Abstract
The iron-sulfur nitroso compound [Fe4S3(NO)7]− is a broad-spectrum antimicrobial agent that has been used for more than 100 years to combat pathogenic anaerobes. Known as Roussin's black salt (RBS), it contains seven moles of nitric oxide, the release of which was always assumed to mediate its cytotoxicity. Using the hyperthermophilic archaeon Pyrococcus furiosus, it is demonstrated through growth studies, membrane analyses, and scanning electron microscopy that nitric oxide does not play a role in RBS toxicity; rather, the mechanism involves membrane disruption leading to cell lysis. Moreover, insoluble elemental sulfur (S0), which is reduced by P. furiosus to hydrogen sulfide, prevents cell lysis by RBS. It is proposed that S0 also directly interacts with the membranes of P. furiosus during its transfer into the cell, ultimately for reduction by a cytosolic NADPH sulfur reductase. RBS is proposed to be a new class of inorganic antimicrobial agent that also has potential use as an inert cell-lysing agent.
Reactive nitrogen species (RNS), such as nitrite, have been used throughout human history to prevent spoilage of fish and meat by microorganisms (5, 25). Their toxicity is thought to be the result of the generation of nitric oxide (NO), which then chemically modifies key enzymes and proteins via primary thiol groups or iron-sulfur clusters (5, 16, 18, 20, 21, 33, 36). However, the specific mechanisms by which RNS inhibit microbial growth is poorly understood (7, 13, 17, 20). Since health concerns over possible connections between nitrite and cancer have caused a steady decline in the amount of nitrite used in American and European foods (29, 34), a more complete understanding of the action of RNS and related molecules is clearly necessary. Herein, we focus on a remarkable molecule known as Roussin's black salt (RBS). RBS is even more bactericidal than nitrite (25, 26); it was discovered in 1858 by the French scientist M. L. Roussin, who synthesized it by heating nitrite, iron, and sulfide (26). It is a broad-spectrum antimicrobial agent that inhibits the growth of both gram-positive and gram-negative bacteria, including many pathogenic strains of anaerobes, including species of Clostridia (1, 2, 5, 13, 22). Remarkably, RBS is toxic to spores as well as vegetative cells (24).
As indicated by its formula, Fe4S3(NO)7Na, RBS has a cube-like structure with seven moles of nitric oxide positioned around an iron-sulfur core (14). The presence of NO groups understandably leads to the assumption that the toxicity of RBS to anaerobic organisms is due to the release of NO (12, 13, 16, 25). Indeed, over many days, RBS slowly decomposes aerobically and does release NO (3, 6, 18, 19), but under anaerobic conditions the release of NO is not observed (18). In contrast to most antibiotics, RBS is stable up to 120°C (25, 26) and therefore is of potential utility in developing genetic systems in microorganisms that thrive at high temperatures and particularly those that grow anaerobically. In addition, in contrast to several bacterial antibiotics, RBS might also be effective against members of the archaea. However, a prerequisite to its use is an understanding of the mechanism of action of RBS, and that was the goal of the present study.
Pyrococcus furiosus is a nonpathogenic anaerobe. It has been studied extensively as a hyperthermophile and as an archaeon (9, 11, 35), making it an excellent model organism with which to investigate the mechanism of RBS. P. furiosus grows optimally near 100°C and utilizes carbohydrates as carbon sources, generating organic acids and CO2 as end products. Protons or elemental sulfur (S0) can be used as terminal electron acceptors producing hydrogen or hydrogen sulfide, respectively, although S0 is the preferred electron sink (28). Herein, it is shown that the growth of P. furiosus is very sensitive to RBS, at least in the absence of S0, but that its mechanism of action does not involve NO.
MATERIALS AND METHODS
RBS.
RBS was obtained from Martin Hughes (Kings College, London, United Kingdom). Stock solutions of 1.8 mM RBS were prepared anaerobically in glass-distilled water or in the P. furiosus growth medium. Concentrations of RBS were estimated using an extinction coefficient at 275 nm of 29,650 (1/cm·M) (3, 6). The stability of RBS was determined at 95°C using an anaerobic sample of RBS (60 μM in P. furiosus growth medium at pH 6.8). For the S-nitrosothiol assays, RBS, nitric oxide, and nitrite (each 1 mM) were prepared in 10 mM 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid (EPPS) buffer, pH 7.0. Under anaerobic conditions at 85°C, each was added to an equal volume of various thiol compounds, also at a concentration of 1 mM. Formation of the S-nitrosothiol was measured by visible spectroscopy at 540 nm (4, 20, 36).
Growth studies.
P. furiosus (DSM 3638) was cultured as described previously (32) using 0.5% (wt/vol) maltose (95% grade; Sigma, St. Louis, MO) as the carbon source with and without S0 (0.1%, wt/vol). Cell density was measured by direct counting using a BX41 Olympus phase-contrast microscope (Olympus, Center Valley, PA) and a Hausser Scientific Partnership counting chamber (Horsham, PA). The toxicity of P. furiosus to RBS was determined by growing the organism to cell densities of ∼5.0 × 107 cells/ml and injecting RBS to final concentrations of 0.5 to 5.0 μM. Elemental sulfur (0.1%, wt/vol), where indicated, was added up to 30 min before the addition of RBS into the culture. Cold-shift assays were conducted by transferring cultures (5.0 × 107 cells/ml) grown at 98°C to 4°C for 1 h. RBS (2 μM) was added, and the cultures were monitored by cell counts.
Membrane preparations.
Membranes were prepared under anaerobic conditions from cells (400-ml cultures) harvested when the cell density reached 5.0 × 107 cells/ml after treatment with RBS (20 μM). Membranes were collected by centrifugation at 113,000 × g for 2 h (Optima L-90K; Beckman-Coulter, Ramsey, MN). The membrane pellet was washed twice with degassed glass-distilled water and resuspended in 2.5 ml of degassed glass-distilled water. Samples of this RBS-tainted membrane suspension (20 to 1200 μl) were then injected into P. furiosus cultures (10 ml) at 98°C, and cell densities were determined after 30 min. For calculations, membrane pellets prepared from 400-ml cultures containing RBS were assumed to have absorbed all of the RBS that was initially added (400 ml of a 20 μM RBS solution contains 8 μmol), such that when the pellet was resuspended to a volume of 2.5 ml, the “membrane” preparation would contain a maximum of 3.2 mM RBS. The RBS-containing membranes were resuspended in 2.5 ml of degassed glass-distilled water containing colloidal sulfur (final concentration, 4 mM) or in 10 mM N-cyclohexyl-2-aminoethanesulfonic acid, pH 10.0 (to prevent the formation of elemental sulfur), containing polysulfide (final concentration, 4 mM), and these were incubated at 85°C for 1 h before membrane toxicity was assessed. Polysulfide was prepared anaerobically by reacting 100 mM sodium sulfide with an excess of elemental sulfur (∼6%, wt/vol) for 24 h at 25°C. The toxicity of sulfur- and polysulfide-treated RBS-containing membranes was assessed by adding the membrane preparation (20 to 1,200 μl) to P. furiosus cultures (10 ml) at 25°C for 30 min. The membrane experiments were repeated using nitrite (800 μM) or NO (40 μM) in place of RBS.
SEM preparation.
RBS (1.0 μM) was injected into a culture of P. furiosus growing at 98°C (cell density of ∼5.0 × 107 cells/ml), and samples (1 ml) were taken after 30 and 60 s. These were cooled to 4°C, fixed with 2% glutaraldehyde, washed three times with phosphate-buffered saline and fixed with 1% osmium tetraoxide (OsO4) for 1 h. Samples were dehydrated sequentially using 25, 50, 75, 85, 90, and 100% ethanol with three washes at each ethanol concentration, gently filtered through a 0.2-μm-pore-size Millipore membrane using a Swinney filter (Millipore, MA), critical-point dried, mounted on a post, and coated with ∼153 Å of gold with a sputter coater (Structure Probe Inc., PA). The coated sample was scanned using a Leo 982 field emission scanning electron microscope ([SEM] Zeiss, MA) at the Center for Ultrastructural Research (University of Georgia, Athens, GA).
Cellular characterization.
Fluorescent reporters, SYTO 9 and propidium iodide, were used in a Live/Dead BacLight Bacterial Viability kit (Molecular Probes, Eugene, OR). Samples (1.0 ml) of a P. furiosus culture grown with and without S0 (0.1%, wt/vol) were centrifuged at 16,000 × g for 5 min, and the cells were resuspended in P. furiosus medium (3 ml) and collected by centrifugation (16,000 × g for 5 min). Cells were resuspended in growth medium (2 ml). The two fluorescent dyes (3 μl of each) were added, and the mixture was incubated at 25°C for 15 min in the dark. Fluorescence was measured with and without RBS (4 μM) using a Shimadzu RF-5301 PC spectrofluorophotometer by excitation at 480 nm and emission at 500 nm.
RESULTS
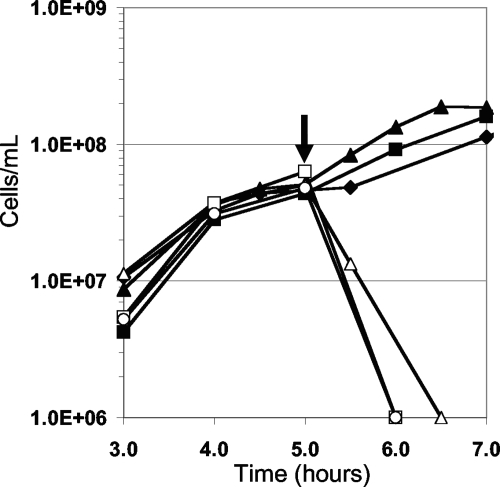
RBS was toxic to a culture of P. furiosus in mid-exponential growth (∼5 × 107/ml) at 98°C. As shown in Fig. 1, the addition of RBS (2.0 μM) resulted in complete cell lysis, as determined by light microscopy. Concentrations of RBS greater than 0.5 μM but less than 2 μM inhibited cell growth for up to 3 h, after which time the culture resumed growth. Cells did not appear to adapt to RBS during the lag phase as two subsequent additions of RBS (0.5 μM) to the same culture (initially containing 0.5 μM RBS) resulted in similar lag phases before growth resumed. In order to investigate whether it was RBS or a product of its degradation that was responsible for the toxicity, the stability of RBS was determined under the growth conditions of P. furiosus. There was no detectable decomposition of RBS as measured by UV/visible spectroscopy when a sample (60 μM) was incubated anaerobically at 98°C for 20 h in the P. furiosus growth medium (without S0). Moreover, this heat-treated sample of RBS was as toxic to P. furiosus as an untreated sample (Fig. 1). RBS is therefore stable at high temperatures and can be used as an antimicrobial agent under such conditions.
FIG. 1.
Effect of RBS on the growth of P. furiosus. The concentrations of RBS are as follows: 0 μM (▴), 0.5 μM (▪), 1.0 μM (⧫), 2.0 μM (Δ), 3.0 μM (○), and 5.0 μM (□). The arrow indicates when RBS was added.
It was assumed that the toxic effects of RBS were mediated by NO and that this involved its reaction with primary thiol groups to generate the S-nitrosothiol derivatives. To investigate the reactivity of RBS, samples of RBS, NO, or nitrite (each 1 mM) were prepared anaerobically in 10 mM EPPS buffer, pH 7.0, and these were incubated at 85°C for 30 min with l-cysteine, dithiothreitol, glutathione, 1-thioglycerol, thioglycolate, coenzyme A, or 2-mercaptoethanol. In contrast to NO and nitrite, both of which reacted with all thiols tested, RBS did not generate a detectable S-nitrosothiol adduct with any of the primary thiols. These results indicated that RBS does not readily generate NO.
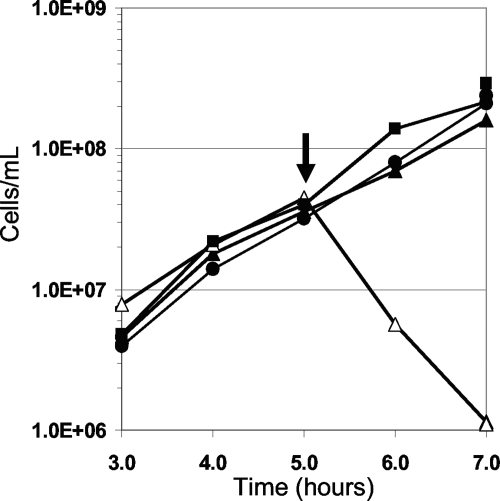
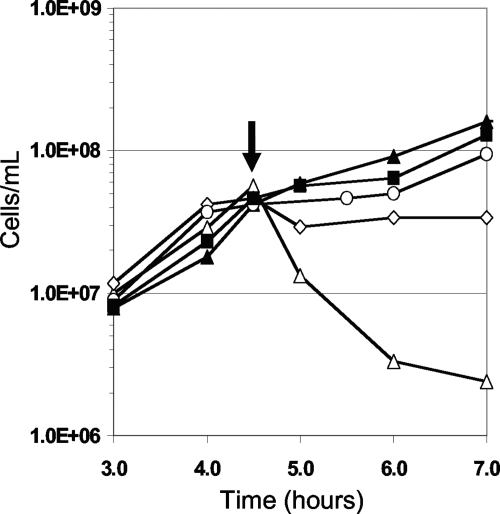
P. furiosus can use insoluble S0 as an electron acceptor, forming H2S instead of reducing protons to H2 (9). Surprisingly, P. furiosus cells in mid-exponential growth were not lysed by the addition of 2.0 μM RBS if S0 was present although they were if S0 was absent (Fig. 2). A concentration of 20 μM was required to cause the degree of cell lysis that was observed with a 10-fold lower concentration of RBS when S0 was not present. To determine if the effect of RBS was mitigated by its direct reaction with S0, RBS (20 μM) was incubated with S0 (1.0%, wt/vol) in the growth medium for 6 h (the time needed for P. furiosus to reach mid-exponential growth) at 98°C and then injected into a P. furiosus culture to a final concentration of 2 μM RBS. RBS remained as toxic as the untreated control. Prior to injection, UV/visible spectroscopy indicated that no reaction had occurred between RBS and S0 compared to the UV thermal stability experiment. To determine if P. furiosus cells had to interact with S0 before S0 had any effect on the toxicity of RBS, S0 was added to cultures up to 30 min before the introduction of RBS (2 μM). Only cells incubated with S0 for at least 20 min at 98°C prior to the addition of RBS did not lyse and continued to grow (Fig. 3). To determine if insoluble particles other than S0 provided any protection from RBS, P. furiosus cells were grown in the presence of excess iron sulfide (FeS; 0.1%, wt/vol). However, when added to the culture at a cell density of 5 × 107 cells/ml, RBS still caused immediate cell lysis. Consequently, unlike insoluble S0, the presence of insoluble FeS did not protect cells from RBS.
FIG. 2.
Effect of RBS on P. furiosus exposed to S0. Symbols are as follows: •, no S0; ▪, S0 added but no RBS; Δ, no S0 plus 2 μM RBS; ▴, S0 added plus 2 μM RBS. The arrow indicates when RBS was added.
FIG. 3.
Effect of RBS on P. furiosus after incubation with S0. Symbols are as follows: ▴, no S0 without RBS Δ, S0 and 2 μM RBS introduced to the culture at the same time; ⋄, 2 μM RBS added after culture was incubated with S0 for 10 min; ○, 2 μM RBS added after culture was incubated with S0 for 20 min; and ▪, 2 μM RBS added after culture was incubated with S0 for 30 min. The arrow indicates when RBS was added.
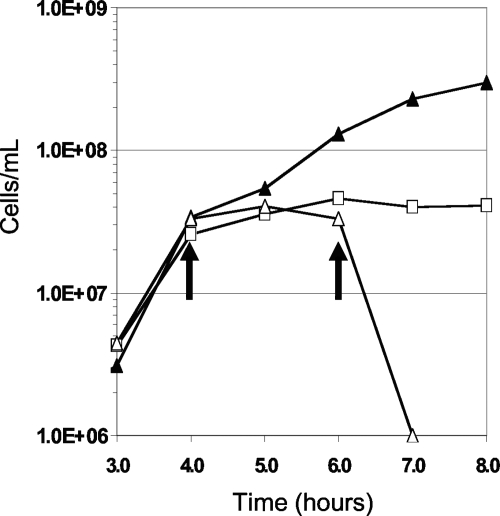
The optimal growth temperature of P. furiosus is near 100°C, and it shows reasonable growth at 72°C, with doubling times of approximately 1 and 5 h, respectively. There is no measurable growth at 4°C, where the metabolic activity approaches zero (4, 18, 20, 26, 36). To determine if rapid cell division was required for the toxic effects of RBS, cultures of P. furiosus were grown to a cell density of 5 × 107 cells/ml at 98°C; cells were then transferred to 4°C for 2 h, and RBS (2.0 μM) was added (Fig. 4). As observed at 98°C, P. furiosus cells lysed immediately upon the addition of RBS. The experiment was repeated with cells grown in the presence of S0 (0.1%, wt/vol). In this case, RBS (2.0 μM) had no effect on cell morphology and did not cause lysis, as was also observed with the culture grown at 98°C.
FIG. 4.
Toxicity of RBS at 4°C. P. furiosus cultures were transferred from 98°C to 4°C after 4 h (indicated by the first arrow) except the control which was maintained at the optimal growth temperature of 98°C. RBS was added to a culture once equilibrated to 4°C at the 6-h mark (indicated by the second arrow). Symbols are as follows: ▴, culture maintained at 98°C and no RBS added; □, culture transferred to 4°C but no RBS added; Δ, culture transferred to 4°C and 2.0 μM RBS added.
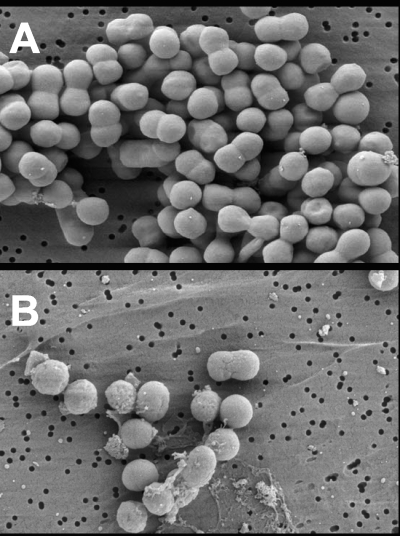
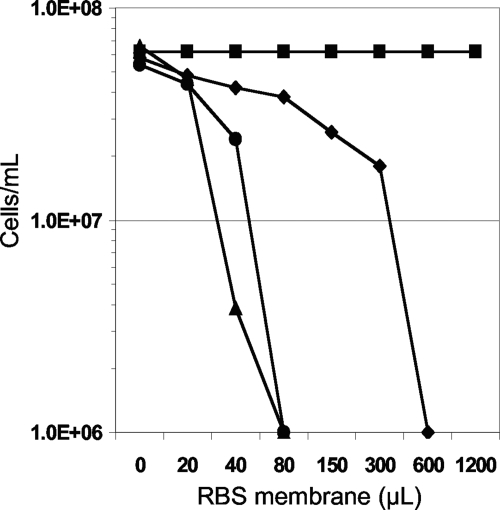
The morphological response of P. furiosus to RBS was examined by SEM. A culture was grown to a cell density of 5 × 107 cells/ml, RBS (1.0 μM) was added, and cell samples were taken. As shown in Fig. 5, the membranes of cells appeared to be rapidly disrupted by RBS, as indicated by the presence of cellular debris within seconds of RBS addition. To further investigate the cellular effects of RBS, membranes were harvested anaerobically from a culture of P. furiosus (400 ml) immediately after RBS (20 μM RBS) had been added. The membranes from the RBS-treated cells, which will be termed RBS-treated membranes, were washed with buffer to remove any residual traces of RBS not bound to or interacting with the membrane. Remarkably, the washed, RBS-treated membranes were toxic to P. furiosus cells since they caused lysis when added to a culture growing at 98°C. Membranes prepared from untreated cells (that had not been exposed to RBS) had no effect on cell growth. In a titration experiment 40 μl of this membrane preparation containing a maximum of ∼3.2 mM RBS (see Materials and Methods) added to the second culture resulted in cell lysis. Since a concentration of 2 μM RBS causes cell lysis, we estimate that the membranes of P. furiosus in the original culture absorbed approximately 16% of the RBS that was added (corresponding to 0.5 mM RBS in the membrane preparation). P. furiosus membranes therefore appear to have a very high affinity for RBS. This experiment was repeated using nitrite (800 μM) or NO (40 μM) in place of RBS. However, membranes isolated from cells treated with these compounds had no effect on growing P. furiosus cells. On the other hand, when RBS-tainted membranes obtained from P. furiosus cells incubated with RBS (20 μM) were treated with S0 for 30 min and used to titrate a fresh P. furiosus culture (Fig. 6), about 15 times as much of the membrane material was required to cause lysis. We estimate that the concentration of RBS in these S0-treated membrane preparations was equivalent to ∼30 μM. It appeared, therefore, that incubation with S0 for 30 min caused RBS to be lost (in the subsequent wash) from RBS-treated membranes such that they contained only ∼7% of the RBS that was absorbed in the absence of S0. When polysulfide (4 mM) was used in place of S0, it did not minimize the toxic effect of RBS-treated membranes (Fig. 6).
FIG. 5.
SEM images of P. furiosus exposed to 1 μM RBS. Images were collected before (A) and 60 s after (B) the addition of 1 μM RBS. Magnification, ×10,000.
FIG. 6.
Transfer of RBS toxicity in P. furiosus membrane fractions. Membranes from cultures treated with 20 μM RBS were added to fresh P. furiosus cultures with increasing injection volumes (μl). Each culture was monitored by cell count after a 30-min incubation with the RBS/membrane preparation. Symbols are as follows: ▪, no membranes added; •, RBS-treated membranes added; ⧫, RBS-treated membranes added after prior incubation with S0 (∼4 mM) at 98°C for 30 min; ▴, RBS-treated membranes added after prior incubation with polysulfide (4 mM) at 98°C for 30 min. Cultures with cell densities below 1 × 106 cells/ml were considered not viable.
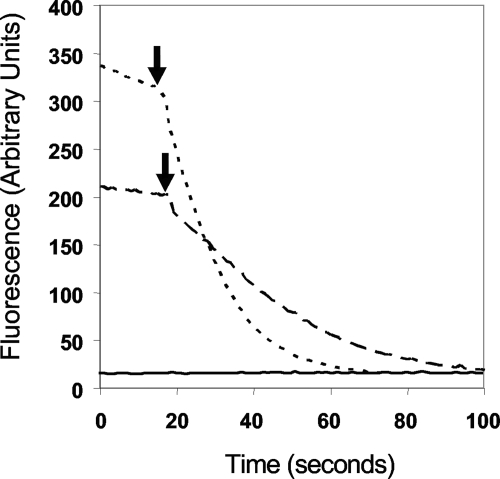
The effect of RBS on the integrity of the membranes of P. furiosus was further investigated using a fluorescent Live/Dead assay, where cell death is indicated by a dramatic loss of fluorescence in the presence of the reagent (Molecular Probes, Eugene, OR). P. furiosus cells were grown in the presence and absence of S0. Surprisingly, both types of cells exhibited a dramatic loss of fluorescence when RBS was added (Fig. 7). However, microscopic analyses confirmed that cells grown without S0 did indeed lyse, yet those grown with S0 did not.
FIG. 7.
Fluorescent Live/Dead assays on P. furiosus cells treated with RBS. The cell concentrations were initially 5.0 × 107 cells/ml. RBS (2 μM) was injected approximately 20 s after the beginning of the recording of the fluorescent scan. Dotted line, P. furiosus cells grown without S0; dashed line, P. furiosus cells grown with S0; solid line, control without cells.
DISCUSSION
The mechanism of action of RBS has remained a mystery for almost 150 years. NO has always been suspected to play a role since each RBS molecule contains seven NO ligands (2, 3, 5, 6, 10, 12, 13, 18, 19, 24, 25). What is known of the antimicrobial interactions of RBS was revealed by extensive work with organisms from the genus Clostridium (2, 5, 13, 24). Other gram-positive and also gram-negative organisms are sensitive to RBS to various degrees (2). Aerobic photolysis of RBS in the presence of endothelial cells leads to the rapid release of NO, causing vasodilatation, but RBS does not appear to have any other effect upon eukaryotic cells (3, 10, 19).
In contrast to what is generally assumed (18), we found no evidence that NO is involved in the toxic effect of RBS under anaerobic conditions. In fact, NO is not released even when RBS is incubated anaerobically at 98°C, and the compound appears to be stable under such conditions. This implies that NO is not involved in the cytotoxic mechanism of RBS and that its effect on anaerobic organisms is due to other properties. P. furiosus is lysed by an RBS concentration of only 2 μM, compared to 0.5 μM to inhibit the growth of vegetative cells of Clostridium perfringens, 3 μM for Listeria monocytogenes, and 1.3 μM for Clostridium sporogenes (5). Lysis of P. furiosus cells by RBS occurs both near the optimum growth temperature, 98°C, and also at 4°C, where metabolic and enzymatic activities are effectively zero. The toxic effects of RBS are therefore unlikely to arise from a product of its metabolism. Our results also clearly indicate that RBS is targeted to the cell membrane. Moreover, it appears to be absorbed into the membranes in an unmodified form since membranes from RBS-treated cells are toxic to cultures not previously exposed to RBS. In contrast, even though nitrite and NO cause P. furiosus cells to lyse, the same treated membranes had no effect on fresh cultures. SEM confirmed that the membrane is indeed compromised by RBS. It is therefore concluded that RBS toxicity is not dependent upon NO release; rather, it is due to a physical interaction with the cell membrane. In fact, we have previously used RBS and assumed it to be a NO generator, showing that Pyrococcus woesei is more sensitive to this compound than P. furiosus (11). In light of the results presented herein, it would appear that the membranes of P. furiosus are more resistant to disruption by RBS than are those of P. woesei, which is consistent with what has been previously reported by other investigators (15).
The toxicity of RBS was dramatically reduced when P. furiosus cells were grown in the presence of S0, and S0 appeared to cause membranes from RBS-treated cells to lose 93% of the RBS that they had absorbed. Yet there appeared to be no chemical reaction between RBS and S0, and the effects of insoluble S0 on the effects of RBS could not be reproduced with soluble polysulfide. Although we cannot rule out that there is a lipid-dependent reaction between RBS and S0, it can be hypothesized that insoluble S0 is able to “dissolve” into the cell membrane at 98°C and, by an as yet unknown mechanism, prevent the disruptive effects of RBS. This also suggests that S0 is not actively transported into the cell, which is consistent with the absence of any S0-regulated transporter, as determined by DNA microarray analyses (8, 23, 27, 30, 31). Presumably S0 interacts with the cell membrane of P. furiosus in such a way that it competes with RBS, while higher concentrations of RBS can, in turn, out-compete S0. Fluorescence and microscopic analyses confirmed that RBS causes cell death by lysis. Even though addition of RBS to S0-grown cells does not cause cell lysis or even loss of viability, the fluorescence-based Live/Dead assay showed that there was a loss of membrane integrity similar to that seen with lysed cells. Under these conditions the Live/Dead assay becomes an indicator of a breach in membrane integrity instead of cell death. Presumably, as the quenching of the fluorescence indicates, RBS partially compromised the cell membrane of S0-grown cells although lysis was not observed. Nevertheless, the interaction between S0 and the cell membrane is still not understood.
Antimicrobial agents that compromise membrane integrity by forming pores, by disruption of the fluid mosaic model, and/or by lowering surface tension between the lipid and water phases have been known for some time (23, 27, 30, 31). The mechanism by which RBS interacts with membranes remains to be determined, as does the reason why it does not appear to have such an effect on eukaryotic cells (3, 10, 19). RBS is an extremely thermostable molecule and can be used even with hyperthermophilic archaea growing at the normal boiling point of water. However, RBS may not be the best choice as a selective molecule for developing a genetic system since mutants that confer resistance might be hard to isolate, given its direct reaction with the membrane seemingly in an unmodified form. Nevertheless, we do show here, 150 years after its discovery, that the anaerobic mechanism of action of this enigmatic molecule has nothing to do with NO but is due instead to its destructive interaction with prokaryotic membranes.
Acknowledgments
We thank Richard Cammack and Martin Hughes for the generous gift of Roussin's black salt, John P. Shields for the SEM work, Frank Jenney and Sonya Clarkson for helpful discussions, Kristina Carswell and Dele Oladapo for technical assistance, and Megan Cade for translating the original French research paper by M. L. Roussin.
This research was supported by grants from the National Institutes of Health (GM 60329) and the Department of Energy (FG05-95ER20175).
Footnotes
Published ahead of print on 5 February 2009.
REFERENCES
- 1.Asan, T., and M. Solberg. 1976. Inhibition of Clostridium perfringens by heated combinations of nitrite, sulfur, and ferrous or ferric ions. Appl. Environ. Microbiol. 31:49-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashworth, J., A. Didcock, L. L. Hargreaves, B. Jarvis, C. L. Walter, and L. F. Larkworthy. 1974. Chemical and microbiological comparisons of inhibitors derived thermally from nitrite with an iron thionitrosyl (Roussin black salt). J. Gen. Microbiol. 84:403-408. [DOI] [PubMed] [Google Scholar]
- 3.Bourassa, J., B. Lee, S. Bernard, J. Schoonover, and P. C. Ford. 1999. Flash photolysis studies of Roussin's black salt anion: Fe4S3(NO)7−. Inorg. Chem. 38:2947-2952. [DOI] [PubMed] [Google Scholar]
- 4.Butler, A. R., F. W. Flitney, and D. L. Williams. 1995. NO, nitrosonium ions, nitroxide ions, nitrosothiols and iron-nitrosyls in biology: a chemist's perspective. Trends Pharmacol. Sci. 16:18-22. [DOI] [PubMed] [Google Scholar]
- 5.Cammack, R., C. L. Joannou, X. Y. Cui, C. Torres Martinez, S. R. Maraj, and M. N. Hughes. 1999. Nitrite and nitrosyl compounds in food preservation. Biochim. Biophys. Acta 1411:475-488. [DOI] [PubMed] [Google Scholar]
- 6.Chmura, A., K. Szacilowski, A. Waksmundzka-Gora, and Z. Stasicka. 2006. Photochemistry of the [Fe4(μ3-S)3(NO)7]− complex in the presence of S-nucleophiles: a spectroscopic study. Nitric Oxide 14:247-260. [DOI] [PubMed] [Google Scholar]
- 7.Davis, K. L., E. Martin, I. V. Turko, and F. Murad. 2001. Novel effects of nitric oxide. Annu. Rev. Pharmacol. Toxicol. 41:203-236. [DOI] [PubMed] [Google Scholar]
- 8.Ding, B., Q. Guan, J. P. Walsh, J. S. Boswell, T. W. Winter, E. S. Winter, S. S. Boyd, C. Li, and P. B. Savage. 2002. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J. Med. Chem. 45:663-669. [DOI] [PubMed] [Google Scholar]
- 9.Fiala, G., and K. O. Stetter. 1986. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100-degrees C. Arch. Microbiol. 145:56-61. [Google Scholar]
- 10.Flitney, F. W., I. L. Megson, D. E. Flitney, and A. R. Butler. 1992. Iron-sulphur cluster nitrosyls, a novel class of nitric oxide generator: mechanism of vasodilator action on rat isolated tail artery. Br. J. Pharmacol. 107:842-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton-Brehm, S. D., G. J. Schut, and M. W. Adams. 2005. Metabolic and evolutionary relationships among Pyrococcus species: genetic exchange within a hydrothermal vent environment. J. Bacteriol. 187:7492-7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurst, R. D., and J. B. Clark. 1996. Investigations into the action of a novel nitric oxide donor on cellular respiration. Biochem. Soc. Trans. 24:460S. [DOI] [PubMed] [Google Scholar]
- 13.Joannou, C. L., X. Y. Cui, N. Rogers, N. Vielotte, C. L. Torres Martinez, N. V. Vugman, M. N. Hughes, and R. Cammack. 1998. Characterization of the bactericidal effects of sodium nitroprusside and other pentacyanonitrosyl complexes on the food spoilage bacterium Clostridium sporogenes. Appl. Environ. Microbiol. 64:3195-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johanson, G., and W. N. Lipscomb. 1958. The structure of Roussin's black salt, CsFe4S3(NO)7·H2O. Acta Crystallogr. 11:594-598. [Google Scholar]
- 15.Kanoksilapatham, W., J. González, D. L. Maeder, J. DiRuggiero, and F. T. Robb. 2004. A proposal to rename the hyperthermophile Pyrococcus woesei as Pyrococcus furiosus subsp. woesei. Archaea 1:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd, D., J. C. Harris, S. Maroulis, A. Mitchell, M. N. Hughes, R. B. Wadley, and M. R. Edwards. 2003. Nitrosative stress induced cytotoxicity in Giardia intestinalis. J. Appl. Microbiol. 95:576-583. [DOI] [PubMed] [Google Scholar]
- 17.Mannick, J. B. 2006. Immunoregulatory and antimicrobial effects of nitrogen oxides. Proc. Am. Thorac. Soc. 3:161-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maraj, S. R., S. Khan, X. Cui, R. Cammack, C. L. Joannou, and M. Hughes. 1995. Interactions of nitric oxide and redox-related species with biological targets. Analyst 120:699-703. [Google Scholar]
- 19.Matthews, E. K., E. D. Seaton, M. J. Forsyth, and P. P. Humphrey. 1994. Photon pharmacology of an iron-sulphur cluster nitrosyl compound acting on smooth muscle. Br. J. Pharmacol. 113:87-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Megson, I. 2000. Nitric oxide donor drugs. Drugs Future 25:701-715. [Google Scholar]
- 21.Mladenka, P., T. Simunek, M. Hubl, and R. Hrdina. 2006. The role of reactive oxygen and nitrogen species in cellular iron metabolism. Free Radic. Res. 40:263-272. [DOI] [PubMed] [Google Scholar]
- 22.Moran, D. M., S. R. Tannenbaum, and M. C. Archer. 1975. Inhibitor of Clostridium perfringens formed by heating sodium nitrite in a chemically defined medium. Appl. Microbiol. 30:838-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair, D. G., B. G. Fry, P. Alewood, P. P. Kumar, and R. M. Kini. 2007. Antimicrobial activity of omwaprin, a new member of the waprin family of snake venom proteins. Biochem. J. 402:93-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payne, M. J., C. Glidewell, and R. Cammack. 1990. Interactions of iron-thiol-nitrosyl compounds with the phosphoroclastic system of Clostridium sporogenes. J. Gen. Microbiol. 136:2077-2087. [DOI] [PubMed] [Google Scholar]
- 25.Perigo, J. A., E. Whiting, and T. E. Bashford. 1967. Observations on the inhibition of vegetative calls of Clostridium sporogenes by nitrite which has been autoclaved in a laboratory medium, discussed in the context of sub-lethally processed cured meats. J. Food Technol. 2:377-397. [Google Scholar]
- 26.Roussin, M. L. 1858. Recherches sur les nitrosulfures doubles de fer (nouvelle classe de sels.). Ann. Chim. Phys. 52:285. [Google Scholar]
- 27.Savage, P. B., C. Li, U. Taotafa, B. Ding, and Q. Guan. 2002. Antibacterial properties of cationic steroid antibiotics. FEMS Microbiol. Lett. 217:1-7. [DOI] [PubMed] [Google Scholar]
- 28.Schut, G. J., S. L. Bridger, and M. W. Adams. 2007. Insights into the metabolism of elemental sulfur by the hyperthermophilic archaeon Pyrococcus furiosus: characterization of a coenzyme A-dependent NAD(P)H sulfur oxidoreductase. J. Bacteriol. 189:4431-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schweinsberg, F., and V. Burkle. 1985. Nitrite: a co-carcinogen? J. Cancer Res. Clin. Oncol. 109:200-202. [DOI] [PubMed] [Google Scholar]
- 30.Singh, A., J. D. Van Hamme, and O. P. Ward. 2007. Surfactants in microbiology and biotechnology: part 2. Application aspects. Biotechnol. Adv. 25:99-121. [DOI] [PubMed] [Google Scholar]
- 31.Van Hamme, J. D., A. Singh, and O. P. Ward. 2006. Physiological aspects. Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol. Adv. 24:604-620. [DOI] [PubMed] [Google Scholar]
- 32.Verhagen, M. F., A. L. Menon, G. J. Schut, and M. W. Adams. 2001. Pyrococcus furiosus: large-scale cultivation and enzyme purification. Methods Enzymol. 330:25-30. [DOI] [PubMed] [Google Scholar]
- 33.Voevodskaya, N. V., V. A. Serezhenkov, C. E. Cooper, L. N. Kubrina, and A. F. Vanin. 2002. Exogenous ferrous iron is required for the nitric oxide-catalysed destruction of the iron-sulphur centre in adrenodoxin. Biochem. J. 368:633-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward, M. H., E. F. Heineman, R. D. McComb, and D. D. Weisenburger. 2005. Drinking water and dietary sources of nitrate and nitrite and risk of glioma. J. Occup. Environ. Med. 47:1260-1267. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg, M. V., G. J. Schut, S. Brehm, S. Datta, and M. W. Adams. 2005. Cold shock of a hyperthermophilic archaeon: Pyrococcus furiosus exhibits multiple responses to a suboptimal growth temperature with a key role for membrane-bound glycoproteins. J. Bacteriol. 187:336-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, Y., and N. Hogg. 2005. S-nitrosothiols: cellular formation and transport. Free Radic. Biol. Med. 38:831-838. [DOI] [PubMed] [Google Scholar]