Abstract
This study demonstrates the susceptibility of a variety of medically important bacteria to inactivation by 405-nm light from an array of light-emitting diodes (LEDs), without the application of exogenous photosensitizer molecules. Selected bacterial pathogens, all commonly associated with hospital-acquired infections, were exposed to the 405-nm LED array, and the results show that both gram-positive and gram-negative species were successfully inactivated, with the general trend showing gram-positive species to be more susceptible than gram-negative bacteria. Detailed investigation of the bactericidal effect of the blue-light treatment on Staphylococcus aureus suspensions, for a range of different population densities, demonstrated that 405-nm LED array illumination can cause complete inactivation at high population densities: inactivation levels corresponding to a 9-log10 reduction were achieved. The results, which show the inactivation of a wide range of medically important bacteria including methicillin-resistant Staphylococcus aureus, demonstrate that, with further development, narrow-spectrum 405-nm visible-light illumination from an LED source has the potential to provide a novel decontamination method with a wide range of potential applications.
Inactivation of microorganisms using methods involving exposure to light is an area of increasing research interest in part because of the intractable problem of microbial antibiotic resistance. Ultraviolet (UV) light is well established as a light inactivation treatment, inducing effects ranging from DNA damage, primarily as a result of UV absorption by DNA at wavelengths of 240 nm to 280 nm (3), to sublethal damage of DNA repair systems caused by near-UV light (24). The use of UV light, however, has limitations due to its detrimental effects on skin tissue and components of the eye (28). On the other hand, visible-light inactivation, which traditionally requires the addition of photosensitizing molecules and is termed photodynamic inactivation (PDI), has been developed as a treatment for cancer and other medical ailments (11). The rapidly increasing problem of microbial antibiotic resistance has ignited research interest into the use of PDI as an alternative antimicrobial treatment. Numerous in vitro PDI studies have been made involving microbial inactivation, with successful results for bacteria, fungi, yeasts, viruses, and parasites (29, 7, 12, 2, 5). Furthermore, recent work has shown that photosensitization of bacterial cells is independent of the antibiotic resistance spectrum (17).
The focus of the present study, however, concerns the photoinactivation of bacterial cells without the involvement of an applied photosensitizer. Previous studies have shown that exposure to visible light, more specifically, blue-light wavelengths, causes inactivation of certain bacterial species, including Propionobacterium acnes, Helicobacter pylori, and some oral pigmented bacteria (1, 8, 22). This inactivation mechanism, known to be oxygen dependent (6), is thought to be a result of the photoexcitation of naturally occurring endogenous porphyrins, which act as endogenous photosensitizers within the bacterial cells. This porphyrin excitation leads to energy transfer and, ultimately, the production of highly cytotoxic, oxygen-derived species, most notably, singlet oxygen (11, 26).
Previous work has demonstrated that Staphylococcus aureus can be photodynamically inactivated using 400- to 420-nm visible-light, with maximum visible-light inactivation at 405 nm (13, 9, 10, 14), through an oxygen-dependent process (15). This inactivation of S. aureus is thought to be the result of a porphyrin-mediated process similar to that demonstrated with P. acnes. A previous study by Maclean et al. (14) utilized a broad-spectrum xenon white-light source combined with a range of optical filters to identify the sensitivity of S. aureus to wavelengths of light within the visible region. The present study investigates specifically the use of 405-nm light as the inactivating wavelength and also extends the scope of previous studies by producing new information on the sensitivities of a range of bacterial pathogens to the bactericidal effects of 405-nm light.
The inactivating light used in the present study was generated from 405-nm light-emitting diodes (LEDs). Investigations were carried out on the use of the 405-nm light from the LED array source for the inactivation of methicillin-resistant S. aureus (MRSA) and a range of other important nosocomial bacterial pathogens—both gram-positive and gram-negative types—in the absence of any chemical pretreatment. The results are discussed with regard to the possible cellular mechanisms involved in this light-based inactivation and to the potential use of this method for environmental decontamination applications in both clinical and nonclinical environments.
MATERIALS AND METHODS
Bacteria.
Bacterial strains and growth media are listed in Table 1. Test strains were inoculated into 100 ml of broth and cultivated at 37°C under rotary conditions (at 125 rpm). After an 18-h incubation period, the broth was centrifuged at 3,939 × g for 10 min, and the resultant pellet was resuspended in 100 ml of phosphate-buffered saline (PBS). The suspension was then diluted in PBS to the required starting population for experimental use.
TABLE 1.
Microorganisms and associated growth mediaa
| Gram status and microorganism | Source or collection no.b | Culture medium |
|---|---|---|
| Gram-positive bacteria | ||
| Staphylococcus aureus | NCTC 4135 | Nutrient broth/agar |
| MRSA | Clinical isolate 16a, GRI | Nutrient broth/agar |
| Staphylococcus epidermidis | NCTC 11964 | Tryptone soya broth/agar |
| Streptococcus pyogenes | NCTC 8198 | Brain heart infusion broth/agar |
| Enterococcus faecalis | NCTC 00775 | Nutrient broth/agar |
| Clostridium perfringens | ATCC 13124 | Thioglycolate agar |
| Gram-negative bacteria | ||
| Acinetobacter baumannii | NCTC 12156 | Nutrient broth/agar |
| Pseudomonas aeruginosa | NCTC 9009 | Nutrient broth/agar |
| Escherichia coli | NCTC 9001 | Nutrient broth/agar |
| Proteus vulgaris | CN 329 | Nutrient broth/agar |
| Klebsiella pneumoniae | NCTC 9633 | Nutrient broth/agar |
All culture media were manufactured by Oxoid, United Kingdom.
NCTC, National Collection of Type Cultures, Health Protection Agency, Collindale, London, United Kingdom; GRI, Glasgow Royal Infirmary; ATCC, American Type Culture Collection, Manassas, VA; CN, Wellcome Collection of Bacteria, Wellcome Research Laboratories, Kent, United Kingdom.
To meet the anaerobic requirements of Clostridium perfringens, alternative culture and preparation methods were used. C. perfringens was plated onto thioglycolate agar and placed within an anaerobic jar containing an AnaeroGen sachet (Oxoid, United Kingdom) and incubated at 37°C for 18 h. After incubation, cells were washed off the plate and suspended in 9 ml of PBS. This was then diluted to give a starting population of 104 to 105 CFU/ml.
LED light source.
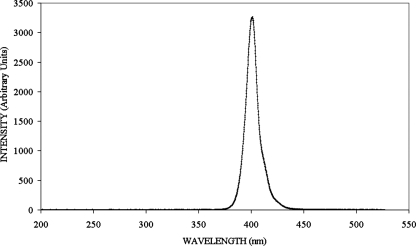
High-intensity 405-nm light was achieved with the LEDs in the form of a close-packed rectangular array of 99 individual LEDs in an 11 by 9 matrix. The light of these 405-nm LEDs is generated in the active region of an InGaN/GaN semiconductor junction. These arrays (OD-405-99-070) are produced by OptoDiode Corp (CA). The emission spectrum of the 405-nm LED array is shown in Fig. 1. It can be seen that the center wavelength for maximum emission is close to 405 nm, and the bandwidth is ∼10 nm at full-width half-maximum. The LED array was bonded to a heat sink and fan to minimize the temperature of the semiconductor junction. The junction temperature was maintained at around 30°C, well below the specified maximum operating temperature of 100°C. This arrangement also ensured that the heat produced by the complete light system was low and had no effect on the test samples exposed to the 405-nm light. The arrangement was mounted in a polyvinyl chloride housing designed to fit on top of a 12-well microplate (without lid), with the LED array positioned directly above a single sample well. The LED array was powered by a direct current supply with the output controllable in the range 0 to 3 A and 0 to 15 V. For all experiments the current was set to 0.5 ± 0.05 A at a voltage of 11.2 ± 0.1 V.
FIG. 1.
Emission spectrum of 99-LED array.
Experimental arrangement.
A 2-ml volume of bacterial suspension, along with a small magnetic follower, was held within one well of a 12-well microplate (the sample dish). The LED array (held in the housing) was then placed on top of the sample dish, with the array in position directly above the sample well. The overlapping edges of the housing ensured that it was held firmly in place. The sample dish and LED were then positioned on a magnetic stirrer which, in conjunction with the magnetic follower, permitted continuous stirring of the sample during light exposure. These 2-ml samples with a depth of 7 mm were exposed to different durations of light treatment and then plated onto the appropriate agar for incubation at 37°C for 24 h (anaerobic incubation was used for C. perfringens samples). Control samples were established; these were continuously agitated but not exposed to 405-nm light.
The distance between the sample and the LED array was approximately 2 cm, and at this distance an irradiance of around 10 mW/cm2 at the surface of the liquid was recorded using a radiant power meter. This procedure was followed for (i) exposure of all bacterial strains used in the study (as in Table 1) and (ii) exposure of S. aureus suspensions at different population densities.
The experimental arrangement was such that no build-up of heat occurred around the light source or was transmitted to the liquid sample. The heat sink and fan bonded to the LED array allowed heat to be easily dissipated, and the LED housing was designed such that it included air vents enabling sufficient ventilation around the light source and sample container, thereby preventing any heat build-up. Temperature measurements of the 2-ml sample volume taken every 30 min throughout the duration of long exposure times (up to a 360-min duration) showed minimal temperature variation, with a mean temperature of 27 ± 1°C.
In order to examine quantitatively the inactivation process, it was necessary to account for any attenuation of the irradiance of the 405-nm light as it passed through a bacterial sample; attenuation is a result of light absorption and scattering. By allowing for attenuation, a mean value for the irradiance through the sample could be determined. Little attenuation in the PBS itself is likely to occur since its optical properties are similar to water or seawater, and the attenuation in these for a wavelength of 405 nm and a depth of 7 mm is less than 0.2% (21). Bacteria suspended in the PBS, on the other hand, will cause some degree of absorption and scattering of the 405-nm light.
Attenuation by the samples used in the study was examined by measuring the irradiance as the light entered the surface of the sample and comparing that value with the irradiance immediately below the sample depth of 7 mm, after allowing for the transmission loss through the base of the sample dish. These measurements showed that for samples containing bacterial populations of 107 CFU/ml and less, no measurable attenuation occurred. For the 109 CFU/ml sample, however, the 10-mW/cm2 irradiance at the sample surface was reduced to 5.6 mW/cm2 after passing through the 7-mm sample depth. Light attenuation through a transparent medium follows an exponential relation referred to as Lambert's law (4), and is written as follows: I(x) = I(0)e−kx, where I(x) and I(0) are the irradiances at the sample surface and at the sample depth x, respectively, and k is the attenuation coefficient. Using the above measurements of irradiance allows k for the 109-CFU/ml sample to be calculated as 0.083 mm−1. The mean value of the irradiance Ī through the sample can now be calculated by applying the integral mean value theorem, as follows (20):
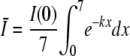 |
Using the data for the 109-CFU/ml sample provides a value for Ī of 7.6 mW/cm2, and this is the value of irradiance used in the analysis of the results for inactivation of the 109-CFU/ml sample.
Statistical analysis.
Each data point on the graphs represents the results from at least two independent experiments, with a minimum of triplicate samples examined for each experiment. These results are documented as mean values with standard deviations included. Significant differences in the results were calculated at the 95% confidence interval using analysis of variance (one way) with Minitab, version 15, statistical software.
RESULTS
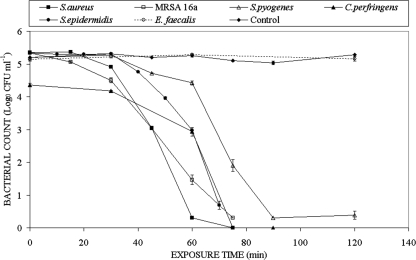
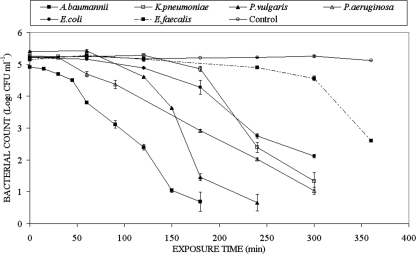
Results for the inactivation of a selection of gram-positive and gram-negative bacterial samples, each with an initial population density 105 CFU/ml, are shown in Fig. 2 and Fig. 3, respectively. The control trend lines in both Fig. 2 and Fig. 3 are averages of the data from all the experiments and demonstrate that the unexposed populations show no significant change throughout the duration of the experiments. In the conventional manner for expression of inactivation data, log10(N/N0) is plotted as a function of exposure time, where N0 and N are the bacterial populations in CFU/ml prior to and following inactivation, respectively. When exposed to 405-nm light, the different gram-positive bacteria behaved in a similar manner, with the exception of Enterococcus faecalis. For the Staphylococcus, Streptococcus, and Clostridium strains, the highest levels of inactivation were recorded, with an approximately 5-log10 reduction in CFU counts observed following exposure between 60 and 90 min to 405-nm light with an irradiance of 10 mW/cm2. Exposure of E. faecalis suspensions, however, to the same irradiance of 10 mW/cm2 for up to 120 min resulted in negligible inactivation. Nevertheless, as shown in Fig. 3, longer exposure times did result in some inactivation of E. faecalis. Significant inactivation, compared to the nonexposed E. faecalis control sample, was demonstrated for a 240-min exposure period, and a 2.6-log10 reduction was recorded following exposure for 360 min.
FIG. 2.
Results of 405-nm light inactivation of MRSA and other medically important gram-positive bacteria at an irradiance of 10 mW cm−2.
FIG. 3.
Results of 405-nm light inactivation of a range of medically important gram-negative bacteria at an irradiance of 10 mW cm−2. Also included is the inactivation curve for the gram-positive bacterium E. faecalis, which shows a significant inactivation effect after a 240-min exposure.
Significant inactivation of the gram-negative bacteria (Fig. 3) was seen following exposure to 405-nm light with an irradiance of 10 mW/cm2. In general, for a given reduction in population, the gram-negative species required longer exposure times than the gram-positive species. For example, a 180-min exposure was required to achieve a 4.2-log10 reduction with Acinetobacter baumannii, and 300-min exposure was required to achieve a 3.1-log10 reduction with Escherichia coli.
The absolute dose in J/cm2, given by the irradiance (W/cm2) times the exposure time (in seconds), and the mean germicidal efficiency, defined as the log10 reduction of a bacterial population [log10(N/N0)] by inactivation per unit dose in J/cm2, for bacterial inactivation was calculated. Details of the inactivation parameters, including germicidal efficiencies, for all bacteria investigated following exposure to the 405-nm light are given in Table 2. S. aureus NCTC 4135 displayed the highest level of inactivation, and the lowest germicidal efficiency was shown by E. faecalis.
TABLE 2.
Energy densities and germicidal efficiencies for the inactivation of a range of bacterial species using narrow-spectrum 405-nm light
| Organisma | Dose (J cm−2) | Log10 reduction | Dose/log10 reduction | GE (% uncertainty)b |
|---|---|---|---|---|
| Staphylococcus aureus | 36 | 5 | 7.2 | 0.14 (±2) |
| MRSA | 45 | 5 | 9 | 0.11 (±0) |
| Staphylococcus epidermidis | 42 | 4.6 | 9.1 | 0.11 (±4) |
| Clostridium perfringens | 45 | 4.4 | 10.2 | 0.10 (±2) |
| Streptococcus pyogenes | 54 | 5 | 10.8 | 0.09 (±5) |
| Acinetobacter baumannii | 108 | 4.2 | 25.7 | 0.04 (±7) |
| Proteus vulgaris | 144 | 4.7 | 30.6 | 0.03 (±5) |
| Pseudomonas aeruginosa | 180 | 4.2 | 42.9 | 0.02 (±4) |
| Klebsiella pneumaniae | 180 | 3.9 | 46.2 | 0.02 (±8) |
| Escherichia coli | 180 | 3.1 | 58.1 | 0.02 (±5) |
| Enterococcus faecalis | 216 | 2.6 | 96 | 0.01 (±0) |
Bacterial species are listed in decreasing order of germicidal efficiency.
GE, germicidal efficiency, calculated as log10(N/N0 per J cm−2). Values including percent uncertainty are for the experimental results in this study (calculated using GE values to three decimal places). A value of 0% uncertainty indicates no differences in the calculated GE values.
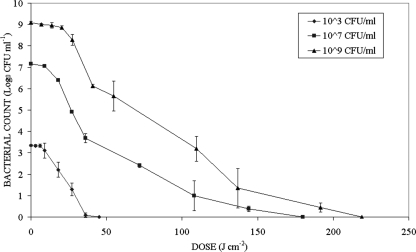
The degree of inactivation as a function of time for S. aureus suspensions with initial population densities of 103, 107, and 109 CFU/ml, following exposure to 405-nm LED light, is shown in Fig. 4. In Fig. 4, the measured population densities are plotted logarithmically as a function of dose rather than exposure time in order to take account of the lower mean irradiance for the 109-CFU/ml sample. The inactivation curves in Fig. 4 demonstrate essentially the same inactivation response as those in Fig. 2 and Fig. 3, irrespective of the different initial population densities. Also in these experiments a sufficient dose was applied to ensure that complete inactivation was achieved for all of the samples. Although the data are not shown, nonexposed control samples showed no significant change throughout these experiments. It is also interesting that to achieve, for example, a 3-log10 reduction, similar doses were required regardless of the initial population density: 36 J/cm2 for 103 and 107 CFU/ml and 41 J/cm2 for 109 CFU/ml. For the 105-CFU/ml population of S. aureus recorded in Fig. 2, the estimated dose for a 3-log10 reduction is 31 J/cm2 (52-min exposure at 10 mW/cm2).
FIG. 4.
Results of 405-nm light inactivation of S. aureus suspensions with different starting populations.
DISCUSSION
This study has successfully demonstrated the bactericidal effect of visible 405-nm light on selected medically important gram-positive and gram-negative bacteria. Experiments were carefully conducted to ensure that the inactivation effects recorded were due only to the direct effects of 405-nm light and not to any indirect temperature effects. The results show, for the range of bacteria tested, that in general gram-positive bacteria require a much lower dose of 405-nm light for inactivation than do gram-negative bacteria. An exception is E. faecalis, which of the bacteria studied was found to be the least susceptible to inactivation by 405-nm light.
The inactivation curves shown in Fig. 2, 3, and 4 have a shape consistent with those obtained in an earlier study (14), where S. aureus was inactivated using visible light of a wavelength greater than 400 nm. Visible-light inactivation—as established for other bacteria such as P. acnes, H. pylori, and some black-pigmented bacteria (1, 8, 22)—has been credited to the photostimulation of endogenous intracellular porphyrins by visible light in the wavelength region of 400 nm to 500 nm and, more specifically, 400 nm to 420 nm in the cases of P. acnes and H. pylori (1, 8). Stimulation of these porphyrins leads to the production of reactive species, predominantly singlet delta oxygen (1O2), which is a well-recognized trigger of cell death (11).
A study by Nitzan et al. (17), which exposed bacteria pretreated with δ-aminolevulinic acid (δ-ALA) to 407- to 420-nm blue light, proposed that the different photoinactivation rates for the various bacteria examined (Staphylococcus, Streptococcus, Bacillus, Escherichia, Acinetobacter, and Aeromonas) were the result of the types of porphyrins produced in the different bacterial cells. They determined the porphyrins produced in a range of gram-positive and gram-negative bacteria upon induction with δ-ALA and found that the predominant porphyrin produced in both S. aureus and Staphylococcus epidermidis was coproporphyrin, whereas there was no predominant porphyrin produced in the gram-negative E. coli, Acinetobacter, and Aeromonas strains. The amount of coproporphyrin produced by the staphylococcal strains was two to three times higher than in the gram-negative strains. Nitzan et al. reported that Streptococcus faecalis, reclassified as E. faecalis (19), did not demonstrate any inactivation for a dose of 100 J/cm2. This is in agreement with the present results (Fig. 2 and 3), where inactivation of E. faecalis was observed only for doses above this value.
In the present study, where no photosensitizing agent was used, the high level of photoinactivation of staphylococcal strains is also likely due to the presence of high levels of coproporphyrin, photosensitized through blue-light illumination. Two other results lend support to this conclusion. One is that P. acnes, which is readily inactivated by visible light through photostimulation of endogenous porphyrins, also contains high levels of coproporphyrin (16), and the other is that S. aureus inactivation through exposure to visible light has been found to be an oxygen-dependent process (15). Some of the variation in germicidal efficiency between bacteria (Fig. 3) may follow from the use of a source of narrow spectral distribution. Because different bacteria produce different porphyrins and because the peak absorption wavelengths of these porphyrins may differ, different wavelengths may be required for their optimum photostimulation. It must also be noted that the bacteria used in the present study were produced under laboratory cultivation conditions and that the levels of cellular constituents such as porphyrins as well as the physiological status of the cells may differ considerably from bacteria that are found in the natural environment, such as those that have been shed from a mammalian host.
The general result from the present study, that visible-light inactivation of gram-negative bacteria is much less efficient than the inactivation of gram-positive bacteria, agrees with results obtained by several other workers in studies involving bacteria pretreated with δ-ALA (17, 18, 25, 23). These studies with δ-ALA used a range of light sources including blue light, 630-nm laser light, and white light.
The results of the present study, compared to the similar results of previous studies, add strength to the probability that the inactivation of the bacteria used in this study through exposure to 405-nm light is the result of the photostimulation of endogenous porphyrin molecules. However, a limitation of the current study is that this aspect was not specifically investigated, and further work is required to fully elucidate the exact role of porphyrins in the inactivation process resulting from exposure to 405-nm irradiation. In addition to this, we are currently developing a mathematical model for inactivation through exposure to 405-nm light, based on the inactivation kinetics of the microorganisms used in the present study.
Visible-light inactivation of bacteria is a much less efficient process than is ultraviolet light inactivation. For example, Wang et al. (27) examined the UV inactivation of E. coli as a function of wavelength and calculated a maximum germicidal efficiency of 430 log10 per J/cm2 at a wavelength of 270 nm. This compares with the highest value of germicidal efficiency found in the present work with 405-nm blue light (for a 105-CFU/ml population density of S. aureus) of 0.14 log10 per J/cm2. In spite of the three orders of magnitude difference in germicidal efficiency, visible-light inactivation has clear advantages in terms of its ease of use and substantially greater safety. In addition to safety benefits, prolonged exposure of materials to 405-nm visible light would not induce the problematic levels of photodegradation that are associated with similar periods of exposure to UV light, particularly in the UV-C region of around 254 to 260 nm typically used with germicidal UV lamps.
A method that can inactivate a wide range of medically important microorganisms, including MRSA, using exposure to visible light and no requirement for pretreatment has potential for widespread application. Further development of this 405-nm narrow-spectrum illumination method could lead to potential applications as decontamination systems for air, contact surfaces, and medical instruments within the clinical environment. Due to visible light's much greater operational safety than UV light, an attractive potential method of environmental decontamination could involve the continuous irradiation of clinical areas, in the presence of patients and staff, with light of the appropriate photodynamic wavelengths in order to contribute to the control of infections in hospitals and other clinical environments. The broad spectrum of the bactericidal effect of the 405-nm-centered wavelengths against a wide range of bacteria also suggests other possible applications such as providing additional measures for controlling food-borne pathogens and food spoilage bacteria as well as for possible applications in water treatment and disinfection industries.
Although the present study has substantially extended the range of bacteria that have been shown to be susceptible to 405-nm visible light, much more work is required to establish if this type of light sensitivity is a widespread phenomenon among diverse types of bacteria. The sensitivity of Mycobacterium spp. would, for example, be of considerable interest due to the medical importance and environmental survival and persistence characteristics of these organisms. It would also be of interest to determine whether types of bacteria that are found in environments that are normally exposed to natural illumination also show similar levels of sensitivity to irradiation around 405 nm or if these types have developed protective features or mechanisms to counteract the inactivating effects of these visible-light wavelengths.
Acknowledgments
We thank The Robertson Trust for their funding support. M.M. thanks the Engineering and Physical Sciences Research Council for their support through a Doctoral Training Grant (awarded 2002 to 2005).
Footnotes
Published ahead of print on 6 February 2009.
REFERENCES
- 1.Ashkenazi, H., Z. Malik, Y. Harth, and Y. Nitzan. 2003. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol. Med. Microbiol. 35:17-24. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann, B., J. Knüver-Hopf, B. Lambrecht, and H. Mohr. 1995. Target structures for HIV-1 inactivation by methylene blue and light. J. Med. Virol. 47:172-178. [DOI] [PubMed] [Google Scholar]
- 3.Blatchley, E. R., III, and M. M. Peel. 1991. Disinfection by ultraviolet irradiation, p. 823-851. In S. S. Block (ed.), Disinfection, sterilisation and preservation, 4th ed. Lea and Febiger, Philadelphia, PA.
- 4.Born, M., and E. Wolf. 1999. Principles of optics. Cambridge University Press, Cambridge, United Kingdom.
- 5.Ferro, S., O. Coppellotti, G. Roncucci, T. B. Amor, and G. Jori. 2006. Photosensitized inactivation of Acanthamoeba palestinensis in the cystic stage. J. Appl. Microbiol. 101:206-212. [DOI] [PubMed] [Google Scholar]
- 6.Feuerstein, O., I. Ginsburg, E. Dayan, D. Veler, and E. I. Weiss. 2005. Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem. Photobiol. 81:1186-1189. [DOI] [PubMed] [Google Scholar]
- 7.Friedberg, J. S., C. Skema, E. D. Baum, J. Burdick, S. A. Vinogradov, D. F. Wilson, A. D. Horan, and I. Nachamkin. 2001. In vitro effects of photodynamic therapy on Aspergillus fumigatus. J. Antimicrob. Chemother. 48:105-107. [DOI] [PubMed] [Google Scholar]
- 8.Ganz, R. A., J. Viveiros, A. Ahmad, A. Ahmadi, A. Khalil, M. J. Tolkoff, N. S. Nishioka, and M. R. Hamblin. 2005. Helicobacter pylori in patients can be killed by visible light. Laser Surg. Med. 36:260-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guffey, J. S., and J. Wilborn. 2006. Effects of combined 405-nm and 880-nm light on Staphylococcus aureus and Pseudomonas aeruginosa in vitro. Photomed. Laser Surg. 24:680-683. [DOI] [PubMed] [Google Scholar]
- 10.Guffey, J. S., and J. Wilborn. 2006. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed. Laser Surg. 24:684-688. [DOI] [PubMed] [Google Scholar]
- 11.Hamblin, M. R., and T. Hasan. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 3:436-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambrechts, S. A. G., M. C. G. Aalders, and J. van Marle. 2005. Mechanistic study of the photodynamic inactivation of Candida albicans by a cationic porphyrin. Antimicrob. Agents Chemother. 49:2026-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maclean, M. 2006. An investigation into the light inactivation of medically important microorganisms. Ph.D. thesis. University of Strathclyde, Glasgow, Scotland, United Kingdom.
- 14.Maclean, M., S. J. MacGregor, J. G. Anderson, and G. Woolsey. 2008. High-intensity narrow-spectrum light inactivation and wavelength sensitivity of Staphylococcus aureus. FEMS Microbiol. Lett. 285:227-232. [DOI] [PubMed] [Google Scholar]
- 15.Maclean, M., S. J. MacGregor, J. G. Anderson, and G. A. Woolsey. 26 June 2008. The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J. Photochem. Photobiol. B. doi: 10.1016/j.jphotobiol.2008.06.006. [DOI] [PubMed]
- 16.Morton, C. A., R. D. Scholefield, C. Whitehurst, and J. Birch. 2005. An open study to determine the efficacy of blue light in the treatment of mild to moderate acne. J. Dermatolog. Treat. 16:219-223. [DOI] [PubMed] [Google Scholar]
- 17.Nitzan, Y., M. Salmon-Divon, E. Shporen, and Z. Malik. 2004. ALA induced photodynamic effects on gram positive and negative bacteria. Photochem. Photobiol. Sci. 3:430-435. [DOI] [PubMed] [Google Scholar]
- 18.Nitzan, Y., and M. Kauffman. 1999. Endogenous porphyrin production in bacteria by δ-aminolaevulinic acid and subsequent bacterial photoeradication. Laser Med. Sci. 14:269-277. [Google Scholar]
- 19.Schleifer, K. H., and R. Kilpper-Bälz. 1984. Transfer of Streptococcus faecalis and Streptococcus faecium to the genus Enterococcus nom. rev. as Enterococcus faecalis comb. nov. and Enterococcus faecium comb. nov. Int. J. Sys. Bacteriol. 34:31-34. [Google Scholar]
- 20.Silverman, R. A. 1989. Essential calculus with applications. Dover Publications, New York, NY.
- 21.Smith, R. C., and K. S. Baker. 1981. Optical properties of the clearest natural waters (200-800 nm). Appl. Opt. 20:177-184. [DOI] [PubMed] [Google Scholar]
- 22.Soukos, N. S., S. Som, A. D. Abernethy, K. Ruggiero, J. Dunham, C. Lee, A. G. Doukas, and J. M. Goodson. 2005. Phototargeting oral black-pigmented bacteria. Antimicrob. Agents Chemother. 49:1391-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szocs, K., F. Gabor, G. Csik, and J. Fidy. 1999. δ-Aminolaevulinic acid-induced porphyrin synthesis and photodynamic inactivation of Escherichia coli B. J. Photochem. Photobiol. B 50:8-17. [DOI] [PubMed] [Google Scholar]
- 24.Tyrrell, R. M., and M. J. Peak. 1978. Interactions between UV radiation of different energies in the inactivation of bacteria. J. Bacteriol. 136:437-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Meulen, F. W., K. Ibrahim, H. J. C. M. Sterenborg, L. V. Alphen, A. Maikoe, and J. Dankert. 1997. Photodynamic destruction of Haemophilus influenzae by endogenously produced porphyrins. J. Photochem. Photobiol. B. 40:204-208. [DOI] [PubMed] [Google Scholar]
- 26.Wainwright, M. 1998. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 42:13-28. [DOI] [PubMed] [Google Scholar]
- 27.Wang, T., S. J. MacGregor, J. G. Anderson, and G. A. Woolsey. 2005. Pulsed ultra-violet inactivation spectrum of Escherichia coli. Water Res. 39:2921-2925. [DOI] [PubMed] [Google Scholar]
- 28.Young, A. R. 2006. Acute effects of UVR on human eyes and skin. Prog. Biophys. Mol. Biol. 92:80-85. [DOI] [PubMed] [Google Scholar]
- 29.Zeina, B., J. Greenman, W. M. Purcell, and B. Das. 2001. Killing of cutaneous microbial species by photodynamic therapy. Br. J. Dermatol. 144:274-278. [DOI] [PubMed] [Google Scholar]