Abstract
Bioprotective alkaloids produced by Epichloë and closely related asexual Neotyphodium fungal endophytes protect their grass hosts from insect and mammalian herbivory. One class of these compounds, known for antimammalian toxicity, is the indole-diterpenes. The LTM locus of Neotyphodium lolii (Lp19) and Epichloë festuce (Fl1), required for the biosynthesis of the indole-diterpene lolitrem, consists of 10 ltm genes. We have used PCR and Southern analysis to screen a broad taxonomic range of 44 endophyte isolates to determine why indole-diterpenes are present in so few endophyte-grass associations in comparison to that of the other bioprotective alkaloids, which are more widespread among the endophtyes. All 10 ltm genes were present in only three epichloë endophytes. A predominance of the asexual Neotyphodium spp. examined contained 8 of the 10 ltm genes, with only one N. lolii containing the entire LTM locus and the ability to produce lolitrems. Liquid chromatography-tandem mass spectrometry profiles of indole-diterpenes from a subset of endophyte-infected perennial ryegrass showed that endophytes that contained functional genes present in ltm clusters 1 and 2 were capable of producing simple indole-diterpenes such as paspaline, 13-desoxypaxilline, and terpendoles, compounds predicted to be precursors of lolitrem B. Analysis of toxin biosynthesis genes by PCR now enables a diagnostic method to screen endophytes for both beneficial and detrimental alkaloids and can be used as a resource for screening isolates required for forage improvement.
Epichloë endophytes systemically colonize cool-season grasses, and comprise sexual Epichloë spp. and their asexual derivatives, the Neoptyphodium spp. Collectively, they produce a range of bioprotective alkaloids, including the ergot alkaloids, pyrrolopyrazine (peramine), aminopyrrolizidines (lolines), and indole-diterpenes (including lolitrems) (5, 63). Most Neotyphodium spp. are of hybrid origin, with two or three Epichloë or Neotyphodium ancestors (11, 44, 59, 66). The alkaloids produced by epichloë endophytes enhance the competitive ability of endophyte-infected grasses by protecting the grass host from insect and mammalian herbivory. Alkaloids such as peramine and the lolines are known for their anti-insect properties (5, 55, 63), while ergovaline and the lolitrems are detrimental to grazing livestock, causing fescue toxicosis and ryegrass staggers, respectively, in pastoral ecosystems (17, 18, 31, 47, 52). Many epichloë endophytes are able to produce multiple classes of alkaloids but, to date, no plant-endophyte combination has been identified that produces lolitrems, lolines, peramine, and ergovaline (5, 10).
A number of naturally occurring endophytes have been identified that do not produce the mammalian toxins, ergovaline and lolitrem B, but retain beneficial agronomic properties (26, 27), such as tolerance to abiotic stress (32). Artificially inoculated associations of these endophytes with elite tall fescue (4) and perennial ryegrass cultivars (16) have been commercially established and shown to enhance animal productivity, while alleviating the negative responses such as fescue toxicosis and ryegrass staggers (1, 2, 50, 51, 68).
The indole-diterpene, lolitrem B, is the major mammalian toxin responsible for ryegrass staggers, a syndrome found in animals grazing N. lolii-infected perennial ryegrass (Lolium perenne) (17, 18). To date, lolitrem production has only been identified in Epichloë festucae-, Neotyphodium lolii-, and Neotyphodium sp. strain FaTG-2-infected grasses (9, 10). The absence of lolitrems in many epichloë endophyte-grass associations does not appear to be due to a grass host effect but rather to the inability of the endophyte to produce the compound (5, 63). Until recently, genetic analysis of the genes required for the production of lolitrem B was not available, so a large effort was put into the chemical analysis of endophyte-infected grasses that resulted in the identification of indole-diterpene compounds, such as lolitriol, lolicines A and B, lolilline and terpendole M, predicted to be precursors or by-products of lolitrem B biosynthesis (5, 19, 37, 38, 40, 46-49). These analyses showed that the ability to produce the alkaloids was dependent on the presence of the endophyte within the plant. The plant genotype and environmental conditions are also known to influence the amount and relative abundance of the alkaloids produced by the endophyte (5, 12, 13, 30), making alkaloid chemotyping of endophyte-infected grasses difficult. For example, loline production was recently shown to be a wound-inducible response in Neotyphodium coenophialum-infected tall fescue (64) and Epichloë glyceriae-infected Glyceria striata (22), where the level of lolines detected in undamaged E. glyceriae-infected plants was minimal compared to the wounded-infected plant (22).
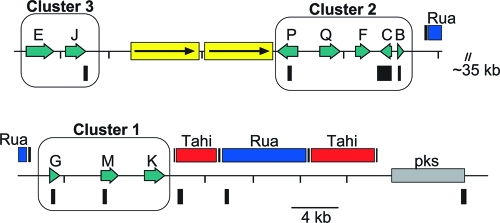
Recently, a cluster of genes at the LTM locus involved in indole-diterpene biosynthesis was isolated from N. lolii and E. festucae (74, 75). The LTM locus is complex in that it contains three clusters separated by retrotransposon relics and other highly repetitive sequences (Fig. 1). The gene cluster consists of at least 10 genes, which encode LtmG, a geranylgeranyl diphosphate synthase; LtmM, an FAD-dependent monooxygenase; LtmC, a prenyl transferase; LtmB, a hypothetical protein of unknown function; LtmF, a prenyl transferase with similarity to dimethylallyl tryptophan synthase; four P450 monooxygenases (LtmJ, LtmK, LtmP, and LtmQ); and LtmE, a multifunctional enzyme that contains two prenyl transferase domains with homology to LtmC and LtmF. The 10 ltm genes are coregulated and highly expressed by the endophyte when associated with the grass host (74, 75).
FIG. 1.
Physical map of the N. lolii LTM locus. The three clusters required for indole-diterpene biosynthesis are boxed and numbered. The regions amplified as probes used for the Southern hybridizations shown in Fig. 2 and 3 are represented by the black boxes below the map. The low-copy direct repeat is indicated as arrows boxed in yellow. The retrotransposons, Tahi and Rua, are depicted as red and blue boxes, respectively. The pks pseudogene is shown as a gray box. The primers used to amplify each fragment can be found in Table 3. (Adapted from reference 75 with permission from Elsevier.)
Based on our knowledge of the biochemical pathway responsible for the production of the indole-diterpene paxilline and its precursors, we predict that LtmG, LtmM, LtmC, and LtmB are required for the biosynthesis of paspaline, the proposed indole-diterpene intermediate that forms a structural backbone essential for the production of more complex indole-diterpenes (56, 57, 73-75). LtmP and LtmQ are predicted to be required for demethylation and hydroxylation of paspaline, respectively, and therefore, are essential for the production of the lolitrem class of indole-diterpenes. LtmF, LtmJ, LtmK, and LtmE appear unique to lolitrem biosynthesis and are predicted to be required for the elaboration of the additional rings that define this class of molecules (75).
Previous studies of the LTM locus (74, 75) have focused on three epichloë endophytes with known chemotypes: the lolitrem B producing E. festucae (isolate Fl1) and N. lolii (isolate Lp19) and nonproducing E. typhina (isolate E8). The observation that lolitrem B production correlated with the presence of the ten ltm genes contained within the LTM locus led us to hypothesize that the capability to produce lolitrem B and indole-diterpene precursors by epichloë endophytes can be predicted by PCR profiling of the essential pathway genes. The aim of the present study was to determine the distribution of the ltm genes among epichloë endophytes, including representative isolates of 10 sexual Epichloë species and a taxonomically diverse set of Neotyphodium isolates. We then utilized the PCR analysis and liquid chromatography-tandem mass spectrometry (LC-MS/MS) data to elucidate the symbiotum indole-diterpene chemotype.
MATERIALS AND METHODS
Fungal strains and growth conditions.
Epichloë and Neotyphodium strains (Table 1) were grown on potato dextrose medium and maintained as previously described (41, 43, 74). Endophyte-infected plant material is listed in Table 2.
TABLE 1.
Biological material
| Biological material | Isolate | Closest nonhybrid ancestor(s)a | Predicted chemical phenotypeb | Grass host | Tribe | Culture no.c | Source or reference(s) |
|---|---|---|---|---|---|---|---|
| E. amarillans | E52 | E. amarillans | No IDT | Sphenopholis obtusata | Aveneae | ATCC 200743 | 44, 60, 61, 62 |
| E. amarillans | E57 | E. amarillans | No IDT | Agrostis hiemalis | Aveneae | ATCC 200744 | 44, 60, 61, 62 |
| E. baconii | E248 | E. baconii | No IDT | Agrostis stolonifera | Aveneae | ATCC 76552 | 29, 44, 61, 62 |
| E. baconii | E1031 | E. baconii | No IDT | Calamagrostis villosa | Aveneae | ATCC 200745 | 29, 44, 60, 61 |
| E. brachyelytri | E1040 | E. baconii | No IDT | Brachyelytrum erectum | Brachyelytreae | ATCC 200752 | 44, 60, 61 |
| E. bromicola | E501 | E. bromicola | No IDT | Bromus erectus | Bromeae | ATCC 200749 | 29, 44, 61 |
| E. bromicola | E799 | E. bromicola | No IDT | Bromus benekenii | Bromeae | ATCC 201559 | 29 |
| E. clarkii | E422 | ETC | No IDT | Holcus lanatus | Poeae | ATCC 90168 | 29, 60, 61 |
| E. elymi | E56 | E. elymi | No IDT | Elymus canadensis | Triticeae | ATCC 201551 | 44, 60, 61, 62 |
| E. elymi | E184 | E. elymi | No IDT | Elymus virginicus | Triticeae | ATCC 200850 | 44, 60, 61 |
| E. festucae | E189 | E. festucae | Lolitrems* | Festuca rubrasubsp.rubra | Poeae | ATCC 90661 | 29, 44, 62 |
| E. festucae | E2368 | E. festucae | No IDT | Festuca spp. | Poeae | C. L. Schardl, University of Kentucky | |
| E. festucae | Fg1 | E. festucae | Lolitrems | Festuca glauca | Poeae | 41 | |
| E. festucae | Fl1 | E. festucae | Lolitrems* | Festuca longifolia | Poeae | ATCC MYA-3407 | 28, 41 |
| E. festucae | Fr1 | E. festucae | No IDT | Festuca rubra | Poeae | 28, 41 | |
| E. festucae | Frc5 | E. festucae | No IDT | Festuca rubrasubsp.commutata | Poeae | 41 | |
| E. festucae | Frc7 | E. festucae | IDT | Festuca rubrasubsp.commutata | Poeae | 41 | |
| E. festucae | Frr1 | E. festucae | No IDT | Festuca rubrasubsp.rubra | Poeae | 41 | |
| E. glyceriae | E277 | E. glyceriae | No IDT | Glyceria striata | Meliceae | ATCC 200747 | 44, 60, 61 |
| E. glyceriae | E2772 | E. glyceriae | No IDT | Glyceria striata | Meliceae | ATCC 200755 | 44, 60, 61 |
| E. sylvatica | E354 | ETC | No IDT | Brachypodium sylvaticum | Brachypodieae | ATCC 200748 | 29, 44, 60, 61 |
| E. sylvatica | E503 | ETC | No IDT | Brachypodium sylvaticum | Brachypodieae | ATCC 200751 | 29, 44, 60, 61 |
| E. typhina | E8 | ETC | No IDT | Lolium perenne | Poeae | ATCC 200736 | 29, 41, 44, 60, 61, 62 |
| E. typhina | E1022 | ETC | No IDT | Poa nemoralis | Poeae | ATCC 201668 | 29, 44 |
| E. typhina | E2463 | ETC | No IDT | Dactylis glomerata | Poeae | C. D. Moon and C. L. Schardl, University of Kentucky | |
| E. typhina | E348 | ETC | No IDT | Phleum pratense | Aveneae | CBS 102648 | 29, 61 |
| E. typhina | E425 | ETC | No IDT | Phleum pratense | Aveneae | ATCC 200851 | 29, 44, 60, 61 |
| E. typhina | E505 | ETC | No IDT | Brachypodium pinnatum | Brachypodieae | ATCC 200739 | 29, 44, 60, 61 |
| N. aotearoae | e899 | Nao | No IDT# | Echinopogon ovatus | Aveneae | ATCC MYA-1229 | 42 |
| N. australiense | e938 | E. festucae, ETC | No IDT | Echinopogon. ovatus | Aveneae | CBS 109347 | 42 |
| N. coenophialum | e19 | E. festucae, ETC, LAE | No IDT | Lolium arundinaceum | Poeae | ATCC 90664 | 44, 65 |
| N. coenophialum | Tf28 | E. festucae, ETC, LAE | IDT | Lolium arundinaceum | Poeae | 9 | |
| N. funkii | e4096 | E. festucae, E. elymi | IDT | Stipa robusta | Stipeae | ATCC MYA-2583 | 45 |
| N. gansuense var. inebrians | e818 | N. inebrians | No IDT | Achnatherum inebrians | Stipeae | ATCC MYA-1228 | 44, 45 |
| N. lolii | AR1 | E. festucae | IDT | Lolium perenne | Poeae | 41 | |
| N. lolii | Lp14 | E. festucae | IDT* | Lolium perenne | Poeae | 9, 41, 62 | |
| N. lolii | Lp19 | E. festucae | Lolitrems* | Lolium perenne | Poeae | 9, 41 | |
| N. lolii | Lp5 | E. festucae | Lolium perenne | Poeae | 9, 41, 62 | ||
| N. lolii | Lp7 | E. festucae | Lolium perenne | Poeae | 9, 41 | ||
| N. lolii | Lp9 | E. festucae | Lolium perenne | Poeae | 9, 41, 62 | ||
| N. melicicola | e822 | E. festucae, N. aotearoae | IDT | Melica racemosa | Meliceae | CBS 108340 | 42 |
| N. siegelii | e915 | E. festucae, E. bromicola | IDT | Lolium pratense | Poeae | ATCC 74483 | 44 |
| Neotyphodium sp. strain FaTG-2 | Tf15 | E. festucae, LAE | Lolium arundinaceum | Poeae | 9, 41 | ||
| Neotyphodium sp. strain FaTG-3 | Tf20 | E. festucae, LAE | Lolium arundinaceum | Poeae | 9, 41 | ||
| Neotyphodium sp. strain HboTG-2 | Hd1 | E. bromicola, ETC | No IDT | Hordeum bogdanii | Triticeae | M. Christensen, AgResearch, New Zealand | |
| Neotyphodium sp. strain LpTG-2 | Lp1 | E. festucae, ETC | IDT | Lolium perenne | Poeae | 9, 41, 62 | |
| Neotyphodium sp. strain LpTG-3 | Lp2 | E. festucae, ETC | Lolium perenne | Poeae | 9, 41, 62 | ||
| N. tembladerae | e1169 | E. festucae, ETC | IDT | Poa huecu | Poeae | ATCC 200844 | 44 |
| N. tembladerae | e4055 | E. festucae, ETC | IDT | Festuca arizonica | Poeae | ATCC MYA-2564 | 44 |
| Neotyphodium sp. | Poa | ETCd | IDT? | Poa sp. | Poeae | M. Christensen, AgResearch, New Zealand |
Abbreviations: ETC, E. typhina complex; LAE, Lolium-associated endophyte as documented in Moon et al. (44).
The predicted phenotype is based on the PCR screen for the presence of the ltm genes. Phenotypes that were confirmed by chemical analysis in this study are indicated in boldface. Chemical phenotypes confirmed in other publications are indicated by an asterisk. No IDT, no indole-diterpene production; IDT, indole-diterpene production; lolitrems, lolitrem production; #, isolate positive as shown by Miles et al. (39).
ATCC and CBS refer to isolates deposited at the American Type Culture Collection (Manassas, VA) or the CentraalBureau voor Schimmelcultures (Utrecht, The Netherlands), respectively.
The possible hybrid status is undetermined.
TABLE 2.
Endophyte-infected plant materiala
| Plant material | Isolate contained | Closest nonhybrid ancestor(s) | Analyzed IDT chemotypeb | Grass host/endophyte inoculation |
|---|---|---|---|---|
| G1730-G1732 | Fr1 | E. festucae | No IDT | L. perenne/PN2207 |
| G1733-G1735 | Frr1 | E. festucae | No IDT | L. perenne/PN2212 |
| G1736-G1738 | Frc5 | E. festucae | No IDT | L. perenne/PN2209 |
| G1739-G1741 | Frc7 | E. festucae | IDT | L. perenne/PN2132 |
| G1742-G1744 | Fg1 | E. festucae | Lolitrems | L. perenne/PN2211 |
| G1745-G1747 | Fl1 | E. festucae | Lolitrems* | L. perenne/PN2278 |
| G1748-G1750 | E189 | E. festucae | Lolitrems* | L. perenne/PN2241 |
| G1751 | AR1 | E. festucae | IDT | L. perenne/PN2279 |
| G1752-G1754 | Lp1 | E. festucae, ETCc | IDT | L. perenne/PN2197 |
All plant materials were derived from L. perenne. The tribe for all plant materials was Poeae, and the reference for all plant materials was the present study.
*, chemotype confirmed in other publications. No IDT, no indole-diterpene production; IDT, indole-diterpene production; lolitrems, lolitrem production.
ETC, E. typhina complex.
Molecular biological techniques.
Genomic DNA from Epichloë and Neotyphodium species were isolated from freeze-dried mycelium by using the methods of Byrd et al. (6) and Yoder (70) and a Plant DNeasy extraction kit (Qiagen, Hilden, Germany).
DNA restriction digestion, Southern blotting, and probe labeling were carried out as previously described in Young et al. (74). Standard PCR amplifications of genomic DNA templates were carried out as previously described (74) using primer pairs listed in Table 3. The primers for each gene were designed to conserved domains identified in peptide sequence alignments of known ltm homologs and sequences predicted to have a role in indole-diterpene production. The primer sequence was subsequently designed with a bias toward the ltm sequence (71). Primers were also designed to the known housekeeping genes, ggsA (which encodes a geranylgeranyl diphosphate synthase) and chsV (which encodes a class V chitin synthase), and used to test the integrity of the genomic DNA stocks used in the PCR screen. PCR products were purified by using a PCR purification kit (Qiagen). DNA for probes were radiolabeled with [α-32P]dCTP (3,000 Ci/mmol; Amersham) by random primed synthesis using a High Prime kit (Roche, Basel, Switzerland), and probes were purified by using ProbeQuant columns (Pharmacia). Standard hybridizations were carried out at 65°C overnight as previously described (72). Low-stringency hybridization was performed with a 4 h prehybridization in 10× Denhardt solution (58) and then hybridized for 48 h at 37°C in 43% (vol/vol) formamide, 5× Denhardt solution, 5× SSC (0.75 M NaCl, 0.075 M trisodium citrate), 0.1% sodium dodecyl sulfate (SDS), 50 mM sodium phosphate (36), and 10 μg of phenol-extracted herring sperm DNA/ml. Posthybridization, the membrane was washed at room temperature in two changes of 2× SSC (0.3 M NaCl, 0.03 M trisodium citrate)-0.1% SDS for 10 min and then with three 15-min washes in 2× SSC-0.1% SDS at 50°C. Membranes that were hybridized multiple times were stripped of their radioactive signal by three to four washes in boiling 0.1% SDS and checked for residual radioactive signal prior to each hybridization.
TABLE 3.
Primers used for probes and PCR analysis
| Gene | Primer 1
|
Primer 2
|
Size (bp) | Application | ||
|---|---|---|---|---|---|---|
| Name | Sequence (5′-3′) | Name | Sequence (5′-3′) | |||
| chsV | ChsV-312 | CTGGCCAGTCGTTTCCACGT | ChsV-289 | GTCGCCGATGTATCGTATTG | 483 | PCR |
| ggsA | CYLp19-16 | CGTCGCCCACAACATCTTTG | CYLp19-1 | CACCATTTCGAGGTAGTC | 214 | PCR |
| ltmC | ltmC-216 | AGATGACATCTGGAGCATGG | ltmC-236 | CTTAAGCGAATTCTACCTTGTGGGTC | 1236 | Probe |
| ltmC | ltmC-278 | GAAACTGCCAATCGAGCATA | ltmC-279 | TTCTTGCAATCATTTTGCAATTG | 403 | PCR |
| ltmE | ltmE-356 | CCGAGTTTGATGACCTGCTG | ltmE-341 | TTCCGCTTCCGAGTAGACTC | 687 | Probe/PCR |
| ltmF | ltmF-359 | GAATTATGTTACTCTTGGGG | ltmF-360 | AAGTTGGCACATAGGTCTTC | 227 | PCR |
| ltmG | ltmG-3 | ACCGACGCCATTAATGAG | ltmG-1 | TGGATCATTCGCAGATAC | 407 | Probe |
| ltmG | ltmG-156 | GCACAAACAATAAATTCGGCCAA | ltmG-157 | AATTTGCCCTCTGTTAAATCCTC | 383 | PCR |
| ltmJ | ltmJ-205 | CCAAGCATCGATTTGTCACC | ltmJ-206 | AATCTGATCGCCATCTTTGC | 242 | Probe/PCR |
| ltmK | ltmK-160 | ATATTGAATTGCTGCGTGAGGAG | ltmK-161 | AGAGGCCAAGAAGCGGCCTGGACA | 568 | PCR |
| ltmM | ltmM-7 | ACTGGGCATCTTCCATAG | ltmM-35 | GTTCGGTGCCTCTAATAC | 448 | Probe |
| ltmM | ltmM-158 | GTGATCGGTGCTGACGGGGTCCA | ltmM-159 | TATCGCCATATTTGCTCCTTGCCC | 669 | PCR |
| ltmP | ltmP-191 | CCAAGGAGGTTTTGAATGTA | ltmP-192 | TTGGATGAGCTCAATCATGC | 374 | Probe |
| ltmP | ltmP-280 | ATGGCTGTCATTCATACAACAGCTATG | ltmP-281 | AGCGTCCCGGACAGGCATATCTCCCA | 508 | PCR |
| ltmQ | ltmQ-313 | CTACCAGGACAGGCGTGACGTCC | ltmQ-282 | CAGAGGTTTAACCCTCTTGACGC | 334 | PCR |
| Rua | rua-4 | ATAGTCTAACTAGAGGGC | rua-23 | TAGATAGGGAAGTTATGC | 382 | Probe |
| Tahi | tahi-16 | AGTCTTTCCTAGCGTAGG | tahi-95 | GTAAGCGGTTAAAAGGGA | 522 | Probe |
PCR products were sequenced by using BigDye chemistry (v31.; Applied Biosystems, Foster City, CA) using gene-specific primers, and the products were separated on an ABI 3730 sequence analyzer. Sequences were aligned by using Sequencher version 4.8 (Gene Codes, Ann Arbor, MI) and annotated using MacVector version 9.5 (MacVector, Inc., Cary, NC). The GenBank accession numbers for Fg1 and Frc7 ltmF are EU530694 and EU544671, respectively.
Plant inoculations and lolitrem analysis.
Inoculation of endophyte-free perennial ryegrass (cv. Nui) seedlings with E. festucae isolates Fr1, Frr1, Frc5, Frc7, Fg1, Fl1, and E189; N. lolii isolates Lp19 and AR1; and Neotyphodium sp. isolate Lp1 were performed as previously described (74) (Table 2). For each association, three independent plants were analyzed for lolitrem B (74) and other indole-diterpenes. Pseudostem fractions from each plant were freeze-dried and milled in a modified (reduced volume) domestic coffee mill. Pseudostem material was chosen for alkaloid analysis since prior experience had shown that alkaloids tend to accumulate to higher levels in this tissue. Plant fractions (50 mg) were extracted in 2-ml polypropylene capped tubes with 1 ml of propan-2-ol for 2 h at ambient temperature and mixed by inversion on a rotary mixer. After centrifugation to sediment residual solids, aliquots of the extract were transferred to septum capped vials for subsequent fluorescence high-pressure LC (HPLC) (74) and LC-MS/MS analysis.
LC-MS/MS.
Alkaloid analysis and identification was performed by using a linear ion trap system and associated liquid chromatography modules (Thermo LTQ and Thermo Surveyor, San Jose, CA). Samples (10 μl) were injected onto a Prodigy ODS-3 column (5-μm pore size, 150 by 4.6 mm; Phenomenex, Torrance, CA) with solvent flow of 1 ml/min at 25°C. The solvents were 40% aqueous acetonitrile with 0.1% acetic acid (by volume) (solvent A) and acetonitrile with 0.1% acetic acid (solvent B) in proportions beginning with 20% solvent B, rising linearly to 50% solvent B at 20 min, and then to 100% solvent B at 40 min and recycling after 60 min.
The ion trap was operated in positive atmospheric pressure chemical ionization mode with an N2 sheath and auxiliary gas (set to 40 and 10, respectively), the source voltage at 6 kV, the capillary temperature at 200°C, and the atmospheric pressure chemical ionization vaporizer temperature at 450°C. The ion trap was tuned to maximum sensitivity with an infusion of paxilline diluted in ca. 65% aqueous acetonitrile with 0.1% acetic acid at 1 ml/min. A parent ion list for targeted indole-diterpenes was programmed to permit data-dependent observation of tandem mass spectrometry (MS2 and MS3) spectra for compound identification purposes and comparison with authentic standards.
For retention time and mass spectral comparison purposes, paxilline was obtained from Sigma (St. Louis, MO), and lolitriol, lolitrem B, lolitrem E, terpendole M, 13-desoxypaxilline, terpendole C, and paspaline were obtained from Sarah Finch (AgResearch, Ltd., New Zealand).
RESULTS
Defining ltm gene profiles of epichloë endophytes by PCR.
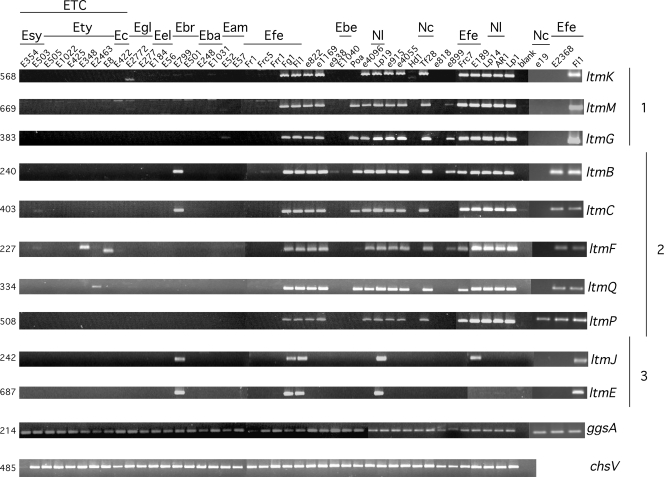
Genomic DNA of 44 isolates from both sexual and asexual epichloë endophytes was subjected to PCR to detect the presence of ltm genes (Fig. 2). As expected, amplification products resulted from the 10 ltm primer pairs with genomic DNA from N. lolii Lp19 and E. festucae Fl1 (Fig. 2). Ten PCR products also were amplified from genomic DNA of one other isolate, E. festucae Fg1, while products corresponding to nine, eight, and five ltm genes were amplified from genomic DNA of E. festucae E189, Frc7, and E2368, respectively. Although E189 did not yield an amplification product with primers designed for ltmE, Southern analysis of E189 genomic DNA showed hybridization with a PCR fragment of ltmE, amplified from Fl1 with primers ltmE356 and ltmE341, which indicated the presence of this gene (data not shown). There was no evidence that the remaining E. festucae isolates—Fr1, Frr1, and Frc5—contained ltm genes apart from a faint band with the ltmB primers with Frc5 DNA. Of the Epichloë spp. included in the analysis, PCR products corresponding to four ltm genes were clearly amplified from E. bromicola strain E799. PCR products corresponding to at least one ltm gene were detected in five Epichloë spp., which included ltmG from E. amarillans E52 but not E. amarillans E57 (Fig. 2). However, most of the sexual Epichloë spp. appeared to lack ltm genes. Faint bands were detected in some samples but these were not analyzed further to determine whether they were from nonspecific amplification. Amplification of the eight genes that comprise clusters 1 and 2 was the most frequently observed pattern, especially among the Neotyphodium species examined that included N. lolii isolates AR1 and Lp14. N. coenophialum e19 has an E. festucae ancestry, but just a single PCR product corresponding to ltmP was amplified from the DNA of this strain (Fig. 2).
FIG. 2.
PCR screen for ltm genes. PCR products were amplified with primer pairs designed to conserved regions of the designated gene. The cluster of each gene is indicated as 1, 2, or 3 on the right of the samples. The primers used to amplify each fragment are listed in Table 3. The PCR product size is indicated in base pairs to the left of the samples. Eam, E. amarillans; Eba, E. baconii; Ebe, E. brachyelytri; Ebr, E. bromicola; Ec, E. clarkii; Eel, E. elymi; Efe, E. festucae; Egl, E. glyceriae; Esy, E. sylvatica; Ety, E. typhina; Nc, N. coenophialum; Nl, N. lolii; ETC, Epichloë typhina complex. The remaining species can be found in Table 1.
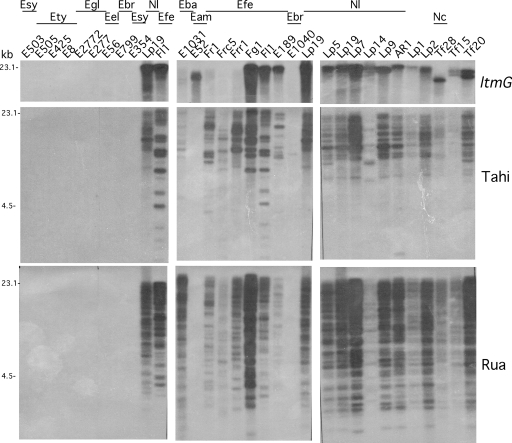
Southern analysis was used to assess the robustness of detecting the epichloë endophytes ltm genes by PCR (Fig. 3), since we could not exclude the possibility of false-negative or false-positive results arising from incompatibility or mispriming of the PCR primers. The two geranylgeranyl diphosphate synthase genes, ggsA, a housekeeping gene and ltmG required for indole-diterpene production, were hybridized to EcoRI-digested genomic DNA isolated from a range of the epichloë endophyte isolates tested by PCR (Fig. 3 and data not shown). A direct correlation was observed between strains that hybridized with ltmG (Fig. 3) and DNA fragments amplified using ltmG primers from genomic DNA of the strains (Fig. 2). Furthermore, the ggsA probe hybridized to genomic DNA from all of the epichloë endophytes, with a common 4.5-kb band seen in E. festucae isolates, or those isolates with E. festucae ancestry (data not shown). Given DNA from all strains hybridized with the ggsA probe and resulted in amplification products with the ggsA primers, the lack of amplification of the ltm genes observed for some strains (Fig. 2 and data not shown) was not due to the quality of the DNA.
FIG. 3.
Detection of ltmG and retrotransposon sequences flanking the LTM locus in epichloë endophytes using Southern analysis. All genomic DNA (2 μg/lane) was digested with EcoRI and hybridized with 32P-labeled fragments. Each probe is indicated to the right of the figure. The location of ltmG, Tahi, and Rua probes at the LTM locus can be found in Fig. 1. The primers used to amplify each fragment are listed in Table 3. Each blot was stripped and checked for residual radiolabel between hybridizations. Lp19 and Fl1 were included on each blot as hybridization controls. The species abbreviations are as for Fig. 2.
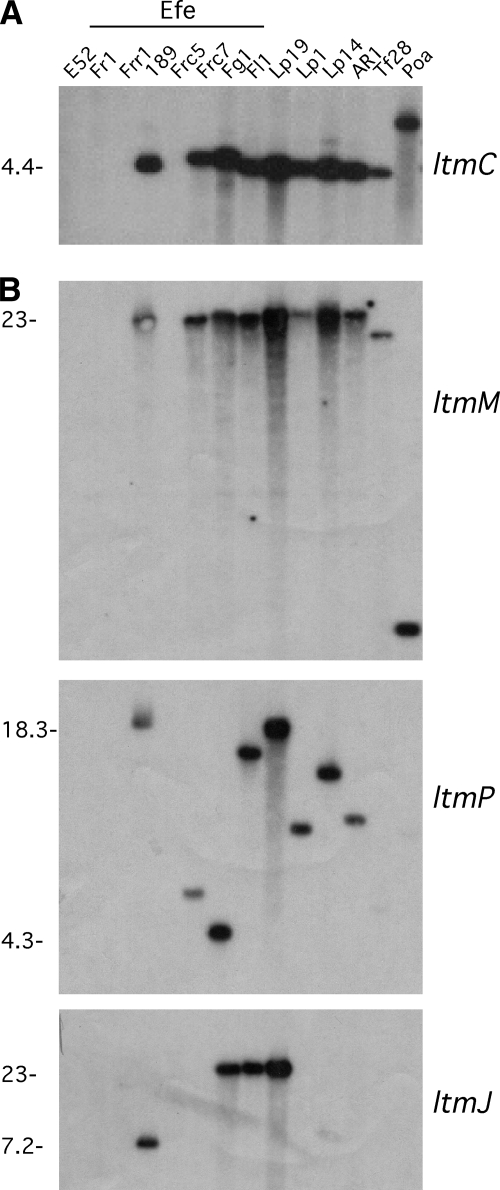
In further support of the reliability of the PCR analysis for the ltm gene detection, the ltmC gene was chosen for low-stringency hybridization to EcoRI-digested genomic DNA from a subset of epichloë endophytes (Fig. 4A). The ltmC gene was selected because it is an essential pathway gene (57) that is not considered to belong to a major family of closely related genes such as the P450- and FAD-dependent monooxygenases. This was supported by the analysis of the E. festucae genome sequence (E2368), which showed there was no significant match to ltmC other than to the gene itself (data not shown). The Southern analysis showed the ltmC probe did not hybridize to E. amarillans E52 or E. festucae Fr1, Frr1, and Frc5, strains that were previously scored as negative for ltmC by PCR (Fig. 4A). However, all of the other isolates tested did show hybridization, which is consistent with the PCR results. The Southern hybridization was repeated under standard hybridization conditions to detect ltm genes from each cluster: ltmM, ltmP, and ltmJ (Fig. 4B). The hybridization patterns for ltmM and ltmP were consistent with the results obtained for ltmC, where the genes were not detected in isolates E52, Fr1, Frr1, and Frc5. The hybridization pattern for ltmP resulted in a greater size variation of hybridizing fragments (Fig. 4B). This result is likely indicative of ltmP being adjacent to an AT-rich repetitive region, as is seen in Lp19, and would therefore reflect the genomic differences found in each strain adjacent to ltm cluster 2 (75). The ltmJ hybridization suggested that only four isolates—E189, Fg1, Fl1, and Lp19—contained sequences from ltm cluster 3 (Fig. 4B).
FIG. 4.
Southern analysis of endophyte strains for ltmC, ltmM, ltmP, and ltmJ genes. Southern analysis of EcoRI-digested genomic DNA hybridized with 32P-labeled probes (Table 3). (A) Autoradiograph of low-stringency hybridization with the complete ltmC gene. (B) Standard hybridization with the probes labeled to the right of the figure. Each blot was stripped and checked for residual radioactivity between hybridizations.
To determine whether the retrotransposon sequences previously found to be located at the N. lolii LTM locus (74, 75) were present in other epichloë endophytes, Southern blots of genomic DNA were hybridized with probes to the Tahi and Rua elements (Fig. 3). These retrotransposon probes hybridized to DNA from E. festucae and E. baconii strains but did not hybridize to genomic digests of other sexual Epichloë isolates tested. The apparent copy number of the retrotransposons varies between isolates. E. baconii E1031 and Neotyphodium sp. strain FaTG-2 Tf15 (of E. baconii and E. festucae hybrid origin) have fewer Tahi elements than are found in E. festucae Fl1 and N. lolii Lp19. E. festucae E189 and Frc5 and N. lolii Lp14 have fewer Tahi and Rua elements than either E. festucae Fl1 or N. lolii Lp19. Hybridization of the pks pseudogene found adjacent to ltm cluster 1 in Fl1 (74) shows no obvious phylogenetic distribution, being present in several different Epichloë species but not necessarily in all isolates tested within a species (data not shown). E. amarillans E52 contains both ltmG and pks sequences, but the retrotransposon probes did not hybridize to genomic digests of this strain.
Correlating indole-diterpene chemotypes with ltm gene PCR profiles.
The PCR and Southern analysis of the LTM locus showed a significant difference between E. festucae isolates with respect to ltm gene composition, ranging from the absence of all genes to the presence of all 10 genes (Fig. 2, 3, and 4). To test the hypothesis that lolitrem biosynthesis requires all 10 ltm genes, a selection of isolates from E. festucae (Fr1, Frr1, Frc5, Frc7, Fg1, Fl1, and E189), N. lolii (AR1, of E. festucae ancestry), and Neotyphodium sp. (Lp1, of E. festucae and E. typhina ancestry) were inoculated into perennial ryegrass (cv. Nui), and tissue extracts from these symbiota were analyzed for indole-diterpenes. The HPLC analysis of these symbiota showed that only Fl1 and E189 endophyte-infected plant material contained lolitrem B (Table 4). LC-MS/MS analysis showed that paspaline, 13-desoxypaxilline, and paxilline were present in perennial ryegrass plants infected with Frc7, Fg1, AR1 (E. festucae ancestry), and Lp1 (E. festucae and E. typhina ancestry). Fl1- and E189-infected plants also contained paspaline and 13-desoxypaxilline, but they did not contain paxilline. In addition, several unknown indole-diterpenes were detected in plant extracts of these isolates (Table 4). Indole-diterpenes were not detected in symbiota infected with Fr1, Frr1, and Frc5, a result consistent with the complete absence of ltm genes in these strains (Fig. 2, 3, and 4 and Table 4). Endophyte-infected perennial ryegrass that contained Fg1, which was positive by PCR for all ten ltm genes, did not produce lolitrem B but did produce lolitriol, and compounds tentatively identified as lolitrem K and lolitrem J. These indole-diterpenes lack the isoprene group needed to form ring I. Frc7, which has a similar ltm gene profile to Lp1 and AR1, did not produce terpendole C and the unknown indole-diterpenes with m/z values of 534 and 518.
TABLE 4.
Occurrence of some indole diterpenes in ryegrass plants infected with selected endophytes
| Compounda | m/z M+H+ | Retention time (min) | MS IDb | Result obtained with endophyte isolate(s)c:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Fr1, Frr1, Frc5 | Lp1, AR1 | Frc7 | Fg1 | Fl1 | E189 | ||||
| Paspaline* | 422 | 32.5 | 1 | - | + | ++ | ++ | + | + |
| 13-Desoxypaxilline* | 420 | 21.6 | 2 | - | ++ | ++ | ++ | ++ | + |
| Paxilline* | 436 | 15 | 2 | - | + | ++ | ++ | Ld | - |
| Unknown | 454 | 9.2 | - | + | ++ | + | + | + | |
| Unknown | 518 | 26.1 | - | ++ | - | - | ++ | + | |
| Unknown | 534 | 21.8 | - | ++ | - | - | ++ | ++ | |
| Terpendole C* | 520 | 27.1 | 1 | - | ++ | - | - | + | + |
| Lolitriol* | 620 | 9.5 | 3 | - | - | - | + | ++ | ++ |
| Lolitrem N | 620 | 8.6 | - | - | - | - | ++ | Ld | |
| Lolitrem K | 602 | 16.1 | - | - | - | ++ | + | - | |
| Lolitrem J | 662 | 14.3 | - | - | - | + | ++ | Ld | |
| Lolitrem E* | 688 | 30.1 | 3 | - | - | - | - | ++ | + |
| Lolitrem B* | 686 | 28.7 | 3 | - | - | - | - | ++ | ++ |
*, compound identified by relative retention and mass spectral comparison to authentic standards. The remaining compounds were tentatively identified by mass, relative retention, and possible relationship(s) to known compounds. Unknown, the mass indicates an as-yet-uncharacterized indole-diterpene.
Order of mass spectral fragmentation used to identify compound compared to authentic standard for occurrences marked “++”.
-, Compounds not detected; Ld, ions at the limit of detection where confirmation of the peak could not be confirmed by mass spectral fragmentation; +, compounds observed at low ion intensity; ++, compounds observed at medium or high intensity. The ltm genes and clusters present in the isolates were as follows: Lp1 and AR1, KMGBCFQP and ltm 1, 2; Frc7, KMGBCF∧QP and ltm 1, 2; Fg1, KMGBCF∧QPJE and ltm 1, 2, 3; Fl1 KMGBCFQPJE and ltm 1, 2, 3; and E189, KMGBCFQPJE and ltm 1, 2, 3. F∧ indicates a nonfunctional ltmF gene. For E189, ltmE was detected by Southern analysis only.
Two asexual perennial ryegrass endophytes, AR1 and Lp1, did not produce detectable levels of lolitrems in planta but were shown to produce the less complex indole-diterpenes, paspaline, 13-desoxypaxilline, paxilline, and terpendole C, a result that is consistent with the presence of functional gene products from ltm clusters one and two. A summary of the predicted chemotype of the species screened to date is shown in Tables 1 and 2.
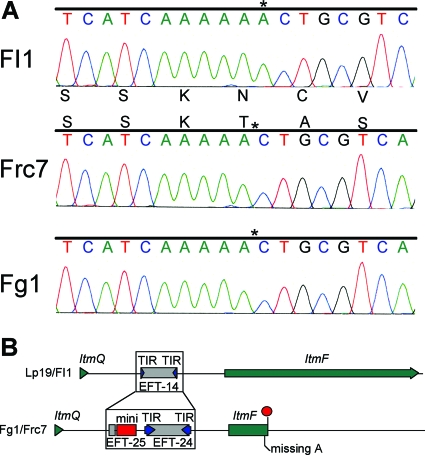
The presence of lolitriol, lolitrem K, and lolitrem J in the Fg1-perennial ryegrass symbiota is consistent with the hypothesis that this strain has a nonfunctional copy of ltmF, which encodes a prenyl transferase (75). A comparison of the Fg1 ltmF nucleotide sequence with that of Fl1 (Fig. 5) and Lp19 (accession no. DQ443465) indicated that Fg1 has a single base deletion within the coding sequence that results in a premature stop codon and, thereby, a nonfunctional gene. Sequence analysis of the Lp19 intergenic region spanning ltmQ and ltmF showed the presence of a 277-bp sequence with hallmarks of a miniature inverted-repeat transposable element (MITE) that was also identified as a repeat sequence within the promoter of ltmP (75). Within this element we identified 35-bp terminal inverted-repeats and a putative TA target site duplication. Two copies of the element, called EFT-14 (for E. festucae transposable element) (14), are positioned 363 and 344 bases upstream from the predicted translational start sites of ltmF and ltmP, respectively. PCR of genomic DNA using primers designed to span the intergenic region from the 3′ end of ltmQ to the beginning of ltmF produced a larger sized band in Fg1 compared to Fl1 and Lp19 (data not shown). Comparison of the nucleotide sequence for the ltmQ-ltmF intergenic promoter region of Fg1 against the previously published sequence from Lp19 showed that the EFT-14 element was not present in Fg1 but three additional repetitive sequences that consisted of two possible MITEs, EFT-24 and EFT-25, and an 18-bp minisatellite sequence (consensus sequence of AYACCCCTAWARAATRCY) (Fig. 5). E. festucae Frc7, a strain that contains the genes from ltm clusters 1 and 2, also has the repetitive sequences and the frameshift within the ltmF coding sequence (Fig. 5).
FIG. 5.
Schematic comparison of the ltmF gene between Lp19, Fl1, Fg1, and Frc7. The sequence chromatograms show the missing A in the Fg1 and Frc7 ltmF sequence that results in a frameshift. The schematic maps were drawn from the sequence contained in accession numbers DQ443465 (Lp19), EU530694 (Fg1), and EU544671 (Frc7). The MITE sequences are labeled as EFT14, EFT24, and EFT25 with the terminal inverted-repeats (TIRs) shown as arrows. The 18-bp minisatellite sequence is labeled mini. The red circle represents the stop codon in the truncated ltmF.
DISCUSSION
The recent cloning of a cluster of genes for lolitrem biosynthesis has allowed us to determine the ltm gene profiles of 44 epichloë endophytes, representing 22 different species, and deduce their indole-diterpene biosynthetic capability. The PCR-based gene analysis allowed us to group these different strains into three main classes: (i) lolitrem producers that have all 10 genes; (ii) indole-diterpene producers that are missing ltmE and ltmJ (of cluster 3) but have at least the core set of genes ltmG, ltmM, ltmC, and ltmB and thus are able to make paspaline and other intermediates; and (iii) indole-diterpene nonproducers without any ltm genes or without the full complement of the four core genes. The majority of the sexual isolates (24 of 28) were predicted to be nonproducers consistent with the absence of the ltm genes from the isolates tested. The presence of remnant ltm sequences in some of these isolates (e.g., E799) suggests that an ancestor of these strains once had the ability to synthesize indole-diterpenes but presumably lost the sequences because they no longer provided an adaptive advantage. Therefore, the inability of these strains to produce lolitrem B is attributed to the absence of the genes rather than mutations within the genes. A similar observation has been made for E. festucae strain E189, a non-loline producer that lacks the genes required for loline production (25). Loss of secondary metabolite production has been identified in other groups of closely related fungi, such as aflatoxin in Aspergillus species (8, 67), and gibberellin (3, 33, 35) and fumonisin (21, 54) biosynthesis in Fusarium (teleomorph Gibberella spp.) species. Analysis of secondary metabolite gene clusters at the gibberellin and fumonisin loci in closely related Fusarium species and for aflatoxin biosynthesis in Aspergillus spp. revealed examples of partial or absent gene clusters (3, 8, 21, 35, 54, 67). However, in some cases, a lack of secondary metabolite production correlated with little or no gene expression and/or sequence variations within the coding and promoter regions of genes within the cluster, which can result in naturally occurring pathway variants (21, 33-35, 53). The LTM locus from the Epichloë and Neotyphodium species we screened contain examples of gene absence (partial or complete cluster) and in three cases, sequence variation in a gene (ltmF and ltmE) that blocks or alters the encoded biosynthetic step.
The members of the E. festucae clade were represented in all three classes of ltm gene distribution, where only some isolates (five of eight) contained ltm genes, and of those with ltm genes, only four isolates had the four core genes responsible for paspaline production. The ltm gene profile for these E. festucae isolates was predicted by PCR and supported by Southern analysis. In turn, the ability of seven E. festucae to produce indole-diterpenes in planta was confirmed by HPLC and LC-MS/MS. Three E. festucae isolates—Fr1, Frr1, and Frc5—lack ltm genes and did not produce indole-diterpenes when in symbiosis with perennial ryegrass. Of the remaining E. festucae isolates, only Fg1 and Fl1 contained the 10 ltm genes, while E2368, Frc7, and E189 contained 5, 8, and 9 genes, respectively. The alkaloid analysis for Fg1 and E189 was inconsistent with predictions from the ltm gene profile, as determined by PCR. Strain Fg1 appears to contain all 10 ltm genes, and yet in planta it produced lolitrems J and K but not lolitrem B (Table 4). This result was subsequently explained based on the presence of a frameshift in ltmF, resulting in a nonfunctional gene. PCR analysis of E189 detected nine ltm genes and did not detect ltmE (encoding a multifunctional prenyl transferase with similarity to LtmC and LtmF at the N- and C-terminal domains, respectively), and yet this strain was still able to produce lolitrem B in planta, albeit at a consistently lower level than did Fl1. However, Fl1 and E189 had different indole-diterpene profiles where in E189-infected plant material only trace amounts of lolitrem N and J were detected, and lolitrem K was not detected at all. Southern analysis determined that at least a partial copy of ltmE is present in E189 (data not shown), but this result does not indicate whether one or both prenyl transferase domains are complete and functional. Sequence analysis of ltmE from E189 and complementation analysis with each ltmE domain in a deletion background will determine whether just one domain is required for lolitrem B production. We also cannot discount the influence that the host genotype has on the alkaloid profile of E189-infected material.
E. festucae is a progenitor to many asexual species (see Table 1) (11, 20, 44), so greater variation of the ltm gene distribution was expected among the Neotyphodium hybrids, which would reflect the ltm amplification patterns identified from E. festucae. Analysis of the eight E. festucae isolates resulted in five different ltm PCR profiles (0, 5, 8, 9, and 10 ltm genes). Of the 16 Neotyphodium endophytes screened by PCR, 12 have an E. festucae ancestor and, of these, 11 contained ltm sequences. N. lolii Lp19 contained all 10 genes, nine isolates were positive for 8 genes, and just ltmP was amplified from N. coenophialum e19 (a hybrid with ancestry from E. festucae, Lolium-associated clade and E. typhina complex). Only three Neotyphodium isolates—N. australiense (e938; Efe x ETC), Neotyphodium isolate Hd1, and N. gansuense var. inebrians (e818)—did not yield amplification products with the ltm primers and, of these, only e938 has E. festucae ancestry (42). Although these data could suggest that a common, predominantly lolitrem producing E. festucae ancestry was involved with the hybridization events that produced Neotyphodium spp. with E. festucae lineage, we must also consider the possibility that there is greater diversity within the Neotyphodium species than tested here. This present study has a strong bias toward endophytes associated with grasses in the genus Lolium and does not present comprehensive analyses from the recently characterized N. tembladerae (also with E. festucae- and E. typhina-like ancestry) identified in host tribes Poeae, Aveneae, Bromeae, and Meliceae in Argentina (20). The two N. tembladerae isolates used in this study, e1169 from Poa huecu (from Argentina) and e4055 from Festuca arizonica (from Arizona), have eight ltm genes and were both isolated from grasses in the Poeae tribe. N. australiense (e938) and N. aotearoae (e899) isolated from Echinopogon ovatus of the tribe Aveneae provided examples of variation in ltm gene composition with respect to their phylogenetic origins, with no evidence for ltm genes in e938 (which has E. festucae ancestry) but at least four—ltmM, ltmG, ltmB, and ltmF—in e899, which resides on a distinct and deeply rooted clade within the epichloë phylogeny (42, 44). In a previous study, alkaloid analysis of an endophyte-infected Echinopogon ovatus from New Zealand did not indicate the presence of lolitrem B by HPLC, but analogs of paxilline were detected by enzyme-linked immunosorbent assay (39). The small quantity of DNA that was isolated from e899, due to its slow growth rate, was not enough to include in Southern analysis to determine whether an ltmC orthologue could be detected, which would provide support that a paxilline analogue could be made by this isolate.
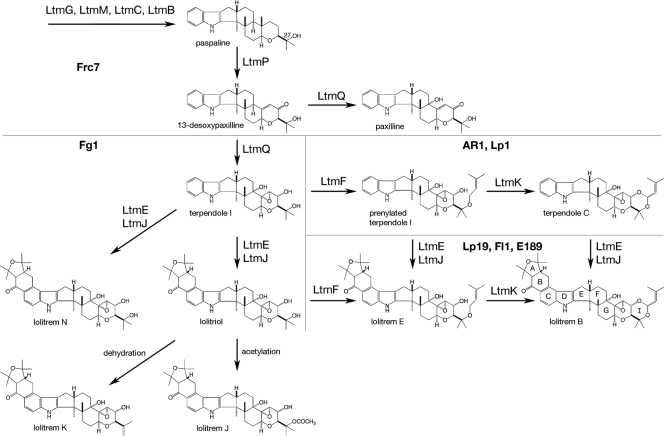
The biosynthetic pathway for lolitrem is predicted to act as a metabolic grid rather than a linear pathway where the A and B rings form independently of the I ring (see the lolitrem B structure in Fig. 6) (19, 46, 48, 49, 69). By comparing the lolitrem intermediates identified in Fl1, Fg1, Frc7, AR1, and Lp1 to the corresponding gene profiles of these strains, we can begin to better define the function of the gene products in the biosynthetic pathway (Fig. 6). However, since the biochemical pathway is so complex, the functions of each gene product required for lolitrem biosynthesis will be confirmed by gene disruption and chemical analysis of the resulting mutants (D. Takemoto and B. Scott, unpublished data). In agreement with the chemotype predicted from the gene composition of the strains Fl1, Fg1, Frc7, E189, AR1, and Lp1, these strains were able to produce at least paspaline and 13-desoxypaxilline. This is consistent with the genes ltmG, ltmM, ltmB, ltmC, and ltmP from Fl1, Fg1, Frc7, E189, AR1, and Lp1 encoding functional gene products. Although paxilline was detected in Lp1, AR1, Frc7, and Fg1, it was absent from Fl1- and E189-infected plant material. Our proposed lolitrem pathway (Fig. 6) indicates that a minor pathway route for the production of paxilline is present for some isolates; thus, we speculate that paxilline is formed due to a diversion of intermediates in isolates that do not contain the complete complement of ltm genes. AR1 and Lp1 lack genes, ltmE and ltmJ, found in ltm cluster 3 but are both able to produce terpendole C that is prenylated to give rise to the I ring but lacks the A and B rings found in lolitrem B (Fig. 6 and Table 4). These observations are consistent with the roles of LtmJ and LtmE catalyzing the formation of the A and B ring structures and roles for LtmF and LtmK in the formation of the I ring structure (Fig. 6). Analysis of Fg1 by PCR indicated the presence of 10 ltm genes, and yet this isolate is not able to produce terpendole C or lolitrem B in planta. Instead, lolitriol and putative lolitrem K and J were detected in Fg1-infected plant material, indicating that gene products for formation of the A and B rings are present and functional but that those required for formation of the I ring are not. Analysis of the ltmF promoter and coding region in Fg1 indicated that this isolate did not contain a functional ltmF gene. These data provide further support that LtmE and LtmJ are likely candidates for the formation of the A and B rings (Fig. 6). Lolitrem J is an acetylated product, accumulating in the absence of isoprenylation required for the formation of an I ring, via action of a further oxidation step. Frc7 lacks ltmE and ltmJ and would be predicted to have a similar indole-diterpene profile to AR1 and Lp1 with the production of terpendole C. However, unlike AR1 and Lp1, Frc7 is unable to produce the terpendole metabolites due to the same mutation in ltmF that was identified in Fg1.
FIG. 6.
Proposed framework for indole-diterpene biosynthesis in epichloë endophytes. The scheme proposed is a working model to explain the chemical diversity of indole-diterpenes found in different epichloë isolates. The identities of the rings in the lolitrem B structure are denoted by letters. Single arrows do not necessarily denote single enzymatic steps.
This research has established, by PCR and LC-MS/MS analysis, that agriculturally important endophytes such as AR1 that have been reported to lack lolitrems (16) have the machinery and ability to produce other indole-diterpenes, such as paspaline, 13-desoxypaxilline, and terpendole C. Paxilline and terpendole C are tremorgenic in mice, but the tremors they induce do not last as long as those caused by lolitrems A, B, and F (48). Field trials with AR1-infected perennial ryegrass showed improved animal productivity over common toxic endophyte (lolitrem B producer)-infected perennial ryegrass, with no evidence of animal toxicity such as ryegrass staggers (1, 2). Indole-diterpene precursors of lolitrem B could provide a selective advantage to the grass that is not related to animal toxicity but may involve protection against insects, such as that found with the nontremorgenic indole-diteprene nodulisporic acid from Nodulisporium species (7).
The LTM loci within E. festucae Fl1 and N. lolii Lp19 are very complex in that the ltm genes are present on three clusters separated by AT-rich remnants of transposable elements that appear to have undergone a repeat-induced point mutation-like mechanism (74, 75). The majority of Epichloë species tested lacked both ltm genes and the transposable elements Rua and Tahi found associated with the LTM locus identified in N. lolii and E. festucae (74, 75). The pks pseudogene found adjacent to cluster 1 of Fl1 was present in some Epichloë species. However, some E. festucae isolates that lacked the ltm genes contained these retrotransposons, indicating that the LTM locus and the retroelements had a separate evolutionary origin. The discontinuous distribution of the ltm genes within epichloë endophytes suggested that a common ancestor has contributed to the evolution of this cluster, as was established within ascomycetes with respect to polyketide synthases (24) and recently proposed with the nonribosomal peptide synthetase genes among epichloë endophytes (23). The Tahi and Rua transposable elements are present in high copy numbers within the E. festucae genome and within Neotyphodium species with an E. festucae progenitor. Both elements are also present in E. baconii, a close relative of E. festucae (11), but absent from other sexual species such as E. amarillans. Analyses show that E. amarillans, E. baconii, and E. festucae cluster together in a well-supported clade within the epichloë phylogeny (11, 44, 45); thus, these data could suggest that the retrotransposon elements, Tahi and Rua, invaded Epichloë after E. amarillans diverged but before the split of E. baconii and E. festucae. However, the rapid evolution of these elements may preclude detection by hybridization in the more distant relatives. The presence of other repetitive elements, such as MITEs, at the LOL locus of N. uncinatum, a hybrid of E. bromicola and E. typhina origin, suggests that transposons may be widespread in epichloë endophytes (15).
The data presented here show that many epichloë endophytes are unable to produce the neurotoxin lolitrem B likely due to a lack of the required biochemical machinery. There is evidence that Epichloë ancestors possessed the ltm gene clusters and that these have been lost over time, a situation similar to that proposed for the LOL locus required for loline production (25). PCR profiling of the ltm genes can be utilized as a tool to predict the indole-diterpene biosynthetic capability of an isolate before it is inoculated into a pasture grass. Additional DNA sequences from epichloë endophytes will allow for further refinement of primer sequences, thereby making the PCR screen even more robust. This technique can provide an advantage over current chemotyping methods that rely solely on the chemical analysis of an expected compound. Although amplification of an ltm gene does not necessarily predict synthesis of a functional gene product in the host grass, the absence of a gene or set of genes provides a robust method for predicting alkaloid compounds in pasture grasses that is independent of host genotype and wound-inducible responses, thereby providing a more targeted approach to chemotyping endophyte-grass associations. Extension of this technique to the other alkaloid biosynthesis genes such as those required for the synthesis of the beneficial lolines and peramine, and detrimental ergot alkaloids will provide the plant breeding industry with a “toolkit” to identify epichloë endophytes of agronomic importance.
Acknowledgments
This research was funded by grants from the New Zealand Foundation for Research, Science, and Technology (C10X0203); the Tertiary Education Commission (National Centre for BioProtection Centre of Excellence); and the U.S. Department of Agriculture (National Research Initiative grant 2005-35319-16141). C.A.Y. was a recipient of FRST Bright Futures Scholarship and supported by the National Centre for BioProtection Centre of Excellence.
We thank Andrea Bryant (Massey University), Karl Fraser (AgResearch), and Shipra Mittal and Kirsty Burr (Noble Foundation) for technical assistance and Michael Christensen and Garrick Latch (AgResearch) for culture information. We thank Damien Fleetwood (AgResearch) and Emily Parker (University of Canterbury) for helpful discussions regarding transposable elements and indole-diterpene biosynthesis, respectively.
Footnotes
Published ahead of print on 30 January 2009.
REFERENCES
- 1.Bluett, S. J., E. R. Thom, D. A. Clark, and C. D. Waugh. 2005. Effects of a novel ryegrass endophyte on pasture production, dairy cow milk production and calf liveweight gain. Aust. J. Exp. Agric. 45:11-19. [Google Scholar]
- 2.Bluett, S. J., E. R. Thom, D. A. Clark, K. A. MacDonald, and E. M. K. Minnee. 2005. Effects of perennial ryegrass infected with either AR1 or wild endophyte on dairy production in the Waikato. N. Z. J. Agric. Res. 48:197-212. [Google Scholar]
- 3.Bömke, C., M. C. Rojas, P. Hedden, and B. Tudzynski. 2008. Loss of gibberellin production is due to a deletion in the gibberellic acid gene cluster in Fusarium verticillioides (G. fujikuroi MP-A). Appl. Environ. Microbiol. 74:7790-7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouton, J. H., Latch, G. C. M., N. S. Hill, C. S. Hoveland, M. A. McCann, R. H. Watson, J. A. Parish, L. L. Hawkins, and F. N. Thompson. 2002. Reinfection of tall fescue cultivars with non-ergot alkaloid-producing endophytes. Agron. J. 94:567-574. [Google Scholar]
- 5.Bush, L. P., H. H. Wilkinson, and C. L. Schardl. 1997. Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiol. 114:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrd, A. D., C. L. Schardl, P. J. Songlin, K. L. Mogen, and M. R. Siegel. 1990. The β-tubulin gene of Epichloë typhina from perennial ryegrass (Lolium perenne). Curr. Genet. 18:347-354. [DOI] [PubMed] [Google Scholar]
- 7.Byrne, K. M., S. K. Smith, and J. G. Ondeyka. 2002. Biosynthesis of nodulisporic acid A: precursor studies. J. Am. Chem. Soc. 124:7055-7060. [DOI] [PubMed] [Google Scholar]
- 8.Chang, P. K., B. W. Horn, and J. W. Dorner. 2005. Sequence breakpoints in the aflatoxin biosynthesis gene cluster and flanking regions in nonaflatoxigenic Aspergillus flavus isolates. Fungal Genet. Biol. 42:914-923. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, M. J., A. Leuchtmann, D. D. Rowan, and B. A. Tapper. 1993. Taxonomy of Acremonium endophytes of tall fescue (Festuca arundinacea), meadow fescue (F. pratensis) and perennial rye-grass (Lolium perenne). Mycol. Res. 97:1083-1092. [Google Scholar]
- 10.Clay, K., and C. Schardl. 2002. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 160:S99-S127. [DOI] [PubMed] [Google Scholar]
- 11.Craven, K. D., P. T. W. Hsiau, A. Leuchtmann, W. Hollin, and C. L. Schardl. 2001. Multigene phylogeny of Epichloë species, fungal symbionts of grasses. Ann. Mo. Bot. Gard. 88:14-34. [Google Scholar]
- 12.Easton, H. S., G. C. M. Latch, B. A. Tapper, and O. J.-P. Ball. 2002. Ryegrass host genetic control of concentrations of endophyte-derived alkaloids. Crop Sci. 42:51-57. [DOI] [PubMed] [Google Scholar]
- 13.Easton, H. S., T. B. Lyons, W. J. Mace, W. R. Simpson, A. C. M. De Bonth, B. M. Cooper, and K. A. Panckhurst. 2007. Differential expression of loline alkaloids in perennial ryegrass infected with endophyte isolated from tall fescue, p. 163-165. In A. J. Popay and E. R. Thom (ed.), Proceedings of the 6th International Symposium on Fungal Endophytes of Grasses. New Zealand Grassland Association, Dunedin, New Zealand.
- 14.Fleetwood, D. J. 2007. Molecular characterisationof the EAS gene cluster for ergot alkaloid biosynthesis in epichloë endophytes of grasses. Ph.D. thesis. Massey University, Palmerston North, New Zealand.
- 15.Fleetwood, D. J., B. Scott, G. A. Lane, A. Tanaka, and R. D. Johnson. 2007. A complex ergovaline gene cluster in epichloë endophytes of grasses. Appl. Environ. Microbiol. 73:2571-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher, L. R. 1999. “Non-toxic” endophytes in ryegrass and their effect on livestock health and production, p. 133-139. In D. R. Woodfield and C. Matthew (ed.), Ryegrass endophyte: an essential New Zealand symbiosis. New Zealand Grassland Association, Napier, New Zealand.
- 17.Gallagher, R. T., E. P. White, and P. H. Mortimer. 1981. Ryegrass staggers: isolation of potent neurotoxins lolitrem A and lolitrem B from staggers-producing pastures. N. Z. Vet. J. 29:189-190. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher, R. T., A. D. Hawkes, P. S. Steyn, and R. Vleggaar. 1984. Tremorgenic neurotoxins from perennial ryegrass causing ryegrass staggers disorder of livestock: structure elucidation of lolitrem B. J. Chem. Soc. Chem. Commun. 9:614-616.
- 19.Gatenby, W. A., S. C. Munday-Finch, A. L. Wilkins, and C. O. Miles. 1999. Terpendole M, a novel indole-diterpenoid isolated from Lolium perenne infected with the endophytic fungus Neotyphodium lolii. J. Agric. Food Chem. 47:1092-1097. [DOI] [PubMed] [Google Scholar]
- 20.Gentile, A., M. S. Rossi, D. Cabral, K. D. Craven, and C. L. Schardl. 2005. Origin, divergence, and phylogeny of epichloë endophytes of native Argentine grasses. Mol. Phylogenet. Evol. 35:196-208. [DOI] [PubMed] [Google Scholar]
- 21.Glenn, A. E., N. C. Zitomer, A. M. Zimeri, L. D. Williams, R. T. Riley, and R. H. Proctor. 2008. Transformation-mediated complementation of a FUM gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings. Mol. Plant-Microbe Interact. 21:87-97. [DOI] [PubMed] [Google Scholar]
- 22.Gonthier, D., J. T. Sullivan, J. K. Brown, L. B. Wurtzel, R. Lawal, K. VandenOever, Z. Buchan, and T. L. Bultman. 2008. Stroma-forming endophyte Epichloe glyceriae provides wound-inducible herbivore resistance to its grass host. Oikos 117:629-633. [Google Scholar]
- 23.Johnson, R., C. Voisey, L. Johnson, J. Pratt, D. Fleetwood, A. Khan, and G. Bryan. 2007. Distribution of NRPS gene families within the Neotyphodium/Epichloë complex. Fungal Genet. Biol. 44:1180-1190. [DOI] [PubMed] [Google Scholar]
- 24.Kroken, S., N. L. Glass, J. W. Taylor, O. C. Yoder, and B. G. Turgeon. 2003. Phylogenomic analysis of type I polyketide synthase genes in pathogenic and saprobic ascomycetes. Proc. Natl. Acad. Sci. USA 100:15672-15675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kutil, B. L., C. Greenwald, G. Liu, M. J. Spiering, C. L. Schardl, and H. H. Wilkinson. 2007. Comparison of loline alkaloid gene clusters across fungal endophytes: predicting the co-regulatory sequence motifs and the evolutionary history. Fungal Genet. Biol. 44:1002-1010. [DOI] [PubMed] [Google Scholar]
- 26.Latch, G. C. M., M. J. Christensen, B. A. Tapper, H. S. Easton, D. E. Hume, and L. R. Fletcher. June 2000. Ryegrass endophytes. U.S. patent 6,072,107.
- 27.Latch, G. C. M., M. J. Christensen, B. A. Tapper, H. S. Easton, D. E. Hume, and L. R. Fletcher. August 2000. Tall fescue endophytes. U.S. patent 6,111,170.
- 28.Leuchtmann, A. 1994. Isozyme relationships of Acremonium endophytes from twelve Festuca species. Mycol. Res. 98:25-33. [Google Scholar]
- 29.Leuchtmann, A., and C. L. Schardl. 1998. Mating compatibility and phylogenetic relationships among two new species of Epichloë and other congeneric European species. Mycol. Res. 102:1169-1182. [Google Scholar]
- 30.Leuchtmann, A., D. Schmidt, and L. P. Bush. 2000. Different levels of protective alkaloids in grasses with stroma-forming and seed-transmitted Epichloë/Neotyphodium endophytes. J. Chem. Ecol. 26:1025-1036. [Google Scholar]
- 31.Lyons, P. C., R. D. Plattner, and C. W. Bacon. 1986. Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science 232:487-489. [DOI] [PubMed] [Google Scholar]
- 32.Malinowski, D. P., and D. P. Belesky. 2000. Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci. 40:923-940. [Google Scholar]
- 33.Malonek, S., M. C. Rojas, P. Hedden, P. Hopkins, and B. Tudzynski. 2005. Restoration of gibberellin production in Fusarium proliferatum by functional complementation of enzymatic blocks. Appl. Environ. Microbiol. 71:6014-6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malonek, S., M. C. Rojas, P. Hedden, P. Gaskin, P. Hopkins, and B. Tudzynski. 2005. Functional characterization of two cytochrome P450 monooxygenase genes, P450-1 and P450-4, of the gibberellic acid gene cluster in Fusarium proliferatum (Gibberella fujikuroi MP-D). Appl. Environ. Microbiol. 71:1462-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malonek, S., C. Bomke, E. Bornberg-Bauer, M. C. Rojas, P. Hedden, P. Hopkins, and B. Tudzynski. 2005. Distribution of gibberellin biosynthetic genes and gibberellin production in the Gibberella fujikuroi species complex. Phytochemistry 66:1296-1311. [DOI] [PubMed] [Google Scholar]
- 36.Maniatis, T., J. Sambrook, and E. F. Fritsch. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Press, Cold Spring Harbor, NY.
- 37.Miles, C. O., S. C. Munday, A. L. Wilkins, R. M. Ede, and N. R. Towers. 1994. Large-scale isolation of lolitrem B and structure determination of lolitrem E. J. Agric. Food Chem. 42:1488-1492. [Google Scholar]
- 38.Miles, C. O., A. L. Wilkins, R. T. Gallagher, A. D. Hawkes, S. C. Munday, and N. R. Towers. 1992. Synthesis and tremorgenicity of paxitrols and lolitriol: possible biosynthetic precursors of lolitrem B. J. Agric. Food Chem. 40:234-238. [Google Scholar]
- 39.Miles, C. O., M. E. di Menna, S. W. L. Jacobs, I. Garthwaite, G. A. Lane, R. A. Prestidge, S. L. Marshall, H. H. Wilkinson, C. L. Schardl, O. J.-P. Ball, and G. C. M. Latch. 1998. Endophytic fungi in indigenous Australasian grasses associated with toxicity to livestock. Appl. Environ. Microbiol. 64:601-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miles, C. O., G. A. Lane, M. E. D. Menna, I. Garthwaite, E. L. Piper, O. J. Ball, G. C. M. Latch, J. M. Allen, M. B. Hunt, L. P. Bush, F. K. Min, I. Fletcher, and P. S. Harris. 1996. High levels of ergonovine and lysergic acid amide in toxic Achnatherum inebrians accompany infection by an Acremonium-like endophytic fungus. J. Agric. Food Chem. 44:1285-1290. [Google Scholar]
- 41.Moon, C. D., B. A. Tapper, and B. Scott. 1999. Identification of Epichloë endophytes in planta by a microsatellite-based PCR fingerprinting assay with automated analysis. Appl. Environ. Microbiol. 65:1268-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moon, C. D., C. O. Miles, U. Jarlfors, and C. L. Schardl. 2002. The evolutionary origins of three new Neotyphodium endophyte species from grasses indigenous to the southern hemisphere. Mycologia 94:694-711. [DOI] [PubMed] [Google Scholar]
- 43.Moon, C. D., B. Scott, C. L. Schardl, and M. J. Christensen. 2000. The evolutionary origins of Epichloë endophytes from annual ryegrasses. Mycologia 92:1103-1118. [Google Scholar]
- 44.Moon, C. D., K. D. Craven, A. Leuchtmann, S. L. Clement, and C. L. Schardl. 2004. Prevalence of interspecific hybrids amongst asexual fungal endophytes of grasses. Mol. Ecol. 13:1455-1467. [DOI] [PubMed] [Google Scholar]
- 45.Moon, C. D., J.-J. Guillaumin, C. Ravel, C. Li, K. D. Craven, and C. L. Schardl. 2007. New Neotyphodium endophyte species from the grass tribes Stipeae and Meliceae. Mycologia 99:895-904. [DOI] [PubMed] [Google Scholar]
- 46.Munday-Finch, S. C., A. L. Wilkins, and C. O. Miles. 1998. Isolation of lolicine A, lolicine B, lolitriol, and lolitrem N from Lolium perenne infected with Neotyphodium lolii and evidence for the natural occurrence of 31-epilolitrem N and 31-epilolitrem F. J. Agric. Food Chem. 46:590-598. [DOI] [PubMed] [Google Scholar]
- 47.Munday-Finch, S. C., C. O. Miles, A. L. Wilkins, and A. D. Hawkes. 1995. Isolation and structure elucidation of lolitrem A, a tremorgenic mycotoxin from perennial ryegrass infected with Acremonium lolii. J. Agric. Food Chem. 43:1283-1288. [Google Scholar]
- 48.Munday-Finch, S. C., A. L. Wilkins, C. O. Miles, H. Tomoda, and S. Omura. 1997. Isolation and structure elucidation of lolilline, a possible biosynthetic precursor of the lolitrem family of tremorgenic mycotoxins. J. Agric. Food Chem. 45:199-204. [Google Scholar]
- 49.Munday-Finch, S. C., A. L. Wilkins, C. O. Miles, R. M. Ede, and R. A. Thomson. 1996. Structure elucidation of lolitrem F, a naturally occurring stereoisomer of the tremorgenic mycotoxin lolitrem B, isolated from Lolium perenne infected with Acremonium lolii. J. Agric. Food Chem. 44:2782-2788. [Google Scholar]
- 50.Parish, J. A., M. A. McCann, R. H. Watson, C. S. Hoveland, L. L. Hawkins, N. S. Hill, and J. H. Bouton. 2003. Use of nonergot alkaloid-producing endophytes for alleviating tall fescue toxicosis in sheep. J. Anim. Sci. 81:1316-1322. [DOI] [PubMed] [Google Scholar]
- 51.Parish, J. A., M. A. McCann, R. H. Watson, N. N. Paiva, C. S. Hoveland, A. H. Parks, B. L. Upchurch, N. S. Hill, and J. H. Bouton. 2003. Use of nonergot alkaloid-producing endophytes for alleviating tall fescue toxicosis in stocker cattle. J. Anim. Sci. 81:2856-2868. [DOI] [PubMed] [Google Scholar]
- 52.Porter, J. K. 1995. Analysis of endophyte toxins: fescue and other grasses toxic to livestock. J. Anim. Sci. 73:871-880. [DOI] [PubMed] [Google Scholar]
- 53.Proctor, R. H., R. D. Plattner, A. E. Desjardins, M. Busman, and R. A. Butchko. 2006. Fumonisin production in the maize pathogen Fusarium verticillioides: genetic basis of naturally occurring chemical variation. J. Agric. Food Chem. 54:2424-2430. [DOI] [PubMed] [Google Scholar]
- 54.Proctor, R. H., R. D. Plattner, D. W. Brown, J. A. Seo, and Y. W. Lee. 2004. Discontinuous distribution of fumonisin biosynthetic genes in the Gibberella fujikuroi species complex. Mycol. Res. 108:815-822. [DOI] [PubMed] [Google Scholar]
- 55.Rowan, D. D., M. B. Hunt, and D. L. Gaynor. 1986. Peramine, a novel insect feeding deterrent from ryegrass infected with the endophyte Acremonium loliae. J. Chem. Soc. Chem. Commun. 12:935-936. [DOI] [PubMed] [Google Scholar]
- 56.Saikia, S., E. J. Parker, A. Koulman, and B. Scott. 2007. Defining paxilline biosynthesis in Penicillium paxilli: functional characterization of two cytochrome P450 monooxygenases. J. Biol. Chem. 282:16829-16837. [DOI] [PubMed] [Google Scholar]
- 57.Saikia, S., E. J. Parker, A. Koulman, and B. Scott. 2006. Four gene products are required for the fungal synthesis of the indole-diterpene, paspaline. FEBS Lett. 580:1625-1630. [DOI] [PubMed] [Google Scholar]
- 58.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 59.Schardl, C. L., and K. D. Craven. 2003. Interspecific hybrisization in plant-associated fungi and oomycetes: a review. Mol. Ecol. 12:2861-2873. [DOI] [PubMed] [Google Scholar]
- 60.Schardl, C. L., and A. Leuchtmann. 1999. Three new species of Epichloë symbiotic with North American grasses. Mycologia 91:95-107. [Google Scholar]
- 61.Schardl, C. L., A. Leuchtmann, K.-R. Chung, D. Penny, and M. R. Siegel. 1997. Coevolution by common descent of fungal symbionts (Epichloë spp.) and grass hosts. Mol. Biol. Evol. 14:133-143. [Google Scholar]
- 62.Schardl, C. L., A. Leuchtmann, H.-F. Tsai, M. A. Collett, D. M. Watt, and D. B. Scott. 1994. Origin of a fungal symbiont of perennial ryegrass by interspecific hybridization of a mutualist with the ryegrass choke pathogen, Epichloë typhina. Genetics 136:1307-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siegel, M. R., G. C. M. Latch, L. P. Bush, F. F. Fannin, D. D. Rowan, B. A. Tapper, C. W. Bacon, and M. C. Johnson. 1990. Fungal endophyte-infected grasses: alkaloid accumulation and aphid response. J. Chem. Ecol. 16:3301-3315. [DOI] [PubMed] [Google Scholar]
- 64.Sullivan, T. J., J. Rodstrom, J. Vandop, J. Librizzi, C. Graham, C. L. Schardl, and T. L. Bultman. 2007. Symbiont-mediated changes in Lolium arundinaceum inducible defenses: evidence from changes in gene expression and leaf composition. New Phytol. 176:673-679. [DOI] [PubMed] [Google Scholar]
- 65.Tsai, H.-F., M. R. Siegel, and C. L. Schardl. 1992. Transformation of Acremonium coenophialum, a protective fungal symbiont of the grass Festuca arundinacea. Curr. Genet. 22:399-406. [DOI] [PubMed] [Google Scholar]
- 66.Tsai, H.-F., J.-S. Liu, C. Staben, M. J. Christensen, G. C. M. Latch, M. R. Siegel, and C. L. Schardl. 1994. Evolutionary diversification of fungal endophytes of tall fescue grass by hybridization with Epichloë species. Proc. Natl. Acad. Sci. USA 91:2542-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van den Broek, P., A. Pittet, and H. Hajjaj. 2001. Aflatoxin genes and the aflatoxigenic potential of koji moulds. Appl. Environ. Microbiol. 57:192-199. [DOI] [PubMed] [Google Scholar]
- 68.Watson, R. H., M. A. McCann, J. A. Parish, C. S. Hoveland, F. N. Thompson, and J. H. Bouton. 2004. Productivity of cow-calf pairs grazing tall fescue pastures infected with either the wild-type endophyte or a nonergot alkaloid-producing endophyte strain, AR542. J. Anim. Sci. 82:3388-3393. [DOI] [PubMed] [Google Scholar]
- 69.Weedon, C. M., and P. G. Mantle. 1987. Paxilline biosynthesis by Acremonium loliae; a step toward defining the origin of lolitrem neurotoxins. Phytochemistry 26:969-971. [Google Scholar]
- 70.Yoder, O. C. 1988. Cochliobolus heterostrophus, cause of southern corn leaf blight. Adv. Plant Pathol. 6:93-112. [Google Scholar]
- 71.Young, C. A. 2005. The indole-diterpene gene cluster from the ryegrass endophyte, Neotyphodium lolii, is required for the biosynthesis of lolitrem B, a bioprotective alkaloid. Ph.D. thesis. Massey University, Palmerston North, New Zealand.
- 72.Young, C., Y. Itoh, R. Johnson, I. Garthwaite, C. O. Miles, S. C. Munday-Finch, and B. Scott. 1998. Paxilline-negative mutants of Penicillium paxilli generated by heterologous and homologous plasmid integration. Curr. Genet. 33:368-377. [DOI] [PubMed] [Google Scholar]
- 73.Young, C. A., L. McMillan, E. Telfer, and B. Scott. 2001. Molecular cloning and genetic analysis of an indole-diterpene gene cluster from Penicillium paxilli. Mol. Microbiol. 39:754-764. [DOI] [PubMed] [Google Scholar]
- 74.Young, C. A., M. K. Bryant, M. J. Christensen, B. A. Tapper, G. T. Bryan, and B. Scott. 2005. Molecular cloning and genetic analysis of a symbiosis-expressed gene cluster for lolitrem biosynthesis from a mutualistic endophyte of perennial ryegrass. Mol. Genet. Genomics 274:13-29. [DOI] [PubMed] [Google Scholar]
- 75.Young, C. A., S. Felitti, K. Shields, G. Spangenberg, R. D. Johnson, G. T. Bryan, S. Saikia, and B. Scott. 2006. A complex gene cluster for indole-diterpene biosynthesis in the grass endophyte Neotyphodium lolii. Fungal Genet. Biol. 43:679-693. [DOI] [PubMed] [Google Scholar]