Abstract
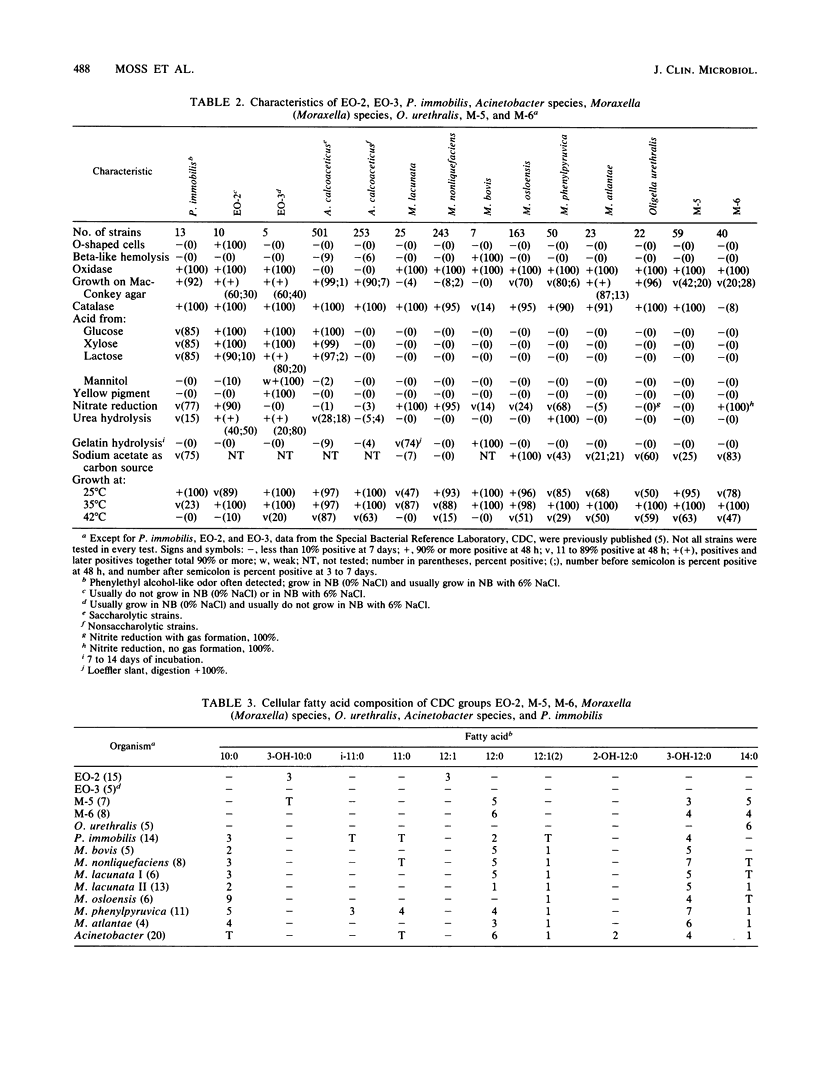
We determined phenotypic characteristics, cellular fatty acid composition, and isoprenoid quinone content of representative strains of CDC groups EO-2, M-5, and M-6, Moraxella (Moraxella) species, Oligella urethralis, Acinetobacter species, and Psychrobacter immobilis. All organisms contained ubiquinone with eight isoprene units as the major isoprenolog, but distinct differences were observed in fatty acid composition. Twenty-eight of the original collection of CDC group EO-2 strains were further identified as P. immobilis, EO-2, or EO-3 by distinctive cellular fatty acid profiles, cellular morphology, and pigment production. The cellular fatty acid compositions of M-5 and M-6 were similar but were clearly different from those of other organisms. The genus Acinetobacter was differentiated from other organisms in the study by small amounts of 2-hydroxydodecanoic acid (2-OH-12:0), and P. immobilis was differentiated by small amounts of decanoic acid (10:0) and a branched-chain 17-carbon acid (i-17:0). All Moraxella species were distinguished by small amounts of decanoic acid (10:0) and the absence of i-17:0. M. bovis, M. nonliquefaciens, and some strains of M. lacunata formed a single fatty acid group, while M. osloensis, M. phenylpyruvica, M. atlantae, and other strains of M. lacunata (M. lacunata II) had species-specific fatty acid profiles. O. urethralis differed from Moraxella species by the presence of large amounts (49%) of cis-vaccenic acid (18:1 omega 7c), small amounts (1%) of 3-hydroxyhexadecanoate (3-OH-16:0), and the absence of 10:0 and 3-hydroxydodecanoate (3-OH-12:0). The combined use of chemical data and a small number of conventional tests permitted rapid identification and differentiation of these organisms from each other and from related organisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryn K., Jantzen E., Bovre K. Occurrence and patterns of waxes in Neisseriaceae. J Gen Microbiol. 1977 Sep;102(1):33–43. doi: 10.1099/00221287-102-1-33. [DOI] [PubMed] [Google Scholar]
- Dees S. B., Moss C. W., Hollis D. G., Weaver R. E. Chemical characterization of Flavobacterium odoratum, Flavobacterium breve, and Flavobacterium-like groups IIe, IIh, and IIf. J Clin Microbiol. 1986 Feb;23(2):267–273. doi: 10.1128/jcm.23.2.267-273.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees S., Thanabalasundrum S., Moss C. W., Hollis D. G., Weaver R. E. Cellular fatty acid composition of group IVe, a nonsaccharolytic organism from clinical sources. J Clin Microbiol. 1980 Jun;11(6):664–668. doi: 10.1128/jcm.11.6.664-668.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M. J., Hollis D. G., Weaver R. E., Galvis C. G. Relationship of CDC group EO-2 and psychrobacter immobilis. J Clin Microbiol. 1987 Oct;25(10):1907–1910. doi: 10.1128/jcm.25.10.1907-1910.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen E., Bryn K., Bergan T., Bovre K. Gas chromatography of bacterial whole cell methanolysates. VII. Fatty acid composition of Acinetobacter in relation to the taxonomy of Neisseriaceae. Acta Pathol Microbiol Scand Suppl. 1975 Dec;83(6):569–580. [PubMed] [Google Scholar]
- Jantzen E., Bryn K., Bergan T., Bovre K. Gas chromatography of bacterial whole cell methanolysates; V. Fatty acid composition of Neisseriae and Moraxellae. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):767–779. doi: 10.1111/j.1699-0463.1974.tb02374.x. [DOI] [PubMed] [Google Scholar]
- Juni E., Heym G. A. Transformation assay for identification of psychrotrophic achromobacters. Appl Environ Microbiol. 1980 Dec;40(6):1106–1114. doi: 10.1128/aem.40.6.1106-1114.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M. A., Patton C. M., Barrett T. J., Moss C. W. Differentiation of Campylobacter and Campylobacter-like organisms by cellular fatty acid composition. J Clin Microbiol. 1987 Apr;25(4):706–713. doi: 10.1128/jcm.25.4.706-713.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Dees S. B. Cellular fatty acids and metabolic products of Pseudomonas species obtained from clinical specimens. J Clin Microbiol. 1976 Dec;4(6):492–502. doi: 10.1128/jcm.4.6.492-502.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W. Gas-liquid chromatography as an analytical tool in microbiology. J Chromatogr. 1981 Jan 9;203:337–347. doi: 10.1016/s0021-9673(00)80305-2. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Kai A., Lambert M. A., Patton C. Isoprenoid quinone content and cellular fatty acid composition of Campylobacter species. J Clin Microbiol. 1984 Jun;19(6):772–776. doi: 10.1128/jcm.19.6.772-776.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Yamamoto H., Iizuka H. Taxonomical studies of Acinetobacter species--cellular fatty acid composition. Z Allg Mikrobiol. 1979;19(4):307–308. doi: 10.1002/jobm.3630190411. [DOI] [PubMed] [Google Scholar]