after exposure, and there was a trend toward a longer duration of colonization in infants (P = 0.081).
Cytokine responses of neonatal, infant, and adult macrophages to killed pneumococci and purified TLR agonists.
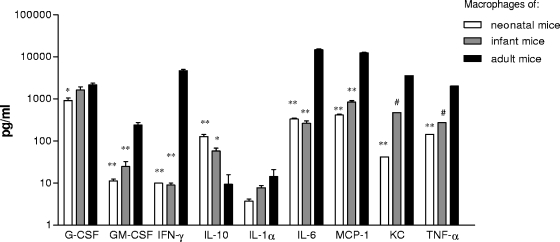
To evaluate the innate responses of 1-week-old neonatal and 2-week-old infant mice to pneumococcus, we compared their macrophage cytokine responses to killed pneumococci to those of adult (5- or 6-week-old) mice. Twenty-four hours after stimulation, the levels of the chemokines G-CSF, GM-CSF, MCP-1, and KC and the cytokines IL-1α, IL-6, TNF-α, and IFN-γ were significantly lower for neonatal mouse macrophages than for macrophages from adult mice (Fig. 2). In contrast, expression of IL-10 was 15-fold higher for macrophages from neonatal mice than for macrophages from adult mice. Cytokine responses from infant macrophages (2-week-olds) were intermediate or, in the case of IFN-γ and IL-6, similar to those for macrophages obtained from neonatal mice.
FIG. 2.
Effect of age on cytokine responses of spleen macrophages stimulated for 24 h with unencapsulated pneumococci. Data here represent the mean plus standard error of the mean (SEM) cytokine responses of macrophages obtained from neonatal, infant, and adult mice. For most samples, triplicate samples were available and were examined by ELISA or Luminex assay. For all cytokines except IL-10, the cytokine production in neonatal macrophages was significantly lower than that in adults. In contrast, IL-10 was consistently higher in neonatal macrophages stimulated with WCA than in adults. *, P < 0.05; **, P < 0.01; #, single value.
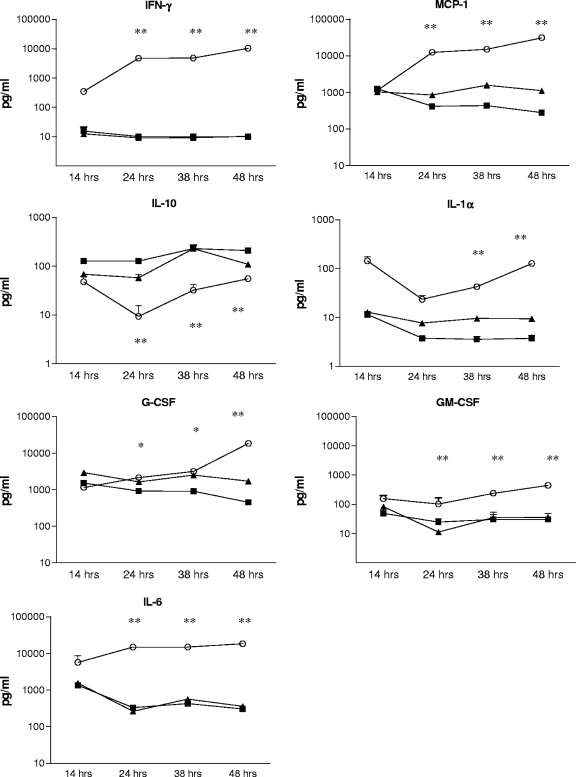
To investigate whether the reduced cytokine responses of neonatal and infant macrophages represented a delay more than a diminished response compared to that of adult macrophages, we performed the same assay with samples of supernatants harvested at 14, 24, 38, and 48 h. There was no evidence that the reduced response from neonatal and infant macrophages represented just a delay; rather, for most cytokines, the response of neonatal and infant macrophages was absent or low at all time points, while cytokine production in adult macrophages increased for all cytokines except for IL-10 (Fig. 3).
FIG. 3.
Time courses of cytokine production of neonatal, infant, and adult macrophages after stimulation with pneumococcal WCA. The mean and SEM cytokine concentrations from triplicate wells (duplicate wells at 14 h) are depicted. Adult macrophages (open circles) produced significantly more IFN-γ, IL-1α, and GM-CSF than did macrophages from neonatal (closed squares) or infant (closed triangles) mice at several time points during the 48-hour stimulation. In contrast, the IL-10 production of macrophages from both neonatal and infant mice upon stimulation with WCA was significantly higher than that of macrophages from adult mice. *, significant difference for neonatal compared to adult macrophages; **, significant difference for both neonatal and infant macrophages compared to adult macrophages.
As a control to ensure that the differences observed were not due to subtle differences in the numbers of macrophages seeded in the tissue culture plates, we repeated the experiments with neonatal and adult macrophages at a series of macrophage concentrations and measured IL-6, KC, and TNF-α levels. Adult macrophages seeded at fourfold lower concentrations still expressed significantly more IL-6, TNF-α, and KC than neonatal cells did, suggesting that the impaired neonatal responses observed were not due to small variations in the plating of cells (data not shown).
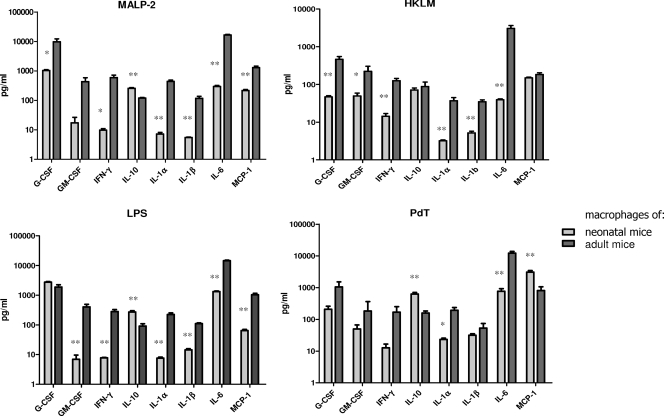
The contrasting responses of neonatal and adult macrophages to stimulation with pneumococcus led us to evaluate in the same system whether responses to TLR agonists are similarly affected by age, as previously reported by others (7, 8, 44). Thus, we compared the macrophage cytokine responses to the TLR2 agonists MALP-2 and heat-killed L. monocytogenes and the TLR4 agonists LPS and PdT. For these experiments, we analyzed the expression of the following cytokines and chemokines: G-CSF, GM-CSF, MCP-1, IL-1α, IL-1β, IL-6, and IFN-γ. We observed similar differences between the cytokine responses of neonatal and adult macrophages for agonists of both TLR2 and TLR4, with significantly lower cytokine responses for most cytokines in neonatal macrophages than in adult macrophages, except for IL-10, whose level was significantly higher in response to MALP-2, LPS, and PdT in neonatal macrophages (Fig. 4).
FIG. 4.
Cytokine production of infant and adult macrophages stimulated for 24 h with the TLR2 agonists MALP-2 and heat-killed L. monocytogenes (HKLM) and the TLR4 agonists PdT and LPS. The mean cytokine concentrations for triplicate wells are depicted, with SEM. Triplicate samples were examined by ELISA or Luminex assay. For all cytokines except IL-10, the cytokine production in neonatal macrophages was significantly lower than that in adult macrophages. In contrast, IL-10 was consistently higher in neonatal macrophages than in adult macrophages for most stimuli. *, P < 0.05; **, P < 0.01.
Comparison of neonatal and adult macrophage function.
We wished to compare several aspects of neonatal and adult macrophage function. First, the ability of macrophages to associate with and phagocytose killed pneumococci was examined. Fluorescein isothiocyanate-labeled pneumococci were incubated with neonatal or adult macrophages, and the association of bacteria and cells was evaluated by flow cytometry. No significant differences in association between cells and bacteria were noted when neonatal and adult macrophages were compared (data not shown). Second, consistent with the findings of many previous investigators (15, 22), we found that MHC II expression was lower on neonatal than on adult macrophages, with or without exposure to WCA (data not shown). Finally, we investigated the ability of neonatal mouse macrophages to induce IL-17A production by immune CD4+ T cells. CD4+ T cells from adult mice immunized twice intranasally with WCV were harvested from spleens and incubated with neonatal or adult macrophages from naïve mice. These cells were then pulsed with WCA. We ascertained that adult CD4+ T cells were not contaminated with macrophages by confirming that stimulation of purified CD4+ T cells without the addition of macrophages produced no measurable IL-17A.
IL-17A production could be detected when both neonatal and adult macrophages were used as APCs, although the response of the CD4+ T cells to antigen presentation by neonatal macrophages was delayed (Fig. 5). Based on the results described above, we evaluated whether this impairment could be reproduced by adding recombinant mouse IL-10 to splenocytes from mice immunized as adults. We observed a dose-dependent effect of exogenous IL-10 on IL-17A expression of splenocytes from immunized adult mice 48 h after stimulation (Fig. 6A). By 72 h, however, splenocytes treated with IL-10 produced IL-17A at a level similar to that of untreated cells (Fig. 6B), again more suggestive of a delay than an absent or abrogated cytokine response.
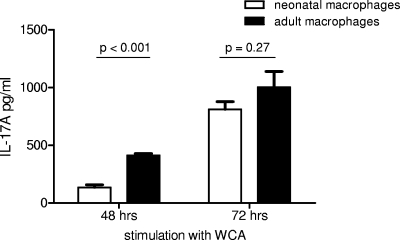
FIG. 5.
IL-17 production by CD4+ T cells from vaccinated adult mice stimulated with WCA at 48 and 72 h in the presence of neonatal or adult macrophages. The mean and SEM cytokine concentrations of triplicate wells are depicted. At 48 h, CD4+ T cells showed significantly less expression of IL-17A when neonatal instead of adult macrophages were used as APCs. At 72 h, this difference in IL-17A expression was not significant.
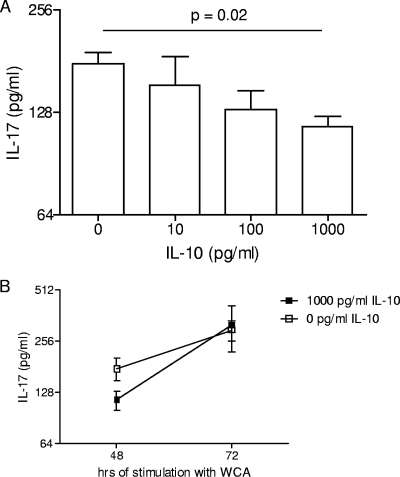
FIG. 6.
Effect of IL-10 on IL-17 production of splenocytes from immunized adult mice. (A) Splenocytes from immunized adult mice were stimulated with WCA in the presence of recombinant IL-10 at the indicated concentrations. IL-17A production measured by ELISA was significantly decreased at 48 h when the highest concentration of IL-10 was added. The mean cytokine concentrations for three replicates are depicted. (B) The reduction in IL-17A production in the presence of 1,000 pg/ml of IL-10 was no longer significant after 72 h of incubation.
Cytokine responses to WCA in whole blood of mice immunized with WCV.
We have recently shown that whole-blood expression of IL-17A in response to stimulation with WCA is a predictor of subsequent protection against pneumococcal colonization (23). We obtained peripheral blood at 5 weeks postimmunization, when all mice were considered adults. IL-17A production in WCA-stimulated whole blood of mice previously immunized once with WCV at 7 days or 6 weeks of age was compared. The IL-17A concentrations in whole blood of mice immunized as neonates were significantly lower than those for mice immunized as adults (Table 1). In addition, we measured several other cytokines found in the literature to be involved in adaptive immunity to S. pneumoniae. The concentration of each cytokine was higher in the blood of mice immunized as adults than in that of mice immunized as neonates; these differences were statistically significant for IL-2, IL-4, and G-CSF, and a trend was also observed for the cytokines MCP-1, TNF-α, G-CSF, and IL-1α (P values of 0.06 to 0.10).
TABLE 1.
Stimulation of whole blood of mice immunized with WCV once at 1 week (neonate) or 6 weeks (adult) of agea
| Cytokine | Median concn (pg/ml)
|
x-Fold increase | P valueb | |
|---|---|---|---|---|
| Neonate | Adult | |||
| IL-17 | 8.1 | 2,480 | 306 | 0.005 |
| IL-2 | 4.0 | 53.4 | 13.4 | 0.02 |
| IL-4 | 4.0 | 29.9 | 7.5 | 0.02 |
| MCP-1 | 207 | 1,160 | 5.6 | 0.06 |
| IL-12 | 4.0 | 14.9 | 3.7 | NS |
| TNF-α | 60.7 | 154 | 2.5 | 0.10 |
| IL-10 | 15.8 | 39.9 | 2.5 | NS |
| IL-6 | 300 | 690 | 2.3 | NS |
| G-CSF | 197 | 437 | 2.2 | 0.05 |
| GM-CSF | 14.9 | 29.7 | 2.0 | NS |
| IFN-γ | 7.1 | 10.8 | 1.5 | NS |
| KC | 19.4 | 26.7 | 1.4 | NS |
| IL-1α | 21.8 | 27.2 | 1.3 | 0.10 |
Peripheral blood was obtained from five to seven mice per group 5 weeks after immunization and then stimulated with WCA for 6 days. The supernatants were analyzed for cytokines using the Luminex method. Data shown in bold indicate statistically significant differences or a trend toward statistically significant differences.
NS, not significant.
IgG production after whole-cell immunization.
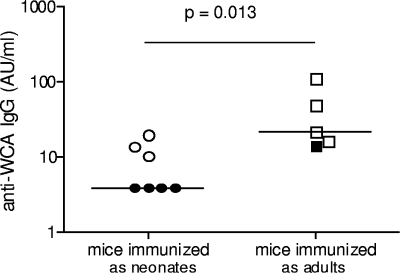
While protection against colonization by WCV occurs independently of antibody in this model, we have also shown that protection against pneumococcal type 3 sepsis can be conferred by passive transfer of antibodies from WCV-immunized animals (25). Thus, we decided to compare the IgG antibody titers to WCA in the sera of neonatal versus adult mice 5 weeks after immunization. As shown in Fig. 7, immunization of neonatal mice resulted in significantly lower anti-WCA antibody levels than did immunization of adult mice.
FIG. 7.
Effect of age at time of intranasal immunization with WCV on generation of serum antipneumococcal IgG antibodies. The mean IgG antipneumococcal antibody titer in sera from mice immunized as neonates was significantly lower than that for mice immunized as adults. The mice represented by the open symbols were fully protected against colonization by WCV, and the closed symbols represent immunized mice that were still colonized at 7 days postchallenge. The median IgG concentration in five to seven mice per group is depicted in arbitrary units/ml.
Ability of neonatal mice to acquire immunity following immunization with WCV.
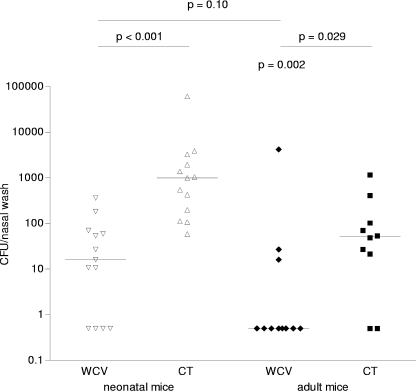
Having observed that immunization of neonatal mice with one dose of WCV results in lower immune responses than those observed in adult mice, we next assessed the protective effect of a single dose of WCV, given at different ages, against pneumococcal colonization. We immunized neonatal mice (6 days of age) and adult mice (6 weeks of age) once with WCV and challenged them with pneumococcal strain 0603 at 6 weeks postimmunization. Since all mice were adults by the time of challenge, no interference was expected from differences in the function of APCs at the time of challenge. As expected, consistent with the results for IL-17A expression in the whole-blood assay, a single dose of WCV was fully protective in adult mice (median density of colonization for WCV- versus CT-immunized mice, 0.5 versus 50.75 CFU [P = 0.021 by MWU test]). A significant reduction in pneumococcal colonization was also observed in mice that received one dose of WCV as neonates compared to control neonatal mice that were immunized with CT alone. Although pneumococci could still be detected in the nasal washes of these WCV-immunized mice, the median reduction in colonization was 100-fold (Fig. 8). It is noteworthy that the density of colonization was significantly higher for the control CT group immunized at 1 week of age (7 weeks of age at challenge) than for the adult control CT group immunized at 7 weeks of age (12 weeks of age at challenge). This difference appears to be related to differential effects of CT on infant versus adult mice; indeed, there was no difference between naïve mice challenged with strain 0603 at 7 versus 12 weeks of age (median density of colonization, 493.8 versus 541.5 CFU, respectively [P = 0.94 by MWU test]). Overall, our results suggest that a single dose of WCV in infancy can confer significant protection against subsequent pneumococcal colonization, although the degree of protection at 7 days postchallenge is inferior to that observed in adult mice.
FIG. 8.
Effect of age on the efficacy of intranasal immunization with WCV on subsequent nasopharyngeal colonization with serotype 6B pneumococci in mice. A single dose of intranasal WCV was administered to neonates or adults. All mice were challenged with a type 6B pneumococcal strain 6 weeks after immunization. The density of nasopharyngeal colonization was evaluated 1 week later. Each symbol represents the density of colonization of an individual animal. Each line represents the median colonization density in each group. Immunization with WCV resulted in a significant reduction in density of pneumococcal colonization compared to that in control CT-immunized mice for mice immunized as both neonates and adults.
DISCUSSION
Worldwide, pneumococcal infections account annually for around 800,000 deaths in children under 5 years of age (43). In developing countries, where the burden of disease is highest, pneumococcal infections appear at an earlier age than in developed countries (32). The reasons for this discrepancy are not entirely clear, but some have suggested that other risk factors, such as crowding, malnutrition, low birth weight, and indoor air pollution, may play a role (30, 33). The advent of pneumococcal conjugate vaccines, which elicit opsonophagocytic anticapsular antibodies in infancy and protect infants and children against invasive disease, may constitute an effective strategy to reduce pneumococcal infections in these settings, as they have in developed countries (2, 3, 12). Phase III studies in South Africa and The Gambia have confirmed the efficacy of conjugate vaccine strategies against pneumococcus in the developing world (9, 19). There remains the concern, however, that because multiple doses of the conjugate are required to elicit protection, some children at highest risk may not be directly protected. For these reasons, there is a need to develop immunization strategies that may protect children at a very early age, preferably with a single immunization.
Among the many possible approaches to this problem, we have been evaluating a killed whole-cell pneumococcal vaccine. This vaccine has many properties that make it suitable for use in developing countries, including coverage irrespective of serotype, low cost of manufacture, stability following lyophilization (thus avoiding cold chain issues), and mucosal administration. In mice, this vaccine elicits two forms of immune protection, namely, protection against sepsis via the generation of noncapsular antipneumococcal antibodies and protection against mucosal colonization via antibody-independent, IL-17-expressing CD4+ T-cell-mediated mechanisms (20-22).
Because prevention (or reduction in duration) of carriage would theoretically be an effective method to reduce pneumococcal disease, the second mechanism of protection is of particular interest. However, because of the relative immaturity of the immune system of neonates, one may wonder whether such immunity could be elicited in the very young. Several studies have documented the relative immaturity of the neonatal immune system, with resulting overall reductions in the secretion of proinflammatory/TH1-cell-polarizing cytokines, such as TNF, IL-1, and IFN-γ (7, 27, 31, 44), and increases in the production of IL-10 (7).
Here we measured innate and adaptive immune responses of infant mice to S. pneumoniae. We observed that, as in young humans, neonatal and infant mice are more susceptible to pneumococcal colonization than adult mice, as evidenced by higher densities and a trend toward longer durations of colonization. In addition, neonatal macrophages showed significantly less production of many cytokines known to be involved in immune defense against pneumococci upon challenge. In particular, we noted a defect in the production of cytokines involved in both neutrophil recruitment (IL-8/KC and G-CSF) and activation (G-CSF), both thought to be of major importance in pneumococcal clearance (17, 20). Some of the defects we noted may also explain the reduced cellular responses we observed: IL-1α has a role in T-cell expansion and survival, whereas TNF-α and IFN-γ stimulate the TH17 and TH1 T-cell pathways, respectively (41). All of these cytokines were significantly reduced in APCs from neonatal mice upon stimulation with pneumococci, as were the responses to purified TLR2 and TLR4 agonists. Finally, IL-10, which is an immune suppressor known to inhibit macrophage function (7, 16) and MHC II expression, was found at higher concentrations in the supernatants of stimulated neonatal mouse macrophages.
From these data, it is tempting to speculate that the reduced immunogenicity and degree of protection observed in neonatal mice after immunization may be related, at least in part, to decreased function and antigen-presenting activity of neonatal APCs, as evidenced by the lower inflammatory (and higher IL-10) response to pneumococci, lower response to TLR agonists, and decreased MHC II expression following pneumococcal stimulation of neonatal macrophages.
Despite these important differences between neonatal and adult macrophages, immunization of neonatal mice with a single dose of WCV significantly reduced colonization density. Nevertheless, unlike in adult mice, pneumococci could still be detected in nasal washes of mice immunized as infants; some of these differences, however, may be due to differences between effects of the adjuvant in infant versus adult mice. One could also hypothesize that the partial protection observed in mice immunized as neonates is the consequence of their delayed (as opposed to absent) cytokine response at the time of immunization.
More generally, it is possible that this global deficiency in cytokine responses may contribute to the increased susceptibility of neonates and infants to pneumococcal colonization and infection. Reduced production of chemokines and cytokines involved in the innate immune response upon pneumococcal challenge may lead to a decrease in the influx of monocytes, macrophages, and neutrophils to the site of infection and a decreased activation of these effector cells in humans (6). This hypothesis is supported by the data of van Benten et al. showing an age-related increase in nasal macrophages and T lymphocytes during upper respiratory tract infection in human infants (39). At this time, however, it is not known whether the impaired response found in neonatal mouse macrophages can be extrapolated to human APCs and to what extent and for what period during early life this impairment results in increased susceptibility to pneumococci. Clearly, more studies on the maturation of innate and adaptive immune responses in human neonates and infants are required to investigate these questions in more detail.
We conclude that neonatal mouse macrophages show impaired innate and adaptive immune responses to pneumococci, which might explain the increased susceptibility to pneumococcal colonization in vivo. However, this relative immaturity of the immune response can be overcome, as a significant degree of protection was observed after one dose of WCV in this murine model of pneumococcal colonization.
Acknowledgments
We thank Krzysztof Trzcinski and Gili Regev-Yochay for helpful discussions. We thank Cassandra Krone for technical support with our neonatal mouse model. We thank Betsy Boush for technical support with flow cytometry.
This work was supported by grants from the Ter Meulen Fund, Royal Netherlands Academy of Arts and Sciences (D.B.), by NIH grants R01 AI048935 (M.L.) and R01 AI066013 (R.M.), and by PATH (R.M.).
Editor: J. N. Weiser
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Balmer, P., R. Borrow, J. Findlow, R. Warrington, S. Frankland, P. Waight, R. George, N. Andrews, and E. Miller. 2007. Age-stratified prevalences of pneumococcal-serotype-specific immunoglobulin G in England and their relationship to the serotype-specific incidence of invasive pneumococcal disease prior to the introduction of the pneumococcal 7-valent conjugate vaccine. Clin. Vaccine Immunol. 141442-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black, S., H. Shinefield, R. Baxter, R. Austrian, L. Elvin, J. Hansen, E. Lewis, and B. Fireman. 2006. Impact of the use of heptavalent pneumococcal conjugate vaccine on disease epidemiology in children and adults. Vaccine 24(Suppl. 2)S2-79-S2-80. [DOI] [PubMed] [Google Scholar]
- 3.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19187-195. [DOI] [PubMed] [Google Scholar]
- 4.Blander, J. M., and R. Medzhitov. 2006. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440808-812. [DOI] [PubMed] [Google Scholar]
- 5.Bogaert, D., R. De Groot, and P. W. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 4144-154. [DOI] [PubMed] [Google Scholar]
- 6.Cailhier, J. F., D. A. Sawatzky, T. Kipari, K. Houlberg, D. Walbaum, S. Watson, R. A. Lang, S. Clay, D. Kluth, J. Savill, and J. Hughes. 2006. Resident pleural macrophages are key orchestrators of neutrophil recruitment in pleural inflammation. Am. J. Respir. Crit. Care Med. 173540-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chelvarajan, R. L., S. M. Collins, I. E. Doubinskaia, S. Goes, J. Van Willigen, D. Flanagan, W. J. De Villiers, J. S. Bryson, and S. Bondada. 2004. Defective macrophage function in neonates and its impact on unresponsiveness of neonates to polysaccharide antigens. J. Leukoc. Biol. 75982-994. [DOI] [PubMed] [Google Scholar]
- 8.Chelvarajan, R. L., Y. Liu, D. Popa, M. L. Getchell, T. V. Getchell, A. J. Stromberg, and S. Bondada. 2006. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J. Leukoc. Biol. 791314-1327. [DOI] [PubMed] [Google Scholar]
- 9.Cutts, F. T., S. M. Zaman, G. Enwere, S. Jaffar, O. S. Levine, J. B. Okoko, C. Oluwalana, A. Vaughan, S. K. Obaro, A. Leach, K. P. McAdam, E. Biney, M. Saaka, U. Onwuchekwa, F. Yallop, N. F. Pierce, B. M. Greenwood, and R. A. Adegbola. 2005. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 3651139-1146. [DOI] [PubMed] [Google Scholar]
- 10.Evans, H. G., T. Suddason, I. Jackson, L. S. Taams, and G. M. Lord. 2007. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc. Natl. Acad. Sci. USA 10417034-17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldblatt, D., M. Hussain, N. Andrews, L. Ashton, C. Virta, A. Melegaro, R. Pebody, R. George, A. Soininen, J. Edmunds, N. Gay, H. Kayhty, and E. Miller. 2005. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J. Infect. Dis. 192387-393. [DOI] [PubMed] [Google Scholar]
- 12.Hansen, J., S. Black, H. Shinefield, T. Cherian, J. Benson, B. Fireman, E. Lewis, P. Ray, and J. Lee. 2006. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr. Infect. Dis. J. 25779-781. [DOI] [PubMed] [Google Scholar]
- 13.Hill, P. C., Y. B. Cheung, A. Akisanya, K. Sankareh, G. Lahai, B. M. Greenwood, and R. A. Adegbola. 2008. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian infants: a longitudinal study. Clin. Infect. Dis. 46807-814. [DOI] [PubMed] [Google Scholar]
- 14.Hogberg, L., P. Geli, H. Ringberg, E. Melander, M. Lipsitch, and K. Ekdahl. 2007. Age- and serogroup-related differences in observed durations of nasopharyngeal carriage of penicillin-resistant pneumococci. J. Clin. Microbiol. 45948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inaba, K., M. Kitaura, T. Kato, Y. Watanabe, Y. Kawade, and S. Muramatsu. 1986. Contrasting effect of alpha/beta- and gamma-interferons on expression of macrophage Ia antigens. J. Exp. Med. 1631030-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janeway, C. A., Jr., P. Travers, M. Walport, and M. Shlomchik. 2001. Immunobiology: the immune system in health and disease, 5th ed. Garland, London, United Kingdom.
- 17.Kadioglu, A., and P. W. Andrew. 2004. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 25143-149. [DOI] [PubMed] [Google Scholar]
- 18.Kadioglu, A., W. Coward, M. J. Colston, C. R. Hewitt, and P. W. Andrew. 2004. CD4-T-lymphocyte interactions with pneumolysin and pneumococci suggest a crucial protective role in the host response to pneumococcal infection. Infect. Immun. 722689-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klugman, K. P., S. A. Madhi, R. E. Huebner, R. Kohberger, N. Mbelle, and N. Pierce. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 3491341-1348. [DOI] [PubMed] [Google Scholar]
- 20.Knapp, S., L. Hareng, A. W. Rijneveld, P. Bresser, J. S. van der Zee, S. Florquin, T. Hartung, and T. van der Poll. 2004. Activation of neutrophils and inhibition of the proinflammatory cytokine response by endogenous granulocyte colony-stimulating factor in murine pneumococcal pneumonia. J. Infect. Dis. 1891506-1515. [DOI] [PubMed] [Google Scholar]
- 21.Lipsitch, M., C. G. Whitney, E. Zell, T. Kaijalainen, R. Dagan, and R. Malley. 2005. Are anticapsular antibodies the primary mechanism of protection against invasive pneumococcal disease? PLoS Med. 2e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu, C. Y., E. G. Calamai, and E. R. Unanue. 1979. A defect in the antigen-presenting function of macrophages from neonatal mice. Nature 282327-329. [DOI] [PubMed] [Google Scholar]
- 23.Lu, Y. J., J. Gross, D. Bogaert, A. Finn, L. Bagrade, Q. Zhang, J. K. Kolls, A. Srivastava, A. Lundgren, S. Forte, C. M. Thompson, K. F. Harney, P. W. Anderson, M. Lipsitch, and R. Malley. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4e1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malley, R., P. Henneke, S. C. Morse, M. J. Cieslewicz, M. Lipsitch, C. M. Thompson, E. Kurt-Jones, J. C. Paton, M. R. Wessels, and D. T. Golenbock. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 1001966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malley, R., M. Lipsitch, A. Stack, R. Saladino, G. Fleisher, S. Pelton, C. Thompson, D. Briles, and P. Anderson. 2001. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect. Immun. 694870-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malley, R., K. Trzcinski, A. Srivastava, C. M. Thompson, P. W. Anderson, and M. Lipsitch. 2005. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. USA 1024848-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marodi, L. 2002. Deficient interferon-gamma receptor-mediated signaling in neonatal macrophages. Acta Paediatr. 91(Suppl.)117-119. [DOI] [PubMed] [Google Scholar]
- 28.McCool, T. L., and J. N. Weiser. 2004. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect. Immun. 725807-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson, T., F. T. Wright, and R. L. Dykstra. 1988. Order restricted statistical inference. Wiley, Chichester, United Kingdom.
- 30.Rudan, I., C. Boschi-Pinto, Z. Biloglav, K. Mulholland, and H. Campbell. 2008. Epidemiology and etiology of childhood pneumonia. Bull. W. H. O. 86408-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satwani, P., E. Morris, C. van de Ven, and M. S. Cairo. 2005. Dysregulation of expression of immunoregulatory and cytokine genes and its association with the immaturity in neonatal phagocytic and cellular immunity. Biol. Neonate 88214-227. [DOI] [PubMed] [Google Scholar]
- 32.Scott, J. A. 2007. The preventable burden of pneumococcal disease in the developing world. Vaccine 252398-2405. [DOI] [PubMed] [Google Scholar]
- 33.Scott, J. A., W. A. Brooks, J. S. Peiris, D. Holtzman, and E. K. Mulhollan. 2008. Pneumonia research to reduce childhood mortality in the developing world. J. Clin. Investig. 1181291-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simell, B., M. Melin, M. Lahdenkari, D. E. Briles, S. K. Hollingshead, T. M. Kilpi, and H. Kayhty. 2007. Antibodies to pneumococcal surface protein A families 1 and 2 in serum and saliva of children and the risk of pneumococcal acute otitis media. J. Infect. Dis. 1961528-1536. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava, A., H. Casey, N. Johnson, O. Levy, and R. Malley. 2007. Recombinant bactericidal/permeability-increasing protein rBPI21 protects against pneumococcal disease. Infect. Immun. 75342-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srivastava, A., P. Henneke, A. Visintin, S. C. Morse, V. Martin, C. Watkins, J. C. Paton, M. R. Wessels, D. T. Golenbock, and R. Malley. 2005. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect. Immun. 736479-6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trzcinski, K., C. Thompson, R. Malley, and M. Lipsitch. 2005. Antibodies to conserved pneumococcal antigens correlate with, but are not required for, protection against pneumococcal colonization induced by prior exposure in a mouse model. Infect. Immun. 737043-7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trzcinski, K., C. M. Thompson, A. Srivastava, A. Basset, R. Malley, and M. Lipsitch. 2008. Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+ T cells. Infect. Immun. 762678-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Benten, I. J., C. M. van Drunen, L. P. Koopman, B. C. van Middelkoop, W. C. Hop, A. D. Osterhaus, H. J. Neijens, and W. J. Fokkens. 2005. Age- and infection-related maturation of the nasal immune response in 0-2-year-old children. Allergy 60226-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Rossum, A. M., E. S. Lysenko, and J. N. Weiser. 2005. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect. Immun. 737718-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weaver, C. T., L. E. Harrington, P. R. Mangan, M. Gavrieli, and K. M. Murphy. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24677-688. [DOI] [PubMed] [Google Scholar]
- 42.Weinberger, D. M., R. Dagan, N. Givon-Lavi, G. Regev-Yochay, R. Malley, and M. Lipsitch. 2008. Epidemiologic evidence for serotype-specific acquired immunity to pneumococcal carriage. J. Infect. Dis. 1971511-1518. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. 1999. Pneumococcal vaccines. WHO position paper. Wkly. Epidemiol. Rec. 74177-183. [PubMed] [Google Scholar]
- 44.Yan, S. R., G. Qing, D. M. Byers, A. W. Stadnyk, W. Al-Hertani, and R. Bortolussi. 2004. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect. Immun. 721223-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, Q., L. Bagrade, J. Bernatoniene, E. Clarke, J. C. Paton, T. J. Mitchell, D. A. Nunez, and A. Finn. 2007. Low CD4 T cell immunity to pneumolysin is associated with nasopharyngeal carriage of pneumococci in children. J. Infect. Dis. 1951194-1202. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, Q., J. Bernatoniene, L. Bagrade, A. J. Pollard, T. J. Mitchell, J. C. Paton, and A. Finn. 2006. Serum and mucosal antibody responses to pneumococcal protein antigens in children: relationships with carriage status. Eur. J. Immunol. 3646-57. [DOI] [PubMed] [Google Scholar]