Abstract
Molecular hydrogen produced biologically from renewable biomass is an attractive replacement for fossil fuels. One potential route for biological hydrogen production is the conversion of biomass into formate, which can subsequently be processed into hydrogen by Escherichia coli. Formate is also a widely used commodity chemical, making its bioproduction even more attractive. Here we demonstrate the implementation of a formate-overproducing pathway in Saccharomyces cerevisiae, a well-established industrial organism. By expressing the anaerobic enzyme pyruvate formate lyase from E. coli, we engineered a strain of yeast that overproduced formate relative to undetectable levels in the wild type. The addition of a downstream enzyme, AdhE of E. coli, resulted in an additional 4.5-fold formate production increase as well as an increase in growth rate and biomass yield. Overall, an 18-fold formate increase was achieved in a strain background whose formate degradation pathway had been deleted. Finally, as a proof of concept, we were able to produce hydrogen from this formate-containing medium by using E. coli as a catalyst in a two-step process. With further optimizations, it may be feasible to use S. cerevisiae on a larger scale as the foundation for yeast-based biohydrogen.
Over the past few years, hydrogen has emerged as a promising renewable energy source as pressure mounts to replace fossil fuels. Among its primary advantages, hydrogen is energetically denser than all other combustible fuels, it combusts into pure water, and its utilization in fuel cells is more efficient than current combustion engines (11). Currently, hydrogen is produced commercially primarily by coal gasification, steam reformation of natural gas, and water electrolysis (25). These methods are not only too costly for hydrogen to compete with gasoline as a fuel, but they are also energy intensive and therefore not environmentally friendly. Given the factors described above, biological hydrogen production may present a useful alternative due to its use of renewable biomass or sunlight as its primary energy source.
Hydrogen can be produced by numerous microbes either photosynthetically or fermentatively using biomass as the carbon and energy supply. While photosynthetic methods use sunlight directly, fermentative approaches are generally faster than their photosynthetic counterparts (51) and require simpler bioreactors (32). Additionally, fermentative processes are typically anaerobic, thus eliminating the need for an oxygen-tolerant hydrogenase, a major hurdle in the photosynthetic field (33).
To date, almost all fermentative biohydrogen approaches utilized organisms which can naturally produce hydrogen via their hydrogenases, such as Escherichia coli, Clostridia, and other anaerobes (7, 19, 22, 52). Although engineering these organisms has its associated benefits given their natural hydrogen production, using the established industrial organism Saccharomyces cerevisiae offers extensive advantages. Yeasts are well suited for industrial scale-up due to knowledge from the ethanol industry, they are a GRAS (generally recognized as safe) organism, their metabolism is well understood, and they are simple to manipulate genetically. Moreover, much research is ongoing to enhance the biomass utilization of these eukaryotes, specifically with respect to cellulose, xylose, and arabinose (37, 39, 43, 48).
Formate is the direct metabolic precursor for hydrogen synthesis in E. coli (36). In these bacteria, formate is cleaved anaerobically by the formate hydrogen lyase (FHL) complex into hydrogen and CO2 (36). FHL contains five membrane-bound proteins and a unique selenocysteine amino acid within its active site, which together make heterologous expression difficult (4, 36, 45). Nonetheless, recent studies have demonstrated that engineered E. coli can catalyze the conversion of formate to hydrogen at high productivity rates (23.6 g H2 h−1 liter−1) and at efficiencies above 90% when formate is supplied in the medium (22, 51). Thus, engineering yeast to overproduce formate can serve as a basis for yeast-based biohydrogen in which the overproduced formate is subsequently converted into hydrogen by E. coli. Additionally, formate is a widely used commodity chemical whose bioproduction is of interest due to the current cost of its chemical synthesis (14).
Pyruvate formate lyase (PFL) is the primary enzyme responsible for formate synthesis in E. coli (36). This anaerobic enzyme is cleaved by oxygen, and catalyzes the radical-based reaction: pyruvate + coenzyme A (CoA) ↔ formate + acetyl-CoA (34). PFL is a dimer of PflB, whose maturation requires the activating enzyme PflAE (encoded by pflA), radical S-adenosylmethionine, and a single electron donor, which in the case of E. coli is flavodoxin (5, 35). During anaerobiosis, the acetyl-CoA generated by PFL is reduced into ethanol by the bifunctional enzyme AdhE, which helps the cell restore redox balance by oxidizing two NADHs in the process (8, 18). It was previously suggested that AdhE protects PFL from oxygen-induced cleavage of the enzyme once anaerobic conditions are lost, although this notion has been disputed (17, 18, 26).
In this study, we engineered yeast to secrete formate through the deletion of the endogenous formate dehydrogenases (FDHs) and expression of PFL and AdhE. We show that the addition of AdhE to this synthetic pathway increases formate concentrations as well as growth rate compared to PFL expression alone. In total, formate concentration in the medium was increased by 18-fold in the FDH deletion background. Finally, we demonstrate that this formate-containing medium can be used for hydrogen production via E. coli. This approach establishes the foundation for a two-organism biohydrogen production scheme in which the industrial advantages of yeast are combined with E. coli's efficient hydrogen production.
MATERIALS AND METHODS
Construction of plasmids.
The strains, plasmids, and primers used in this study are shown in Table 1. The pflB, pflA, and adhE genes were amplified using their respective 5′ and 3′ primers. The genes were cloned by the “biobrick” assembly strategy of prefix and suffix insertions (29), using the restriction enzymes EcoRI, XbaI, SpeI, and PstI. P0511-BB and L0101-BB were created in a previous study (2). The final constructs, PPS3163-BB, PPS3164-BB, and PPS3165-BB, consisted of pGal1-gene-Adh1_Terminator and were transferred into yeast integrating shuttle vectors (pRS304-6) (38). pflB and adhE contain internal PstI recognition sites, and hence that restriction enzyme was not used in their cloning.
TABLE 1.
Strains, plasmids, and cloning primers used in this study
| Strain, plasmid or primer | Genotype, description, or sequence | Source or reference |
|---|---|---|
| Strains | ||
| S. cerevisiae | ||
| PSY580a (wild type) | Mataura3Δ52 trp1Δ63 leu2Δ1GAL2+ | 47 |
| PSY3643 | PSY580a Δfdh1::loxP-kanMX-loxP | This work |
| PSY3644 | PSY580a Δfdh1::loxP | This work |
| PSY3645 | PSY580a Δfdh1::loxP Δfdh2::loxP-kanMX-loxP | This work |
| PSY3646 (ΔFDH1 ΔFDH2) | PSY580a Δfdh1::loxP Δfdh2::loxP | This work |
| PSY3647 | PSY3646 LEU2::pflB | This work |
| PSY3648 (PFL) | PSY3646 LEU2::pflBTRP1::pflA | This work |
| PSY3649 (PFL and AdhE) | PSY3646 LEU2::pflBTRP1::pflAURA3::AdhE | This work |
| E. coli | ||
| W3110 | F− λ− IN(rrnD-rrnE)1 rph-1 | ATCC 27325 |
| Plasmids | ||
| pUG6 | Deletion plasmid; kanMX marker | 15 |
| pSH65 | Cre-expressing plasmid; phleomycin gene marker | 15 |
| V0120-BB | Empty biobrick vector | 29 |
| P0511-BB | pGal1 | 2 |
| L0101-BB | Adh1 terminator | 2 |
| PPS3160-BB | V0120; pflB | This work |
| PPS3161-BB | V0120; pflA | This work |
| PPS3162-BB | V0120; adhE | This work |
| PPS3163-BB | V0120; pGal1-pflB-Adh1_Term | This work |
| PPS3164-BB | V0120; pGal1-pflA-Adh1_Term | This work |
| PPS3165-BB | V0120; pGal1-adhE-Adh1_Term | This work |
| pRS304 | S. cerevisiae integration vector; trp1 marker | 38 |
| pRS305 | S. cerevisiae integration vector; leu2 marker | 38 |
| PRS306 | S. cerevisiae integration vector; ura3 marker | 38 |
| PPS3166 | pRS304; pGal1-pflA-Adh1_Term | This work |
| PPS3167 | pRS305; pGal1-pflB-Adh1_Term | This work |
| PPS3168 | pRS306; pGal1-adhE-Adh1_Term | This work |
| Cloning primers | ||
| FDH1/2-hom1 | ATGTCGAAGGGAAAGGTTTTGCTGGTTCTTTACGAAGGTGCAGCTGAAGCTTCGTACGC | This work |
| FDH1/2-hom2 | TTATTTCTTCTGTCCATAAGCTCTGGTGGCATAAGAACCAGCATAGGCCACTAGTGGATCTG | This work |
| 5′ pflB | CCTTGAATTCGCGGCCGCATCTAGAATGTCCGAGCTTAATGAAAAG | This work |
| 3′ pflB | AAGGACTAGTTTACATAGATTGAGTGAAGGTACGAGTAATAACGTCCTGCTG | This work |
| 5′ pflA | CCTTGAATTCGCGGCCGCATCTAGAATGTCAGTTATTGGTCGCAT | This work |
| 3′ pflA | AAGGACTAGTTTAGAACATTACCTTATGACCGTACTGCTCAAGAATGCC | This work |
| 5′ adhE | CCTTGAATTCGCGGCCGCATCTAGAATGGCTGTTACTAATGTCG | This work |
| 3′ adhE | AAGGACTAGTTTAAGCGGATTTTTTCGCTTTTTTCTCAGCTTTAGCCGG | This work |
Construction of S. cerevisiae strains.
The background strain used in this study is PSY580a (47). FDH1 and FDH2 deletions were performed in series using the Gueldener strategy (15) with pUG6 as the integration template and pSH65 for removal of the resistance marker. Due to sequence similarity, the same primers were used for both FDHs as previously described (28).
PPS3166, PPS3167, and PPS3168 were sequenced for verification, linearized by restriction digest, and integrated into the trp1, leu2, and ura3 loci, respectively. All integrations and deletions were performed using standard yeast transformation techniques and were verified via PCR.
Cultivation conditions.
S. cerevisiae cells were initially cultivated at 30°C in synthetic complete (SC) yeast medium containing 2% galactose under aerobic conditions. For formate secretion experiments, the yeast cells were diluted to an optical density at 600 nm (OD600) of 0.05 in a volume of 100 ml in 250-ml flasks using the same medium composition. The cells were grown in an anaerobic chamber (Coy Laboratories) at 30°C and shaken at 150 rpm. Ergosterol and Tween 80, two essential anaerobic factors, were dissolved in ethanol and added at final concentrations of 10 mg liter−1 and 420 mg liter−1, respectively (44). To prevent medium acidification, SC medium (pH 6.5) containing 125 mM MES (morpholineethanesulfonic acid) buffer (pH 6.5) was used when indicated. SC medium contains 0.5% ammonium sulfate as its primary nitrogen source. When urea was used as an alternate nitrogen source, 1% urea was added to SC medium lacking in ammonium sulfate. Formate was supplemented from a 1 M stock when required.
Samples were taken for measurements of cell density, pH, and formate concentration. Formate and pH were measured using centrifuged medium (4,000 rpm for 10 min). Formate was detected spectrophotometrically using an enzymatic assay (R-Biopharm AG).
Intracellular metabolite analysis.
Samples for intracellular metabolite measurements were prepared according to the method of Lange et al. (6, 20, 46). Briefly, 10-ml samples were quickly drawn at a cell density of an OD600 of 1.0 and immediately quenched in 40 ml cold 60% (vol/vol) methanol. Two subsequent washes were performed using the cold quenching solution, followed by metabolite extraction using the boiling ethanol method (180 s at 95°C) as mentioned in the study by Lange et al. (20). A frozen binary solution of 60% (vol/vol) ethanol was used to maintain the yeast samples and quenching solution at −40°C. Samples were lyophilized, resuspended in 180 μl water, and centrifuged twice before use. The supernatant was stored at −80°C until processing via high-performance liquid chromatography (HPLC).
HPLC measurements were performed using a Waters 2695 HPLC separation module fitted with a Luna C18 5μ column (250 by 4.5 mm; Phenomenex). Samples of 50 to 80 μl were injected and eluted at a flow rate of 1 ml/min, starting with 100% mobile phase buffer A and gradually increasing to 100% mobile phase buffer B (9). Specifically, the relative fraction of buffer B in the mobile phase was increased at a rate of 15%/min until it reached 60%; at 0.6%/min until it reached 80%, during which the majority of separation occurred; and at 20%/min until it reached 100%. Buffer A contained 10 mM tetrabutylammonium hydroxide, 10 mM KH2PO4, and 0.25% methanol at pH 7.0 (9). Buffer B contained 2.8 mM tetrabutylammonium hydroxide, 100 mM KH2PO4, and 30% methanol at pH 5.5 (9). ATP, ADP, and NAD+ were analyzed at 260 nm, and NADH was analyzed at 340 nm using a photodiode array detector (Waters 996) (9, 40). Standard curves of the metabolites were performed to enable quantification.
Hydrogen assay.
E. coli strain W3110 was grown aerobically to saturation in LB medium. Cells were harvested, washed with phosphate buffer, and inoculated anaerobically to an OD600 of 6.0 in the presence of spent yeast medium. Airtight, 44-ml Suba seal vials (Sigma) were used, leaving a 5-ml headspace. After 8 h, gas content was analyzed via gas chromatography (Shimadzu GC14A) using a TCD detector at 180°C and a ShinCarbon ST column (Restek Corporation) at 40°C.
RESULTS
Construction of a formate-producing pathway in S. cerevisiae.
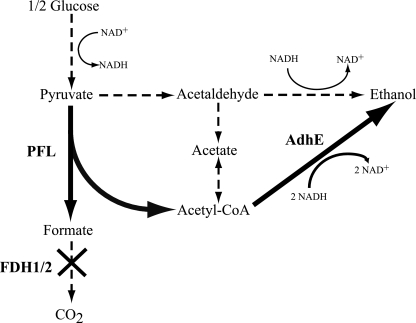
S. cerevisiae contains two highly homologous FDHs, FDH1 and FDH2, which catalyze the reaction formate + NAD+ ↔ NADH + CO2 (28). Since the goal of the experiments was to engineer yeast to secrete formate, we created a ΔFDH1 ΔFDH2 double-deletion mutant (PSY3646) which served as a parent strain for this study. Using this mutant, we integrated pflA, pflB, and adhE (all from E. coli) to create the formate-overproducing strains. The three exogenous genes were put under the control of the inducible promoter, pGal1. Together, PFL and AdhE essentially serve as an alternative fermentation route to the S. cerevisiae pyruvate-to-ethanol pathway, which is catalyzed by pyruvate decarboxylases (PDCs) and alcohol dehydrogenases. Both the artificial and endogenous pathways help maintain a functional redox balance anaerobically by regenerating NAD+ (Fig. 1).
FIG. 1.
Schematic representation of central yeast metabolism, including the synthetic pathway for formate production. Some of the intermediates and metabolic branching points are not illustrated for simplicity. Bold arrows represent heterologous reactions. Dashed lines represent native pathways. PFL is from E. coli, FDH1/2 represents the native FDH1 and -2 from S. cerevisiae, and AdhE represents acetaldehyde/alcohol dehydrogenase E from E. coli. The X represents the deletion of the FDH1 and -2 genes.
FDH deletion eliminates formate consumption in media under anaerobic conditions.
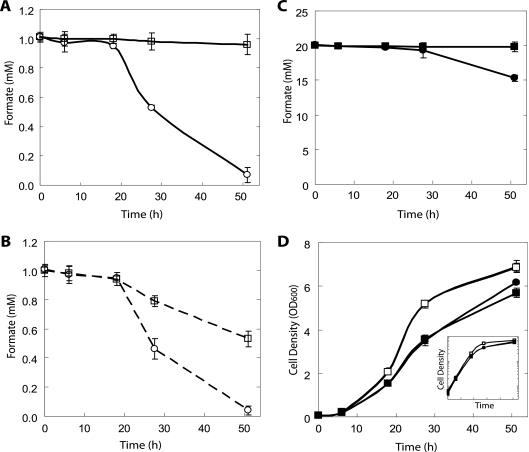
Previously, it has been demonstrated that the deletion of both FDH genes almost completely inhibits formate consumption from formate-containing media (28). However, this previous study was performed aerobically using glucose, whereas we use galactose as the carbon source under anoxic conditions due to the anaerobic requirements of PFL. Additionally, in our study we used a background strain of yeast different from that reported. Therefore, we verified that our FDH deletion strain (PSY3646) had essentially undetectable consumption under our conditions in both 1 mM and 20 mM starting formate concentrations (Fig. 2A and C).
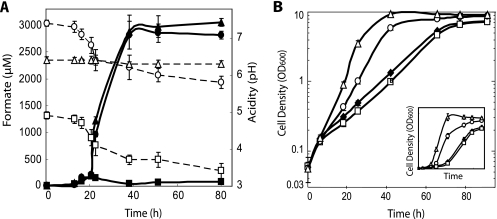
FIG. 2.
Effect of FDH1 and FDH2 deletions on residual formate levels in anaerobic conditions, using galactose as a carbon source. Liquid cultures of S. cerevisiae were grown under pH-buffered or unbuffered conditions with different initial formate concentrations. (A) 1 mM initial formate, pH buffered; (B) 1 mM initial formate, no pH buffering; (C) 20 mM initial formate, pH buffered; (D) growth curves of pH-buffered experiments. The inset depicts the growth on a logarithmic scale. When pH buffering was used, final values reached 6.35 ± 0.05, starting from a pH of 6.5. Final pH values without buffering reached 3.5 ± 0.1. ○, PSY580a (wild type) with 1 mM initial formate; □, PSY3646 (ΔFDH1 ΔFDH2) with 1 mM initial formate; •, PSY580a with 20 mM initial formate; ▪, PSY3646 with 20 mM initial formate. The dashed line indicates no pH buffering. The growth rates of both strains in 1 mM initial formate were almost identical: hence the overlap in panel D. The error bars indicate standard deviations of three replicates.
When fermenting anaerobically under unbuffered conditions, yeast cells acidify the medium as biomass increases. As a result, residual formate levels in unbuffered medium were decreased due to the higher vapor pressure (52 mm Hg at 30°C) of the undissociated acid (pKa, 3.74), which is abundant at low pH, in comparison to water (31.8 mm Hg at 30°C) (Fig. 2A and B) (27). Therefore, we buffered the medium to pH 6.5 to eliminate evaporation of nonionized formic acid. This served another purpose by enabling the use of the spent medium for hydrogen production via E. coli, given that anaerobically grown E. coli cells require buffering to neutralize their fermentation products and cannot tolerate the acidic pH generated by unbuffered yeast.
In agreement with a previous aerobic study, higher formate concentrations resulted in reduced biomass yield under our anaerobic conditions as well (Fig. 2D) (28). This explains the increased biomass yield of the wild-type strain (PSY580a) compared to PSY3646 in 20 mM formate toward the end of the fermentation, at which the formate levels have been noticeably decreased by the FDHs of PSY580a. Formate's biomass inhibition is likely due to its effect as a weak acid, including its harmful effects in the mitochondria (10). Nonetheless, substantial biomass can still be produced as shown by the high-cell density attained even at a 20 mM formate concentration.
Formate production using engineered S. cerevisiae.
Initially we evaluated the production of formate in PSY3647 (which expresses pflB alone), PSY3648 (pflB and pflA), and PSY3649 (pflB, pflA, and AdhE). Due to the oxygen sensitivity of PFL, increased formate production was observed only under anaerobic conditions (data not shown). Based on the levels of formate accumulation in the medium, function of PFL required pflA (PFL-activating enzyme) expression, but not flavodoxin (fldA or fldB) expression, despite the role of flavodoxin as a cofactor for PFL activation in E. coli (5, 35).
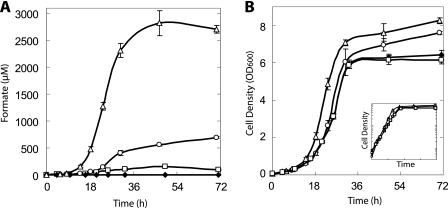
In order to assay for formate secretion, the engineered yeast cells were grown anaerobically, using pH-buffered SC medium with 2% galactose. Under these conditions, pH values remained almost unchanged throughout fermentation (starting pH 6.5 and final pH 6.35 ± 0.05), thereby negating the loss of formate due to vaporization. We observed that PSY3646 (ΔFDH1 ΔFDH2) secreted low levels of formate presumably since S. cerevisiae cells generate small amounts of this compound through tetrahydrofolate-mediated one-carbon metabolism (Fig. 3A) (30). Formate was not detected at any stage of growth using PSY580a (wild type), likely due to its active FDHs which degrade this endogenously produced metabolite.
FIG. 3.
Formate secretion and growth of the engineered S. cerevisiae strains. Formate concentrations and cell densities were assayed at different time points using the different S. cerevisiae strains listed below. (A) Residual formate concentrations in the media. (B) Growth curves of the different strains. The inset illustrates the growth on a logarithmic scale. The initial pH was 6.5 and reached a final value of 6.35 ± 0.05. ⧫, PSY580a; □, PSY3646 (ΔFDH1 ΔFDH2); ○, PSY3648 (PFL); ▵, PSY3649 (PFL and AdhE). The error bars indicate standard deviations of three replicates.
Using the PFL-containing strain (PSY3648), formate secretion was increased 4.5-fold with respect to PSY3646 (Fig. 3A and Table 2). Strikingly, the combination of both enzymes PFL and AdhE generated a dramatic increase in formate compared to PFL alone: 2,820 ± 237 μM versus 696 ± 21 μM. With respect to PSY3646, PSY3649 (PFL and AdhE) fermentation displayed an 18-fold increase in residual formate.
TABLE 2.
Comparison of growth rates and increase in formate concentrations in mediuma
| Strain | Log-phase growth rate (h−1)b | Final cell density (OD600)b | Maximal formate concn (μM)b | Fold increase in formate vs parent |
|---|---|---|---|---|
| PSY580a | 0.15 ± 0.01 | 6.59 ± 0.26 | 0 ± 0 | |
| PSY3646 (parent) | 0.15 ± 0.00 | 6.15 ± 0.22 | 154 ± 3 | 1 |
| PSY3648 | 0.15 ± 0.01 | 7.60 ± 0.09 | 696 ± 21 | 4.5 |
| PSY3649 | 0.18 ± 0.01 | 8.25 ± 0.14 | 2820 ± 237 | 18 |
All strains were grown in SC medium with 2% galactose.
Values are means ± standard deviations of three replicates.
A difference in growth rates and biomass yield between the strains was also observed (Fig. 3B and Table 2). PSY3648 and PSY3649 both had larger biomass yields than PSY3646 and PSY580a, whereas PSY3649 also had a faster growth rate. This growth phenotype was also observed when the strains were grown in unbuffered SC medium (data not shown).
Comparing intracellular ATP/ADP and NAD+/NADH ratios between the strains.
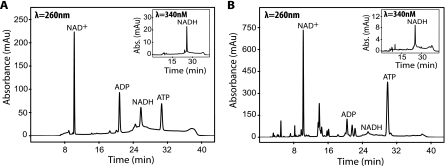
In order to further understand the growth differences between the formate-overproducing strains (PSY3649 and PSY3648) compared to the background strains (PSY580a and PSY3646), we assayed their intracellular ATP/ADP and NAD+/NADH ratios (Fig. 4 and Table 3). These two measurements give an indication of the yeast strains' energetic and redox states, respectively. No significant difference in energetic ratios was observed between the strains (Table 3). Values regarding differences in redox balance indicate that PSY3649 (4.59 ± 0.97) has a slightly increased NAD+/NADH ratio in comparison to PSY580a (3.43 ± 0.77) (P < 0.11, Student's t test). The large standard deviations in our experiment coincide with the reality that measuring intracellular NAD+/NADH ratios in yeast is still challenging, with relatively large variations in reported ratios (6, 16, 40, 42, 46).
FIG. 4.
Quantification of intracellular yeast metabolites using HPLC. (A) Standard chromatogram containing 10 nmol each of ATP, ADP, NAD+, and NADH in water. (B) Chromatogram of a representative S. cerevisiae sample (PSY3649). All samples were processed at an OD600 of 1.0. ATP, ADP, and NAD+ were quantified using their respective absorptions (milliabsorbance units [mAU]) at 260 nm. NADH was quantified at 340 nm due to its enhanced signal/noise ratio at this wavelength (insets in panels A and B).
TABLE 3.
Comparison of intracellular NAD+/NADH and ATP/ADP concentration ratiosa
| Strain | Cell density (OD600) | ATP/ADP ratio | NAD+/NADH ratio |
|---|---|---|---|
| PSY580a | 1.05 ± 0.18 | 2.97 ± 0.38 | 3.43 ± 0.77 |
| PSY3646 | 1.00 ± 0.12 | 2.80 ± 0.38 | 3.67 ± 0.75 |
| PSY3648 | 0.95 ± 0.13 | 2.92 ± 0.51 | 4.08 ± 0.84 |
| PSY3649 | 0.94 ± 0.15 | 2.75 ± 0.41 | 4.59 ± 0.97 |
All strains were grown in unbuffered SC medium with 2% galactose. Values are means ± standard deviations of at least four replicates.
Formate production with urea as an alternative nitrogen source.
Adding urea to the medium increases the starting pH of the yeast culture. Therefore, we hypothesized that replacing the nitrogen source component of the medium (ammonium sulfate) with urea would enable formate production without the use of a pH buffer. Indeed, residual formate concentrations were only slightly decreased when using unbuffered urea medium (2,806 ± 74 μM) in comparison to buffered urea medium (3,041 ± 71 μM) (Fig. 5A). These formate concentrations are comparable to those achieved with ammonium sulfate (Table 2). In contrast, formate levels were almost completely abolished when no buffering was used together with ammonium sulfate-containing medium (Fig. 5A), likely due to the evaporation of formic acid at the low pH associated with this fermentation.
FIG. 5.
Formate secretion and growth using urea as an alternative nitrogen source. Formate concentrations and cell densities were assayed at different time points throughout growth. (A) Residual formate concentrations and pH of the medium using strain PSY3649 (PFL and AdhE). Solid symbols indicate formate concentrations, open symbols indicate pH values. ▵, PSY3649 with urea and pH buffering; ○, PSY3649 with urea but without buffering; □, PSY3649 without urea and without buffering. (B) Growth curves of the different engineered S. cerevisiae strains using urea as the nitrogen source on a logarithmic scale. The inset displays the growth on a linear scale. ⧫, PSY580a; □, PSY3646 (ΔFDH1 ΔFDH2); ○, PSY3648 (PFL); ▵, PSY3649. The error bars indicate standard deviations of three replicates.
Similarly to the previous experiment, a fast-growth phenotype was again noticed when comparing the PFL-containing strains to PSY580a and PSY3646 (Fig. 5B and Table 4). However, growth of all strains was slower in this case due to the use of urea, a poorer nitrogen source than ammonium sulfate (41). Therefore, the use of urea presents a tradeoff between the opportunity of not using buffer and a longer fermentation.
TABLE 4.
Comparison of growth rates with urea as the nitrogen sourcea
| Strain | Log-phase growth rate (h−1) | Final cell density (OD600) |
|---|---|---|
| PSY580a | 0.06 ± 0.00 | 7.48 ± 0.33 |
| PSY3646 | 0.06 ± 0.00 | 8.24 ± 0.21 |
| PSY3648 | 0.10 ± 0.00 | 8.64 ± 0.15 |
| PSY3649 | 0.16 ± 0.01 | 9.13 ± 0.16 |
All strains were grown in SC medium with 1% urea. Values are means ± standard deviations of three replicates.
Hydrogen production using yeast-derived formate.
Spent medium was collected from yeast cultures to evaluate the feasibility of using yeast-derived formate for hydrogen production via E. coli as a catalyst. Cultures of anaerobically grown E. coli cells were harvested and cultured at an OD600 of 6.0 in closed vials in the presence of the spent yeast medium. Hydrogen was assayed by gas chromatography after a long incubation (8 h) such that the majority of the formate was metabolized by the high-density E. coli culture (data not shown). As mentioned, E. coli does not grow in the highly acidic environment generated by the fermentation of S. cerevisiae in unbuffered SC medium (pH 3.5 ± 0.1). Therefore, the use of previously buffered yeast medium (final pH 6.35 ± 0.05) or unbuffered medium containing urea as the nitrogen source (final pH >5.5) was essential in order to maintain a suitable pH for hydrogen production.
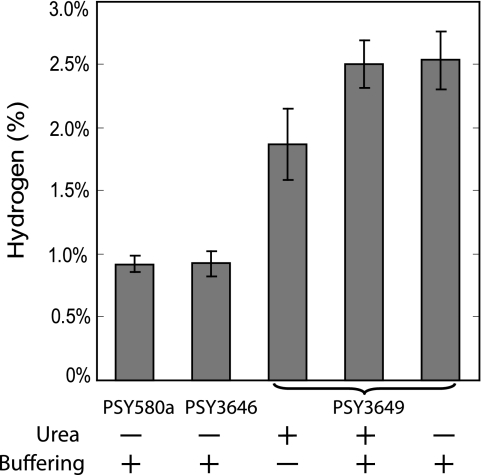
Hydrogen was observed in the headspace of the non-formate-overproducing strains due the consumption of the yeast fermentation products and their subsequent conversion into formate and later hydrogen by PFL and FHL, respectively. However, the amount of hydrogen detected in the headspace of PSY3649 was significantly larger than those for PSY3646 and PSY580a, resulting in a 2.7-fold increase when using buffered medium (Fig. 6). This increase can be attributed to the increased formate secretion of the engineered strain, since no difference was observed between PSY580a and PSY3646. The use of unbuffered urea medium resulted in increased hydrogen production compared to PSY3646 and PSY580a; however, this increase was further enhanced when the medium was buffered. The nitrogen source of the yeast medium did not have a noticeable effect on hydrogen production, which is evident given the similar formate concentrations of unbuffered urea medium, buffered urea medium, and buffered SC (ammonium sulfate containing) medium, which were 2,988 μM, 3,015 μM, and 2,951 μM, respectively.
FIG. 6.
Hydrogen production using cultured yeast media as a substrate. E. coli were grown anaerobically in a closed environment on the spent media of different yeast strains (PSY580a, wild type; PSY3646, ΔFDH1 ΔFDH2; PSY3649, PFL and AdhE). Hydrogen was assayed from the headspace. The error bars indicate standard deviations of three replicates.
DISCUSSION
In this work, we report that S. cerevisiae can be engineered to overproduce formate via a synthetic pathway. We observe that pflA is required for PFL function, while flavodoxin is not. We also show that PFL alone enables formate overproduction and that the addition of AdhE enhances formate levels 4.5-fold. In addition to formate overproduction, the artificial pathway also enhanced growth rate and biomass yield. This growth phenotype may be in part due to the alleviation of anaerobic redox stress by AdhE. Finally, we demonstrate that this yeast-derived formate, a molecule that has economic value in its own right, can be used for hydrogen production in a two-step process using E. coli.
Based on our results, PFL function in S. cerevisiae requires expression of both the structural gene encoding the PFL homodimer (pflB) and its activating enzyme (pflA), but not E. coli flavodoxin (fldA or fldB). Inactive PFL is converted into its active form under anaerobiosis by the stabilization of a glycyl radical in its active site, a process which is mediated by PflAE (5, 35). Additionally, in E. coli, flavodoxin is thought to be a cofactor that acts as a single electron donor in this process of PFL activation (35). In vitro, flavodoxin can be replaced by photoreduced 5-deazariboflavin (53), indicating that another electron donor may be used. Taken together, our findings imply that S. cerevisiae contains a cytosolic, single-electron donor capable of activating PFL. This could perhaps be the previously identified flavodoxin-like protein YLR011wp, found in this organism (21).
One surprising finding was that AdhE increases yeast growth rate and overall formate secretion. This was not necessarily expected since the enzyme itself does not produce formate. One possible hypothesis is that AdhE decreases acetyl-CoA levels and in turn increases PFL kinetics by distancing it from chemical equilibrium (Fig. 1). Additionally, since PFL does not regenerate NAD+, an essential metabolic requirement in anaerobiosis, the fact that AdhE generates two NAD+ molecules may allow higher flux through the PFL-AdhE artificial pathway. This added flux acts as an additional fermentation pathway in yeast. In turn, this likely increases the glycolytic flux and the consequent ATP production rate, which may partially explain the observed increase in growth rate and biomass yield (Tables 2 and 4).
In line with the above findings, we tested to see if the difference in growth rate could be attributed to a difference in the energetic or redox state of the yeast. Despite our intuition that there would be differences in the ATP/ADP ratio given our observation that the ratio can change dramatically between the exponential phase growth and saturation (data not shown), all strains had very similar energetic states (Table 3). Therefore, this ratio does not explain the differences in growth rate and biomass yield. With respect to our other hypothesis that the redox stress may be eased in the presence of AdhE, our data suggest that there is a slight increase in the NAD+/NADH ratio of the AdhE-containing strain (4.59 ± 0.97 compared to 3.43 ± 0.77 in PSY580a; P < 0.11 by Student's t test). This suggests that AdhE presents an alternative to glycerol synthesis as a means of recycling excess NADH in anaerobically grown S. cerevisiae. Consequently, this may explain in part why PSY3649 grows faster and reaches higher biomass yields than the other strains.
Limiting the acidity of the medium is necessary both for inhibition of formate evaporation and for hydrogen production with E. coli. We demonstrated that the use of a pH buffer or urea as a nitrogen source prevents the majority of formic acid evaporation (Fig. 2). Formate yields on both nitrogen sources were comparable, with a slight increase in the case of pH-buffered urea (3,041 ± 71 μM versus 2,820 ± 237 μM). However, the use of urea without pH buffer resulted in slower growth and in a final pH (pH 5.8 ± 0.15) which was suboptimal for hydrogen production; the optimal hydrogen production pH was estimated to be 6.5 (51). Taken together, finding an ideal combination between urea, ammonium sulfate, and pH buffer concentrations may enhance the yield of the process while reducing the cost associated with excess buffer.
In a final experiment, we constructed a two-cell system for hydrogen production in which spent medium from the different yeast strains was used by E. coli to produce hydrogen. The increase in hydrogen production using the spent medium of PSY3649 (PFL and AdhE), in comparison to that of PSY3646 (ΔFDH1 ΔFDH2), was 2.7-fold (Fig. 6). This corresponds to a difference of 2.8 mM formate between the spent media of the two yeast strains. The actual efficiency of this formate to hydrogen conversion is difficult to calculate due to the presence of additional fermentation products, some of which are metabolized into CO2 and hydrogen. Regardless, this yield may potentially be enhanced by further engineering of the E. coli as previously demonstrated (23, 51).
Despite the 18-fold increase in formate compared to PSY3646 (ΔFDH1 ΔFDH2), PSY3649 (PFL and AdhE) only produced 1.5% of its maximal theoretical yield of two formates per galactose. Accordingly, our method as presented in this work is currently not competitive with E. coli as this organism can produce formate at 33% efficiency from glucose (50). E. coli strains have also been engineered to achieve a 90% theoretical yield of hydrogen from glucose (1.82 mol H2/mol glucose) (52); however, this process has many limitations, including a lack of sustainability due to inhibition of FHL by E. coli's other fermentation products and the need for a metabolite extraction system (50, 52). In addition, the specific hydrogen productivity was an order of magnitude slower using glucose instead of formate, which again highlights the potential benefits of a two-step process in which E. coli cells convert formate instead of glucose into hydrogen.
Perhaps the most promising approach to increasing the efficiency of formate production is by deleting the PDCs. It has been shown that knocking out the PDCs can increase yields in similar studies where there is enzymatic competition over pyruvate, as in our approach (Fig. 1) (13, 31). PDC-negative strains cannot grow in excess glucose and cannot synthesize sufficient amounts of cytosolic acetyl-CoA, a phenotype which can be rescued aerobically by the supplementation of a C2 carbon source (12). We constructed these deletion mutants in the background of PSY3648 (PFL) and PSY3649 (PFL and AdhE), hoping that the acetyl-CoA generated by PFL would enable growth. Despite our observation that the PDC knockouts can grow aerobically on galactose (data not shown), albeit very slowly, the strains were unable to grow or metabolize anaerobically. This anaerobic inviability may be due to a lack of cytosolic acetyl-CoA synthesis due to insufficient flux through PFL, improper redox balance, growth inhibition by galactose, or a combination of the above.
Several additional considerations should be taken into account in optimizing formate and hydrogen yields. Yeast can use formate to generate formyl-tetrahydrofolate (24); therefore, manipulation of the folate pathways might also result in an increase in formate production. Additionally, increasing expression of PFL and AdhE by means of high-expression plasmids may offer another possibility to compete against the endogenous PDCs. In this regard, it should be noted that the major downstream product of the PDCs is ethanol, which is also a valuable by-product given its role as a biofuel. Finally, carbon dioxide is emitted in conjunction with hydrogen in this method, and while it is neutral to hydrogen fuel cells (1), it can be separated in order to increase the component of hydrogen in the gas. This can be done by means of membranes, water-gas shift reactions, methanation, absorption in amine solutions, and adsorption (1, 3, 49).
In summary, our findings set the basis for using yeast in the production of formate and hydrogen. We focused on yeast because of its prominence as an industrial organism, with the potential for scale-up having been well established. Further research including flux analysis, strain evolution, and metabolic engineering should enable an increase in yields and a better understanding of the enhanced growth phenotype. Finally, given formate's role in hydrogen production and the fact that yeasts are currently the most robust organism in the fermentation-based energy industry, this approach may offer promise in advancing the biohydrogen field.
Acknowledgments
We thank David A. Drubin, David F. Savage, Caleb J. Kennedy, and Jeffrey C. Way for helpful comments on the manuscript. We thank Dave F. Savage for assistance with gas chromatography. We thank Edwin Tan for assistance with the HPLC.
Support for this work was provided by funds from the Harvard Integrated Life Sciences Program (HILS) and from Harvard University.
Footnotes
Published ahead of print on 6 February 2009.
REFERENCES
- 1.Ahmed, S., and M. Krumpelt. 2001. Hydrogen from hydrocarbon fuels for fuel cells. Int. J. Hydrogen Energy 26:291-301. [Google Scholar]
- 2.Ajo-Franklin, C. M., D. A. Drubin, J. A. Eskin, E. P. Gee, D. Landgraf, I. Phillips, and P. A. Silver. 2007. Rational design of memory in eukaryotic cells. Genes Dev. 21:2271-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, R. 2001. Future directions of membrane gas-separation technology. Membr. Technol. 2001:5-10. [Google Scholar]
- 4.Boyington, J. C., V. N. Gladyshev, S. V. Khangulov, T. C. Stadtman, and P. D. Sun. 1997. Crystal structure of formate dehydrogenase H: catalysis involving Mo, molybdopterin, selenocysteine, and an Fe4S4 cluster. Science 275:1305-1308. [DOI] [PubMed] [Google Scholar]
- 5.Buis, J. M., and J. B. Broderick. 2005. Pyruvate formate-lyase activating enzyme: elucidation of a novel mechanism for glycyl radical formation. Arch. Biochem. Biophys. 433:288-296. [DOI] [PubMed] [Google Scholar]
- 6.Canelas, A. B., W. M. van Gulik, and J. J. Heijnen. 2008. Determination of the cytosolic free NAD/NADH ratio in Saccharomyces cerevisiae under steady-state and highly dynamic conditions. Biotechnol. Bioeng. 100:734-743. [DOI] [PubMed] [Google Scholar]
- 7.Chin, H. L., Z. S. Chen, and C. P. Chou. 2003. Fedbatch operation using Clostridium acetobutylicum suspension culture as biocatalyst for enhancing hydrogen production. Biotechnol. Prog. 19:383-388. [DOI] [PubMed] [Google Scholar]
- 8.Clark, D. P. 1989. The fermentation pathways of Escherichia coli. FEMS Microbiol. Rev. 5:223-234. [DOI] [PubMed] [Google Scholar]
- 9.Di Pierro, D., B. Tavazzi, C. F. Perno, M. Bartolini, E. Balestra, R. Calio, B. Giardina, and G. Lazzarino. 1995. An ion-pairing high-performance liquid chromatographic method for the direct simultaneous determination of nucleotides, deoxynucleotides, nicotinic coenzymes, oxypurines, nucleosides, and bases in perchloric acid cell extracts. Anal. Biochem. 231:407-412. [DOI] [PubMed] [Google Scholar]
- 10.Du, L., Y. Su, D. Sun, W. Zhu, J. Wang, X. Zhuang, S. Zhou, and Y. Lu. 2008. Formic acid induces Yca1p-independent apoptosis-like cell death in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 8:531-539. [DOI] [PubMed] [Google Scholar]
- 11.Dunn, S. 2002. Hydrogen futures: toward a sustainable energy system. Int. J. Hydrogen Energy 27:235-264. [Google Scholar]
- 12.Flikweert, M. T., M. de Swaaf, J. P. van Dijken, and J. T. Pronk. 1999. Growth requirements of pyruvate-decarboxylase-negative Saccharomyces cerevisiae. FEMS Microbiol. Lett. 174:73-79. [DOI] [PubMed] [Google Scholar]
- 13.Geertman, J. M., A. J. van Maris, J. P. van Dijken, and J. T. Pronk. 2006. Physiological and genetic engineering of cytosolic redox metabolism in Saccharomyces cerevisiae for improved glycerol production. Metab. Eng. 8:532-542. [DOI] [PubMed] [Google Scholar]
- 14.Global Industry Analysts. 2006. Formic acid. Global Industry Analysts, San Jose, CA.
- 15.Gueldener, U., J. Heinisch, G. J. Koehler, D. Voss, and J. H. Hegemann. 2002. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res. 30:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hynne, F., S. Dano, and P. G. Sorensen. 2001. Full-scale model of glycolysis in Saccharomyces cerevisiae. Biophys. Chem. 94:121-163. [DOI] [PubMed] [Google Scholar]
- 17.Kessler, D., W. Herth, and J. Knappe. 1992. Ultrastructure and pyruvate formate-lyase radical quenching property of the multienzymic AdhE protein of Escherichia coli. J. Biol. Chem. 267:18073-18079. [PubMed] [Google Scholar]
- 18.Kessler, D., I. Leibrecht, and J. Knappe. 1991. Pyruvate-formate-lyase-deactivase and acetyl-CoA reductase activities of Escherichia coli reside on a polymeric protein particle encoded by adhE. FEBS Lett. 281:59-63. [DOI] [PubMed] [Google Scholar]
- 19.Kumar, N., and D. Das. 2001. Continuous hydrogen production by immobilized Enterobacter cloacae IIT-BT 08 using lignocellulosic materials as solid matrices. Enzyme Microb. Technol. 29:280-287. [Google Scholar]
- 20.Lange, H. C., M. Eman, G. van Zuijlen, D. Visser, J. C. van Dam, J. Frank, M. J. de Mattos, and J. J. Heijnen. 2001. Improved rapid sampling for in vivo kinetics of intracellular metabolites in Saccharomyces cerevisiae. Biotechnol. Bioeng. 75:406-415. [DOI] [PubMed] [Google Scholar]
- 21.Liger, D., M. Graille, C. Z. Zhou, N. Leulliot, S. Quevillon-Cheruel, K. Blondeau, J. Janin, and H. van Tilbeurgh. 2004. Crystal structure and functional characterization of yeast YLR011wp, an enzyme with NAD(P)H-FMN and ferric iron reductase activities. J. Biol. Chem. 279:34890-34897. [DOI] [PubMed] [Google Scholar]
- 22.Maeda, T., V. Sanchez-Torres, and T. K. Wood. 2007. Enhanced hydrogen production from glucose by metabolically engineered Escherichia coli. Appl. Microbiol. Biotechnol. 77:879-890. [DOI] [PubMed] [Google Scholar]
- 23.Maeda, T., V. Sanchez-Torres, and T. K. Wood. 2008. Metabolic engineering to enhance bacterial hydrogen production. Microb. Biotechnol. 1:30-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNeil, J. B., A. L. Bognar, and R. E. Pearlman. 1996. In vivo analysis of folate coenzymes and their compartmentation in Saccharomyces cerevisiae. Genetics 142:371-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nath, K., and D. Das. 2004. Improvement of fermentative hydrogen production: various approaches. Appl. Microbiol. Biotechnol. 65:520-529. [DOI] [PubMed] [Google Scholar]
- 26.Nnyepi, M. R., Y. Peng, and J. B. Broderick. 2007. Inactivation of E. coli pyruvate formate-lyase: role of AdhE and small molecules. Arch. Biochem. Biophys. 459:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohe, S. 1999. Computer aided data book of vapor pressure, 2nd ed. Data Book Publishing, Inc., Tokyo, Japan.
- 28.Overkamp, K. M., P. Kotter, R. van der Hoek, S. Schoondermark-Stolk, M. A. Luttik, J. P. van Dijken, and J. T. Pronk. 2002. Functional analysis of structural genes for NAD(+)-dependent formate dehydrogenase in Saccharomyces cerevisiae. Yeast 19:509-520. [DOI] [PubMed] [Google Scholar]
- 29.Phillips, I., and P. A. Silver. 2006. A new biobrick assembly strategy designed for facile protein engineering. In DSpace. MIT Artificial Intelligence Laboratory: MIT Synthetic Biology Working Group, Massachusetts Institute of Technology, Cambridge, MA. http://hdl.handle.net/1721.1/32535.
- 30.Piper, M. D., S. P. Hong, G. E. Ball, and I. W. Dawes. 2000. Regulation of the balance of one-carbon metabolism in Saccharomyces cerevisiae. J. Biol. Chem. 275:30987-30995. [DOI] [PubMed] [Google Scholar]
- 31.Porro, D., M. M. Bianchi, L. Brambilla, R. Menghini, D. Bolzani, V. Carrera, J. Lievense, C.-L. Liu, B. M. Ranzi, L. Frontali, and L. Alberghina. 1999. Replacement of a metabolic pathway for large-scale production of lactic acid from engineered yeasts. Appl. Environ. Microbiol. 65:4211-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rupprecht, J., B. Hankamer, J. H. Mussgnug, G. Ananyev, C. Dismukes, and O. Kruse. 2006. Perspectives and advances of biological H2 production in microorganisms. Appl. Microbiol. Biotechnol. 72:442-449. [DOI] [PubMed] [Google Scholar]
- 33.Savage, D. F., J. Way, and P. A. Silver. 2008. Defossiling fuel: how synthetic biology can transform biofuel production. ACS Chem. Biol. 3:13-16. [DOI] [PubMed] [Google Scholar]
- 34.Sawers, G., and D. P. Clark. July 2004, posting date. Chapter 3.5.3. EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. http://www.ecosal.org
- 35.Sawers, G., and G. Watson. 1998. A glycyl radical solution: oxygen-dependent interconversion of pyruvate formate-lyase. Mol. Microbiol. 29:945-954. [DOI] [PubMed] [Google Scholar]
- 36.Sawers, R. G. 2005. Formate and its role in hydrogen production in Escherichia coli. Biochem. Soc. Trans. 33:42-46. [DOI] [PubMed] [Google Scholar]
- 37.Sedlak, M., and N. W. Ho. 2004. Production of ethanol from cellulosic biomass hydrolysates using genetically engineered Saccharomyces yeast capable of cofermenting glucose and xylose. Appl. Biochem. Biotechnol. 116:403-416. [DOI] [PubMed] [Google Scholar]
- 38.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skipper, N., M. Sutherland, R. W. Davies, D. Kilburn, R. C. Miller, Jr., A. Warren, and R. Wong. 1985. Secretion of a bacterial cellulase by yeast. Science 230:958-960. [DOI] [PubMed] [Google Scholar]
- 40.Sporty, J. L., M. M. Kabir, K. W. Turteltaub, T. Ognibene, S. J. Lin, and G. Bench. 2008. Single sample extraction protocol for the quantification of NAD and NADH redox states in Saccharomyces cerevisiae. J. Sep. Sci. 31:3202-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ter Schure, E. G., N. A. van Riel, and C. T. Verrips. 2000. The role of ammonia metabolism in nitrogen catabolite repression in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 24:67-83. [DOI] [PubMed] [Google Scholar]
- 42.Theobald, U., W. Mailinger, M. Baltes, M. Rizzi, and M. Reuss. 1997. In vivo analysis of metabolic dynamics in Saccharomyces cerevisiae. I. Experimental observations. Biotechnol. Bioeng. 55:305-316. [DOI] [PubMed] [Google Scholar]
- 43.van Maris, A. J., D. A. Abbott, E. Bellissimi, J. van den Brink, M. Kuyper, M. A. Luttik, H. W. Wisselink, W. A. Scheffers, J. P. van Dijken, and J. T. Pronk. 2006. Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: current status. Antonie van Leeuwenhoek 90:391-418. [DOI] [PubMed] [Google Scholar]
- 44.Verduyn, C., E. Postma, W. A. Scheffers, and J. P. van Dijken. 1990. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J. Gen. Microbiol. 136:395-403. [DOI] [PubMed] [Google Scholar]
- 45.Vignais, P. M., and A. Colbeau. 2004. Molecular biology of microbial hydrogenases. Curr. Issues Mol. Biol. 6:159-188. [PubMed] [Google Scholar]
- 46.Visser, D., G. A. van Zuylen, J. C. van Dam, M. R. Eman, A. Proll, C. Ras, L. Wu, W. M. van Gulik, and J. J. Heijnen. 2004. Analysis of in vivo kinetics of glycolysis in aerobic Saccharomyces cerevisiae by application of glucose and ethanol pulses. Biotechnol. Bioeng 88:157-167. [DOI] [PubMed] [Google Scholar]
- 47.Winston, F., C. Dollard, and S. L. Ricupero-Hovasse. 1995. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11:53-55. [DOI] [PubMed] [Google Scholar]
- 48.Wisselink, H. W., M. J. Toirkens, M. del Rosario Franco Berriel, A. A. Winkler, J. P. van Dijken, J. T. Pronk, and A. J. van Maris. 2007. Engineering of Saccharomyces cerevisiae for efficient anaerobic alcoholic fermentation of l-arabinose. Appl. Environ. Microbiol. 73:4881-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yong, Z., and A. E. Rodrigues. 2002. Hydrotalcite-like compounds as adsorbents for carbon dioxide. Energy Convers. Manag. 43:1865-1876. [Google Scholar]
- 50.Yoshida, A., T. Nishimura, H. Kawaguchi, M. Inui, and H. Yukawa. 2007. Efficient induction of formate hydrogen lyase of aerobically grown Escherichia coli in a three-step biohydrogen production process. Appl. Microbiol. Biotechnol. 74:754-760. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida, A., T. Nishimura, H. Kawaguchi, M. Inui, and H. Yukawa. 2005. Enhanced hydrogen production from formic acid by formate hydrogen lyase-overexpressing Escherichia coli strains. Appl. Environ. Microbiol. 71:6762-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshida, A., T. Nishimura, H. Kawaguchi, M. Inui, and H. Yukawa. 2006. Enhanced hydrogen production from glucose using ldh- and frd-inactivated Escherichia coli strains. Appl. Microbiol. Biotechnol. 73:67-72. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, W., K. K. Wong, R. S. Magliozzo, and J. W. Kozarich. 2001. Inactivation of pyruvate formate-lyase by dioxygen: defining the mechanistic interplay of glycine 734 and cysteine 419 by rapid freeze-quench EPR. Biochemistry 40:4123-4130. [DOI] [PubMed] [Google Scholar]