Abstract
In biomolecular systems, the mechanical transfer of free energy occurs with both high efficiency and high speed. It is shown here that such a transfer can be achieved only if the participating free-energy-storing elements exhibit opposing relationships between their content of free energy and the force they exert in the transfer direction. A kinetic equilibrium of forces (KEF) results, in which the transfer of free energy is mediated essentially by thermal molecular motion. On the basis of present evidence, KEF is used as a guiding principle in developing a mechanical model of the crossbridge cycle in muscle contraction. The model allows the basic features of molecular events to be visualized in terms of plausible structures. Real understanding of the process will require identification of the elements that perform the functions described here. Besides chemomechanical energy transduction, KEF may have a role in other biomolecular processes in which free energy is transferred mechanically over large distances.
In biomolecular systems, free energy frequently needs to be transferred over substantial distances by conformational changes of the protein backbone. In the case of the basic events in muscle contraction, for example, free energy gained from the binding of ATP to the active site of myosin has to be transferred over a distance of several nanometers to the myosin–actin interface, to dissociate the two proteins (1). It is notable that the transfer is not only thermodynamically efficient but also very rapid, indicating the absence of large activation energies.
By using a simplified representation of the interaction between myosin, actin, and ATP, it is first shown that rapid and loss-free mechanical transfer of free energy in biomolecular systems is possible only when the regions between which free energy is exchanged fulfill certain dynamic conditions. The resulting molecular state under which free energy is transferred essentially by thermal molecular motion will be termed kinetic equilibrium of forces (KEF).
A mechanical model of the myosin head constructed along these lines will then be described, incorporating the currently known properties of the proteins (2–4). The molecular events reflect the properties of discrete energy-storing sites, linked by the relative movement of rigid protein domains. Models of this type have previously been used in considering aspects of the crossbridge cycle on which muscle contraction is based (5–7) or of the interaction between kinesin and microtubules (8).
On the basis of the model, the interaction of myosin, actin, and ATP will be considered in a general way, followed by certain aspects of the complete crossbridge cycle, with emphasis on the origin of the large conformational change of the myosin head and the mechanism of ADP release.
KEF.
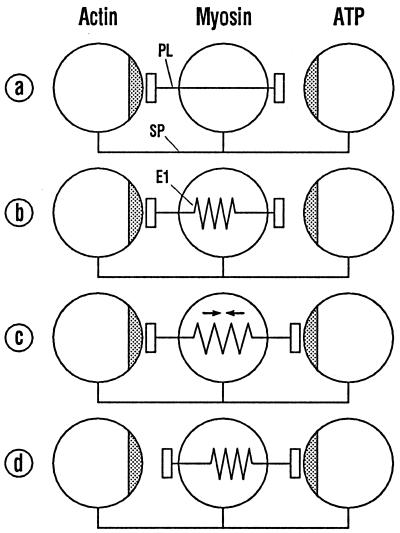
Fig. 1 illustrates the dynamic requirements for an efficient mechanical transfer of free energy in biomolecular systems. The molecules actin, myosin, and ATP (see above), which interact during the crossbridge cycle, are schematically represented as spheres. Their distances are held constant by a spacer SP. The actin and the ATP spheres have binding sites (stippled) for the myosin molecule lying between them. The corresponding binding sites of myosin are located on the ends of a movable plunger PL, which passes through the myosin. The attractive forces between the plunger ends and the respective partner molecules are supposed to be equal and to have the same dependence on distance. Free energy is defined as the integral of the scalar product of force over displacement.
Figure 1.
Scheme for the interaction between myosin, actin, and ATP. The partners are depicted as spheres whose separation is kept constant by the spacer SP. Free binding energy is transferred by shifting of the myosin plunger PL that interacts with actin and ATP. With a stiff plunger (a) a high activation energy is to be expected. With an elastic plunger (b–d) an activation energy can be avoided.
In Fig. 1a, the distance between the binding sites on the right is greater than that on the left. The myosin–ATP bond thus contains more free energy than the myosin–actin bond. Because of the greater distance, however, ATP exerts a smaller force on the plunger than would be required to detach the plunger from actin. Thus, the rigid plunger in Fig. 1a is unable, of its own accord, to detach itself from actin. Thermal molecular motion can supply the required energy in the form of activation energy, but transfer of free energy from right to left by this means will be greatly slowed.
In Fig. 1 b–d, an elastic element E1 is incorporated into the plunger within the myosin sphere. The elastic characteristics are chosen so as to balance the attractive force between the plunger and ATP at all distances. A minimal distance between the plunger and ATP leads to the limiting situation in Fig. 1c, in which the free energy generated by the attraction between the plunger and ATP is entirely stored in the elastic element. Because the forces are balanced and the arrangement is symmetrical, the energy in the elastic element can pass back to either side without loss. The limiting situation shown in Fig. 1d, with the free energy entirely stored in the plunger–actin bond, can also occur transiently.
The state in which the free energy diffuses without loss within limits set by the system is termed a “kinetic equilibrium of forces,” with the abbreviation KEF (9). The example considered here shows that the requirements for KEF are (i) the free-energy-storing elements participating in the transfer must exhibit oppositely directed relationships between their content of free energy and the force they exert along the direction of transfer; and (ii) the forces exerted by the cooperating storing elements must exhibit the same dependence on the transfer coordinate.
Because the free energy diffuses under random thermal molecular motion backwards and forwards along the transfer coordinate, an ideal KEF system exhibits no net transport over time. A fast and effective net transport is achieved if the maximal free energy of the coupled elements declines slightly in the desired direction of transport. Because a KEF system constantly changes in structure, the only meaningful pictorial representation is in terms of limiting situations between which the system stochastically oscillates for a period of time.
Molecular energy stores in which the exerted force increases with increasing free energy, such as elastic element E1, are referred to here as parallel or PA elements. Energy stores with the opposite dependence, such as myosin/ATP or myosin/actin, are termed antiparallel or AP elements. Combinations of PA and AP elements that fulfill the KEF conditions are termed KEF systems.
Because changes in free energy are defined by the integral of the scalar product of force over displacement (see above), the capacity of the free-energy-storing elements depends not only on the magnitude but also on the range of the force exerted by the elements. PA elements with relatively long-range forces can be generated by the elastic deformation of the protein backbone. The elements' characteristics can be adjusted via the structure of the protein. AP elements appropriate to KEF systems may be based largely on electrostatic interactions and cooperative systems of hydrogen bonds. Under physiological conditions, however, these usually have ranges of less than 1 nm. A mechanical amplification of the range, at the cost of the magnitude of the force, may therefore be necessary in linking AP and PA elements (see below). For very short distances, molecular attraction generally turns into repulsion. Given the short range of such repulsive forces, their influence on the distribution of free energy discussed here is small; they will therefore not be considered further.
The Mechanical Model of the Myosin Head.
Muscle contraction is based on the relative motion of myosin and actin filaments (10–12). Knowledge of the molecular processes in muscle contraction has grown considerably during the past few years. The identification of the structure of actin (13) and of the myosin head (2) was of crucial importance. It was shown that the 50-kDa domain of the myosin head is divided by a deep cleft (2). It was concluded that force is generated primarily by a change of the cleft width when myosin and actin combine (14). Later, it was demonstrated that the incorporation of ADP vanadate into the myosin ATPase changes the cleft angle by a few degrees only but simultaneously causes much larger angle changes in the so-called converter domain (15, 16). From this, it was concluded that the myosin head may exist in two conformations that differ much more in the angle between the cleft axis and the axis of the relatively slender lever arm (which is connected to the converter domain) than in the cleft angle (17). Agreement has not yet been reached as to whether the 50-kDa cleft is closed in the strongly bound (18) or open (19) state. The latter is assumed here, because it allows a simpler mechanism for the control of phosphate release (see below).
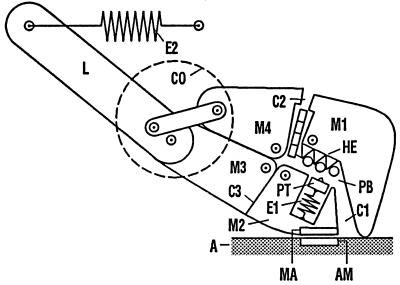
In Fig. 2, the structure of the myosin head reflects the current state of knowledge. The central part of the head is divided into the rigid domains M1–M4. They are held together in the center by four interconnected hinges represented by small circles. M3 and M4 are connected via the mechanical gear located in the converter domain CO, with the lever arm L, which in turn is coupled to the myosin filament (not shown) via the elastic element E2. Between M1 and M2 lies the phosphate-binding pocket PB, which is connected at one end with the 50-kDa cleft C1 and at the other with the ATPase cleft C2. C3 is a flexible area in the vicinity of SH1 and SH2 (20–22), which is also treated as a cleft (SH cleft).
Figure 2.
Mechanical model of the myosin head. M1-M4, domains of the central region; CO, converter region with the mechanical gear; L, lever arm; PB, phosphate-binding pocket; C1, 50-kDa cleft; C2, ATPase cleft; C3, SH cleft; PT, piston attracting the phosphate group of ATP; HE, helix linking the phosphate group to M1; E1, E2, elastic elements.
The adenosine group of ATP, represented as a segmented rectangle, is located in cleft C2. The triphosphate group, shown as a chain of three circles, has entered the phosphate-binding pocket PB and is linked to M1 via the helix HE without, however, affecting its attraction for the movable piston PT. PT carries the water molecule required for ATP hydrolysis, shown as a small triangle. The piston is linked via the elastic element E1 and the myosin domain M2 to the actin-binding site of myosin (MA), which faces a corresponding site on actin (AM). PT, E1, and MA together form the elastic plunger PL seen in Fig. 1 b–d. The task of the spacer SP in Fig. 1 is performed by the myosin domain M1: it merely abuts on the actin filament A, whereas it is linked to the triphosphate of ATP via HE. Because the piston PT has separated from the triphosphate group, Fig. 2 corresponds to the situation in Fig. 1b.
Events in the Vicinity of the Phosphate-Binding Pocket.
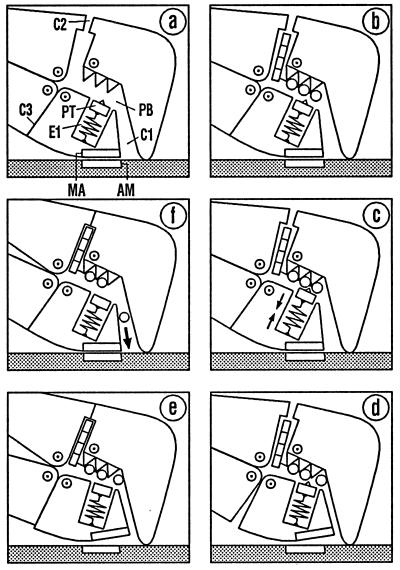
In Fig. 3, the events in the vicinity of the phosphate-binding pocket PB are considered first, by using the terminology of Fig. 2. The diagram is to be read in clockwise sequence. In Fig. 3a, PB is empty, which corresponds to the rigor state. In Fig. 3b, ATP has entered the myosin head through the open ATPase cleft C2. The piston PT is still in its starting position. The phosphate group, therefore, is only weakly bound. Under the restoring force of the elastic element E1, however, PT can approach the phosphate group, generating a first KEF with the limiting situations shown in Fig. 3 b and c. At a moment when the binding energy of the phosphate group happens to be concentrated in E1 (Fig. 3c), the elastic element succeeds in transferring the free energy to the myosin–actin bond, MA and AM moving apart (Fig. 3d). This generates a second KEF with the limiting situations shown in Fig. 3 c and d, in which clefts C1 and C3 open and close reciprocally. When E1 chances to be relaxed, so that the free energy is located entirely in the myosin–actin bond (Fig. 3d), the filament system can detach the head from the actin filament with only slight expenditure of free energy (Fig. 3e, without actin), with closure of clefts C2 and C3.
Figure 3.
Events in the vicinity of the phosphate-binding pocket. The diagram is to be read in clockwise sequence. (a–c) Entry of ATP via C2 initiates stretching of the elastic element E1. In d, E1 has succeeded in opening the myosin–actin bond MA/AM. Detachment of the myosin head triggers ATP hydrolysis and closes clefts C2 und C3 (e, without actin). Reattachment starts with the conformation of the free head (e, with actin). Returning to the strong binding state (f) opens cleft C1 so that Pi can leave the head. A further change of the conformation is required for ADP release (see Fig. 4).
The ATP cleavage step is assumed to be triggered by the formation of a particularly strong bond between the phosphate group and the piston bearing the water molecule. This bond is strongest when it is not weakened by the myosin–actin bond, i.e., when myosin and actin are dissociated from each other (Fig. 3e, but without actin). Induction of ATP hydrolysis by the separation of actin and myosin was demonstrated as early as 1971 (23). Further, it was shown that hydrolysis of ATP bound to myosin takes place with an equilibrium constant close to 1 (24). In terms of the KEF theory, this could be explained by the fact that, as hydrolysis proceeds, the decreasing attraction between the phosphate group and the phosphate-binding pocket (as AP element) is constantly compensated for by a matching decrease in the repulsion between the phosphate ion Pi and ADP (as PA element). This process, however, cannot be represented satisfactorily by mechanical elements. It was demonstrated that the ATP hydrolysis products at first remain bound in the myosin head (23). This may be caused by a slight inherent tendency of the cleft C1 to close, suppressing the cleavage step. Such a tendency is to be expected, because of the complementarity of the hydrophobic inner surfaces of C1 (14).
During the reattachment to actin (Fig. 3e, with actin), the head is only weakly bound at first, and the state of the hydrolysis products and the clefts has not yet changed. Only when the cleft C1 opens during the transition to the strong bond (Fig. 3f) is the hydrolysis equilibrium shifted so that the Pi ion can leave the myosin head via C1 (25). According to the model, release of Pi is not linked to an appreciable loss of free energy. The ADP at first stays in the myosin head, because the ATPase cleft C2 is still closed. A further conformational change is required for ADP release and opening of C2, as shown in the next section.
The Complete Crossbridge Cycle.
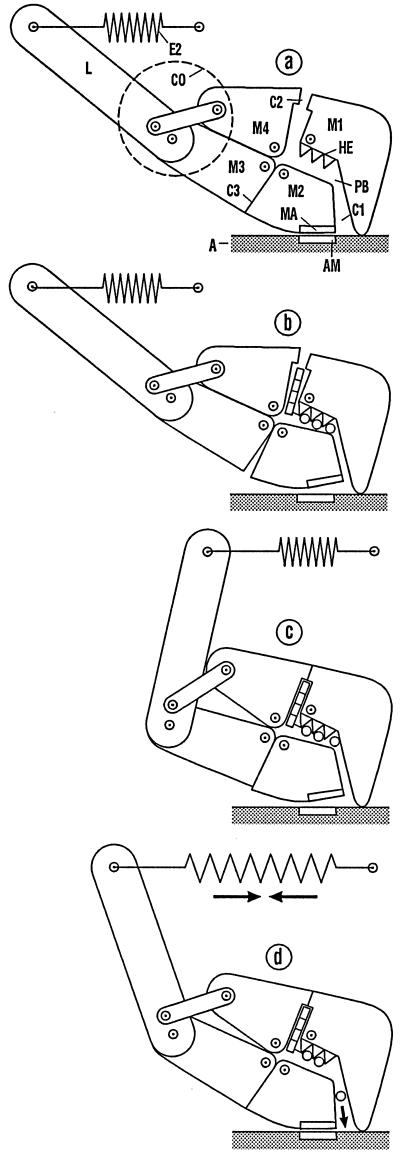
Fig. 4 describes the complete crossbridge cycle by using the mechanical model of the myosin head presented in Fig. 2. The details of the phosphate-binding pocket considered in Fig. 3 have been omitted to simplify the figures.
Figure 4.
The complete crossbridge cycle. Incorporation of ATP shifts the head from the strong binding state (a) into the weak binding state (b). Detachment and reattachment result in another weak binding state (c) at another actin position with a conformation optimal for the power stroke. To save space, diagrams c and d are shifted to the right compared with their true alignment relative to a and b. The transition to the strong binding state (d) (power stroke) stretches the elastic element E2, and allows Pi to escape. Transfer of the free energy stored in E2 to the filaments takes the system back to a and results in the exchange of ADP for ATP.
In Fig. 4a, the head is in the same conformation as in Fig. 2. The phosphate-binding pocket PB, however, is still empty. In Fig. 4b, an ATP has entered the myosin head via the ATPase cleft C2. Via the intermediate states described in the previous section, ATP has weakened the actin–myosin bond (see Fig. 3d). Because only the orientation of the myosin domain M2 has changed during the transition from the strong to the weak bond, no negative power stroke has occurred (22).
Fig. 4c (but without actin) represents the head detached from actin. Through detachment, the head acquires the freedom to follow the weak attractive interaction between the adenosine part of the ATP and the ATPase cleft C2. Because the myosin domains M3 and M4 are coupled via the converter CO, not only C2 but also the SH cleft C3 closes. This results in a large change of conformation.
During the weak reattachment of the myosin head at a downstream actin position (Fig. 4c), the conformation is at first unchanged. Hence, the head is still strongly curved and therefore possesses the optimal conformation for the power stroke. The transition to Fig. 4d (power stroke) takes place as a KEF between the elastic element E2 as PA element and the myosin–actin bond MA/AM as AP element. The large difference in amplitudes of the two free-energy-storing elements is compensated for by the mechanical amplification of the converter CO. The Pi ion can leave the myosin head via the 50-kDa cleft C1 (see above).
The return to the conformation corresponding to Fig. 4a and the resultant release of ADP take place only when the free energy stored in the elastic element E2 has been taken over by the filament system. In this way, a premature and hence useless entry of ATP into the myosin head is avoided. In addition, the conformational change on ADP release is essential for adapting the proportion of attached heads to the load of the muscle (26).
Discussion
As in the well-known model of Huxley and Simmons (5), the power stroke consists in the stretching of an elastic element (E2) by an increasing attraction between the myosin head and actin. Because of the small range of the attractive forces (see above), several spatially separated interaction sites of increasing strength were assumed by these authors to be invoked successively during the power stroke. The problem of a too-long response time (5, 27) is solved by the mechanical amplification in the model presented here. Thus, both KEF conditions can be fulfilled so that a much shorter response time is to be expected.
As in the preliminary model of Rayment et al. (28), the large change of conformation of the myosin head in the model presented here is induced by the closure of the ATPase cleft in the detached state of the head. This process, however, is not associated with a significant accumulation of free energy in the myosin head, and the ATPase cleft remains closed throughout the power stroke (29).
In experiments performed by Whittaker et al. (30), the myosin heads were connected with the actin filament only. If the adenosine group of the ADP is bound sufficiently tightly by the myosin head, as it appears to be in the nonstriated muscle myosin used, C2 closes. This induces a change of conformation corresponding to the transition from Fig. 4 a to d, which can be reversed by removal of the ADP. This change of conformation is not expected to contribute significantly to the power stroke (31).
A distinction is often made between weakly and strongly bound myosin–actin states (1, 32–34). In the model depicted by Fig. 4, strongly and weakly bound myosin–actin states refer to different situations of the same MA/AM interaction. In the crossbridge cycle, there are two strongly bound (a and d) and two weakly bound (b and c) limiting situations.
The isometric state of contraction is represented by a dynamic alternation between Fig. 4 c and d. In the case of slow contraction of the muscle under load, it can be assumed that the myosin head moves by one actin monomer per ATP hydrolyzed. In rapid or unloaded shortening, much larger relative movements of actin and myosin filaments occur than would be expected on the basis of the finite ATPase rate (35). Howard's explanation is that, “each individual crossbridge must spend a significant time detached whereas other (attached) crossbridges move the filament and the next binding site forward.”
In a recent study of single-molecule interactions, steps of approximately 5 nm were observed (36), a dimension which corresponds within the accuracy of the measurements to the axial separation between neighboring actin monomers. Another group observed a similar step size but found evidence for the production of multiple steps for each ATP split (37). These authors attempted to explain this effect in terms of a gradual release of the free energy during successive actomyosin interactions and noted a conflict with the “widely accepted view that force generation is directly coupled to release of bound ligands,” which of course can occur only once per ATP. On our present model, Pi release occurs during force production but is not its cause. The head could therefore repeat the power stroke by using the free energy resulting from the hydrolysis of one ATP, as long as the detachment process (under KEF) leaves a sufficient fraction of the free energy stored in the actin–myosin bond or in the elastic element E2. For a short period of time, free energy that has been lost could thus be recovered sufficiently rapidly from thermal molecular motion, without contravening the second law of thermodynamics.
In the model presented here, the direction of movement of the myosin head is fixed by the properties of the actin filament together with the construction of the converter domain. The same is to be expected for myosin/actin systems that serve other tasks than muscle contraction, e.g., movement of vesicles. Until recently, it has been assumed that all types of myosin move to the “plus” end of actin. It has recently been shown that myosin VI moves in the opposite direction (38). From sequence analysis and cryoelectron microscopy, the authors concluded that the change of the myosin direction is a consequence of a structural change in the converter domain. A mechanical model for myosin motors with opposite polarity of force generation has been proposed (39). In the crossbridge cycle represented by Fig. 4, reversal of myosin's direction of movement on actin results if the lever arm L is an extension of the myosin domain M4 instead of M3. The mechanical linker in the converter domain then runs from L to M3.
In the basic event in muscle contraction, the free energy generated by the binding of the triphosphate group in the myosin ATPase is released as mechanical energy via the filament system. Therefore, it is not surprising that the transfer of the free energy from the ATPase to the filament system takes place mechanically. A similar situation holds in the case of motor systems based on kinesin and dynein (8, 35, 40, 41). It is to be expected, therefore, that the kinetic equilibrium of forces is important in these systems also. KEF theory may likewise be relevant for biochemical processes that are not designed for force production, but in which free energy is nevertheless transferred mechanically over large distances.
Acknowledgments
I thank Kenneth Holmes and John Wray for many stimulating discussions and for critically reading the manuscript.
Abbreviations
- KEF
kinetic equilibrium of forces
- PA
parallel
- AP
antiparallel
- AM
myosin-binding site of actin
- MA
actin-binding site of myosin
References
- 1.Eisenberg E, Greene L E. Annu Rev Physiol. 1980;42:293–309. doi: 10.1146/annurev.ph.42.030180.001453. [DOI] [PubMed] [Google Scholar]
- 2.Rayment I, Rypniewsky W R, Schmidt-Bäse K, Smith R, Tomchick D R, Benning M M, Winkelmann D A, Wesenberg G, Holden H M. Science. 1993;261:50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- 3.Cooke R. Physiol Rev. 1997;77:671–697. doi: 10.1152/physrev.1997.77.3.671. [DOI] [PubMed] [Google Scholar]
- 4.Geeves M, Holmes K. Annu Rev Biochem. 1999;68:687–728. doi: 10.1146/annurev.biochem.68.1.687. [DOI] [PubMed] [Google Scholar]
- 5.Huxley A F, Simmons R M. Nature (London) 1971;233:533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- 6.Goody R S, Holmes K C. Biochim Biophys Acta. 1983;726:13–39. doi: 10.1016/0304-4173(83)90009-5. [DOI] [PubMed] [Google Scholar]
- 7.Wray J S, Goody R S, Holmes K C. Adv Exp Med Biol. 1988;226:49–59. [PubMed] [Google Scholar]
- 8.Peskin C S, Oster G. Biophys J. 1995;68:202S–211S. [PMC free article] [PubMed] [Google Scholar]
- 9.Becker E W. Z Naturforsch. 1992;47c:628–633. doi: 10.1515/znc-1992-7-823. [DOI] [PubMed] [Google Scholar]
- 10.Huxley A F, Niedergerke R. Nature (London) 1954;173:971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- 11.Huxley H E, Hanson E J. Nature (London) 1954;173:973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- 12.Huxley H E. Science. 1969;164:1356–1366. [PubMed] [Google Scholar]
- 13.Holmes K C, Popp D, Gebhard W, Kabsch W. Nature (London) 1990;347:44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- 14.Fisher A J, Smith C A, Thoden J, Smith R, Sutoh K, Holden H M, Rayment I. Biophys J. 1995;68:19S–28S. [PMC free article] [PubMed] [Google Scholar]
- 15.Smith C A, Rayment I. Biochemistry. 1996;35:5404–5417. doi: 10.1021/bi952633+. [DOI] [PubMed] [Google Scholar]
- 16.Houdusse A, Cohen C. Structure (London) 1996;4:21–32. doi: 10.1016/s0969-2126(96)00006-8. [DOI] [PubMed] [Google Scholar]
- 17.Holmes K C. Curr Biol. 1997;7:R112–R118. doi: 10.1016/s0960-9822(06)00051-0. [DOI] [PubMed] [Google Scholar]
- 18.Gulick A M, Rayment I. BioEssays. 1997;19:561–569. doi: 10.1002/bies.950190707. [DOI] [PubMed] [Google Scholar]
- 19.Holmes K C. Acta Crystallogr A. 1998;54:789–797. doi: 10.1107/s0108767398010307. [DOI] [PubMed] [Google Scholar]
- 20.Huston E E, Grammer J C, Yount R G. Biochemistry. 1988;27:8945–8952. doi: 10.1021/bi00425a011. [DOI] [PubMed] [Google Scholar]
- 21.Rayment I, Holden H M. Trends Biochem Sci. 1994;19:129–134. doi: 10.1016/0968-0004(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 22.Houdusse A, Kalabokis V N, Himmel D, Szent-Györgyi A G, Cohen C. Cell. 1999;97:459–470. doi: 10.1016/s0092-8674(00)80756-4. [DOI] [PubMed] [Google Scholar]
- 23.Lymn R W, Taylor E W. Biochemistry. 1971;10:4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- 24.Bagshaw C R, Trentham D R. Biochem J. 1973;133:323–328. doi: 10.1042/bj1330323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yount R G, Lawson D, Rayment I. Biophys J. 1995;68:44s–49s. [PMC free article] [PubMed] [Google Scholar]
- 26.Duke T A J. Proc Natl Acad Sci USA. 1999;96:2770–2775. doi: 10.1073/pnas.96.6.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenberg E, Hill T L. Prog Biophys Mol Biol. 1978;33:55–82. doi: 10.1016/0079-6107(79)90025-7. [DOI] [PubMed] [Google Scholar]
- 28.Rayment I, Holden H M, Whittaker M, Yohn C B, Lorenz M, Holmes K C, Milligan R A. Science. 1993;261:58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- 29.Franks-Skiba K, Hwang T, Cooke R. Biochemistry. 1994;33:12720–12728. doi: 10.1021/bi00208a025. [DOI] [PubMed] [Google Scholar]
- 30.Whittaker M, Wilson-Kubalek E M, Smith J E, Faust L, Milligan R A, Sweeney H L. Nature (London) 1995;378:748–751. doi: 10.1038/378748a0. [DOI] [PubMed] [Google Scholar]
- 31.Cremo C R, Geeves M A. Biochemistry. 1998;37:1969–1978. doi: 10.1021/bi9722406. [DOI] [PubMed] [Google Scholar]
- 32.Geeves M A, Goody R S, Gutfreund H. J Muscle Res Cell Motil. 1984;5:351–361. doi: 10.1007/BF00818255. [DOI] [PubMed] [Google Scholar]
- 33.Taylor E W. J Biol Chem. 1991;266:294–302. [PubMed] [Google Scholar]
- 34.Brenner B. Adv Biophys. 1991;27:259–269. doi: 10.1016/0065-227x(91)90024-8. [DOI] [PubMed] [Google Scholar]
- 35.Howard J. Nature (London) 1997;389:561–567. doi: 10.1038/39247. [DOI] [PubMed] [Google Scholar]
- 36.Mehta A D, Finer J T, Spudich J A. Proc Natl Acad Sci USA. 1997;94:7927–7931. doi: 10.1073/pnas.94.15.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitamura K, Tokunaga M, Iwane A H, Yanagida T. Nature (London) 1999;397:129–134. doi: 10.1038/16403. [DOI] [PubMed] [Google Scholar]
- 38.Wells A L, Lin A W, Chen L-Q, Safer D, Cain S M, Hasson T, Carragher B O, Milligan R A, Sweeney H L. Nature (London) 1999;401:505–508. doi: 10.1038/46835. [DOI] [PubMed] [Google Scholar]
- 39.Schliwa M. Nature (London) 1999;401:431–432. doi: 10.1038/46692. [DOI] [PubMed] [Google Scholar]
- 40.Howard J, Hudspeth A J, Vale R D. Nature (London) 1989;342:154–158. doi: 10.1038/342154a0. [DOI] [PubMed] [Google Scholar]
- 41.Grummt M, Woehlke G, Henningsen U, Fuchs S, Schleicher M, Schliwa M. EMBO J. 1998;17:5536–5542. doi: 10.1093/emboj/17.19.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]